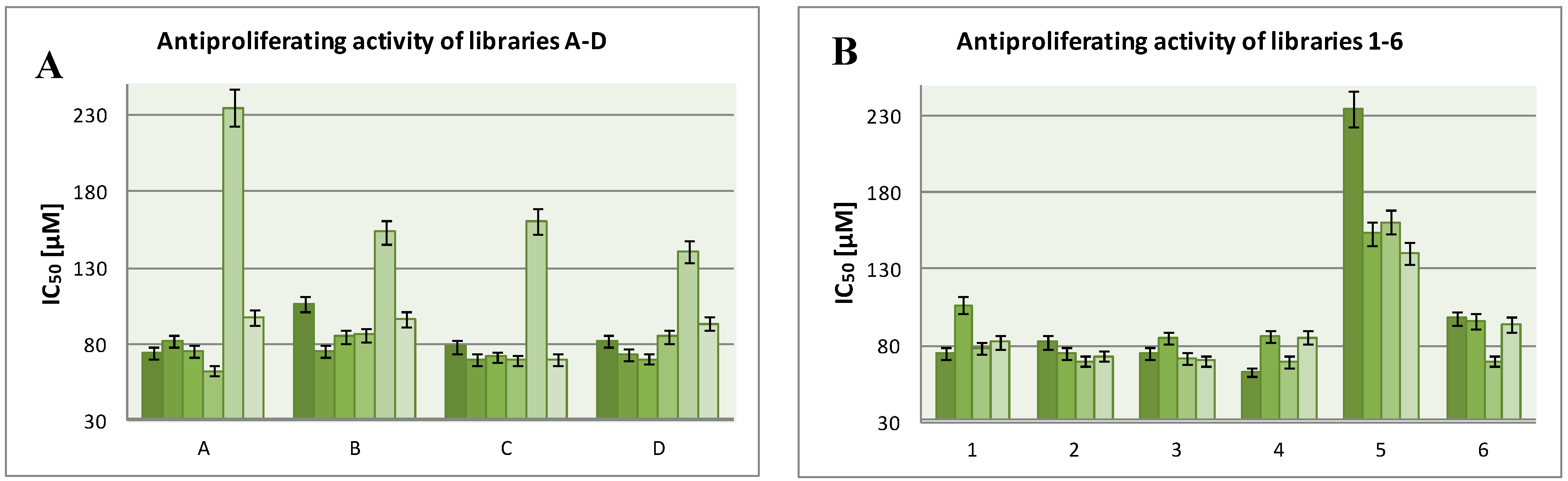
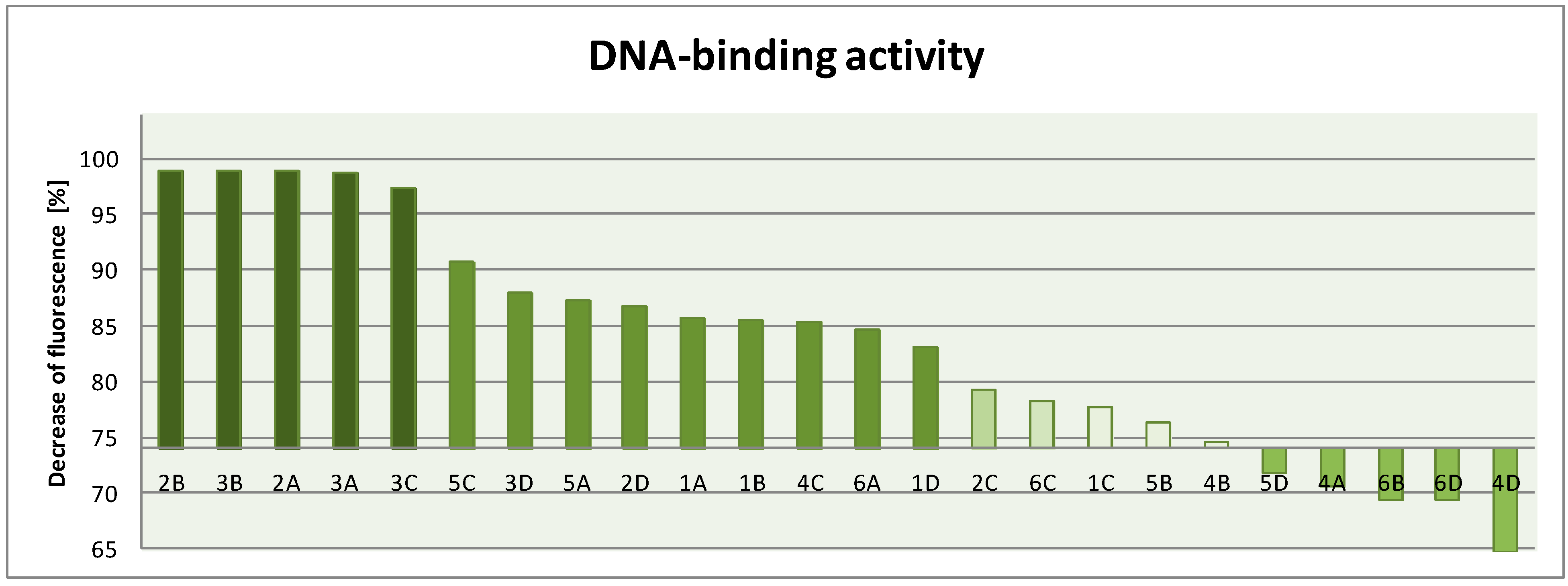
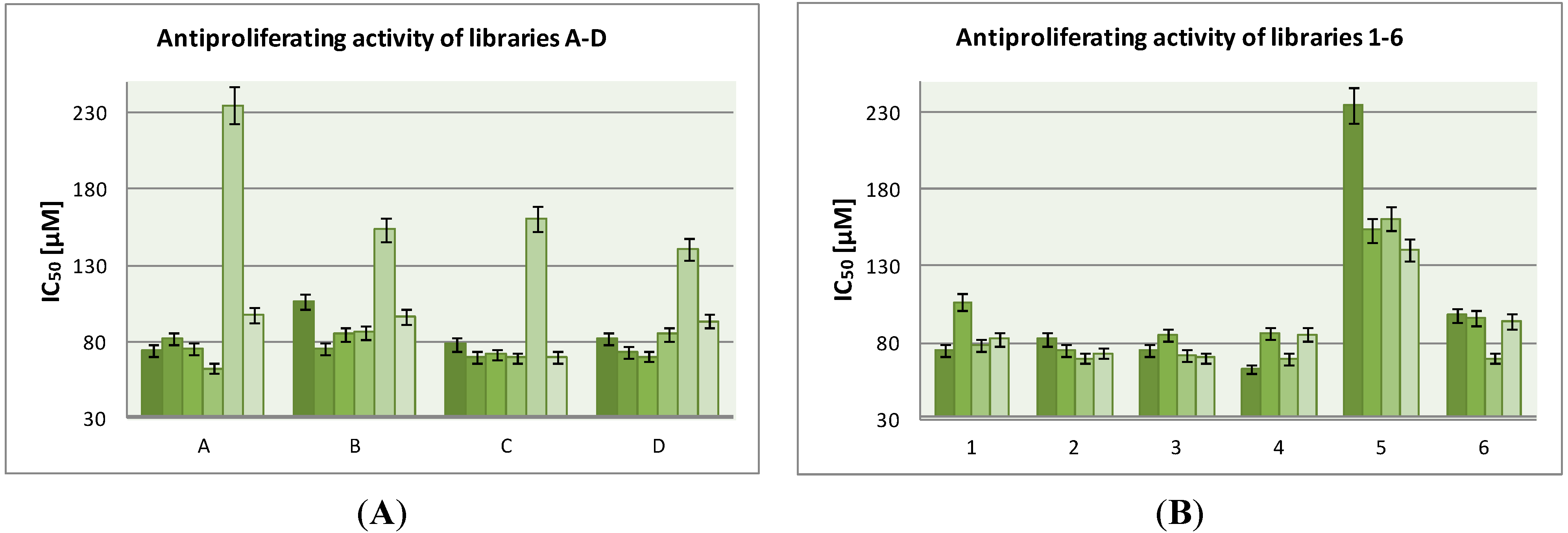
3.2. General Procedure
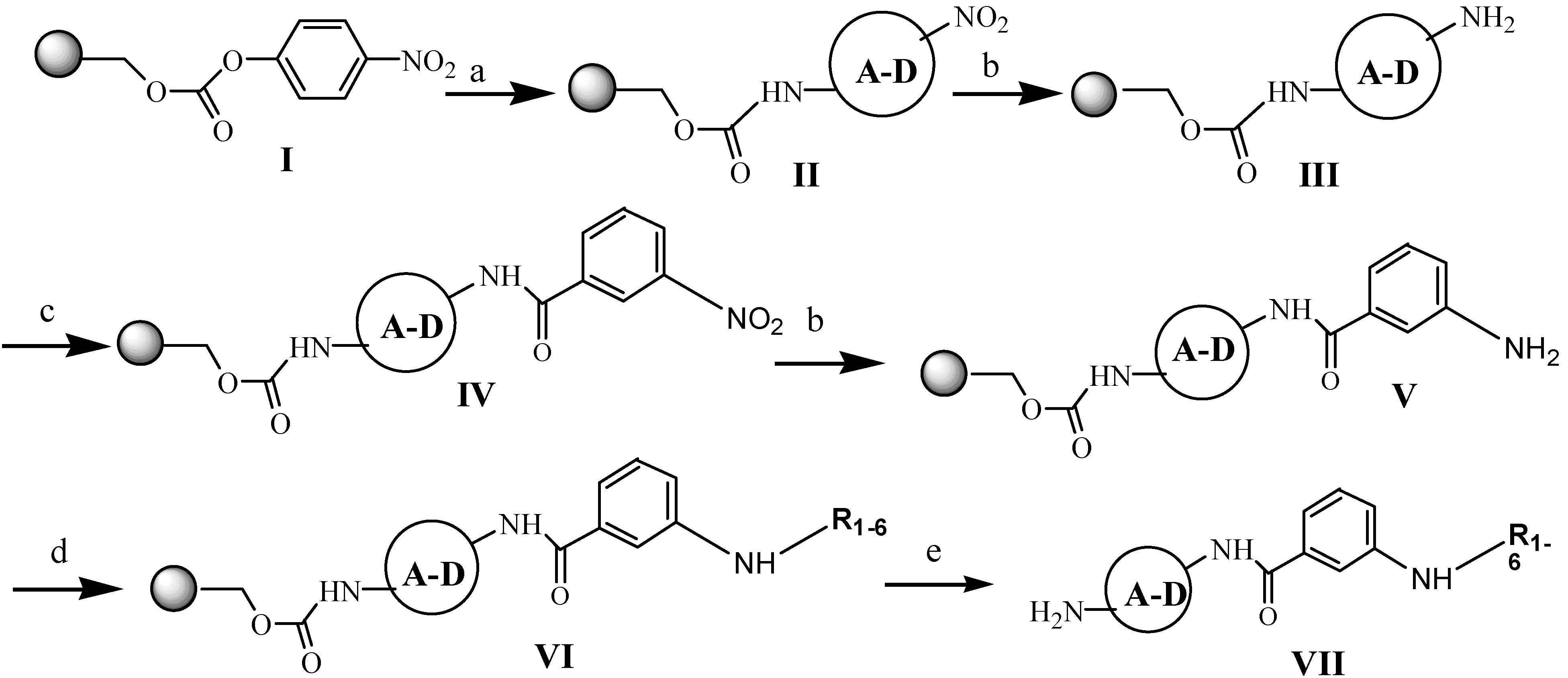
The solid-phase syntheses of the new compounds are shown in
Scheme 1. 4-Nitrophenyl Wang resin
1 (0.5 g; 0.41 mmol; 0.81 mmol/g) was suspended and swelled for 10 min in dry DCM (10 mL), then the resin was treated with nitroamines (
A,
B,
C,
D; 1.64 mmol; 4 eq) dissolved in DCM (20 mL) and pyridine (177.22 μL; 2.2 mmol). [
A (0.226 g);
B (0.225 g);
C (0.235 g);
D (0.226 g)] Then the mixture was stirred for 20 h. The products
II were washed five times with DCM (20 mL) and three times with DMF (20 mL). Then reduction of nitro groups of
II with the dihydrate of tin (II) chloride in DMF (1 M; 20 mL) during 4 h at room temperature were carried out. The resins
III were filtered off and then washed five times with DMF (20 mL) and three times with DCM (20 mL). Then the aliquots of 3-nitrobenzoyl chloride (0.304 g; 1.64 mmol; 4 eq) with DMAP (0.0025 g; 0.0205 mmol) were dissolved in DCM (20 mL) and added to resin
III. The stirring was continued at room temperature overnight. After that, the resin-bound nitro compounds
IV were washing five times with DCM (20 mL) and three times with DMF (20 mL). The treatment of thes compounds
IV with 1 M SnCl
2 allowed to obtain the intermediates
V. To prepare the resin-bond compounds
VI there were used the substrates prepared as described below. The resins
VII were washed with DCM (5 × 20 mL), DMF (5 × 20 mL) and DCM (5 × 20 mL), dried and then treated with TFA/DCM (95:5). Subsequent evaporation of the solvent and the several portions of DCM yielded the products as glaze solids.
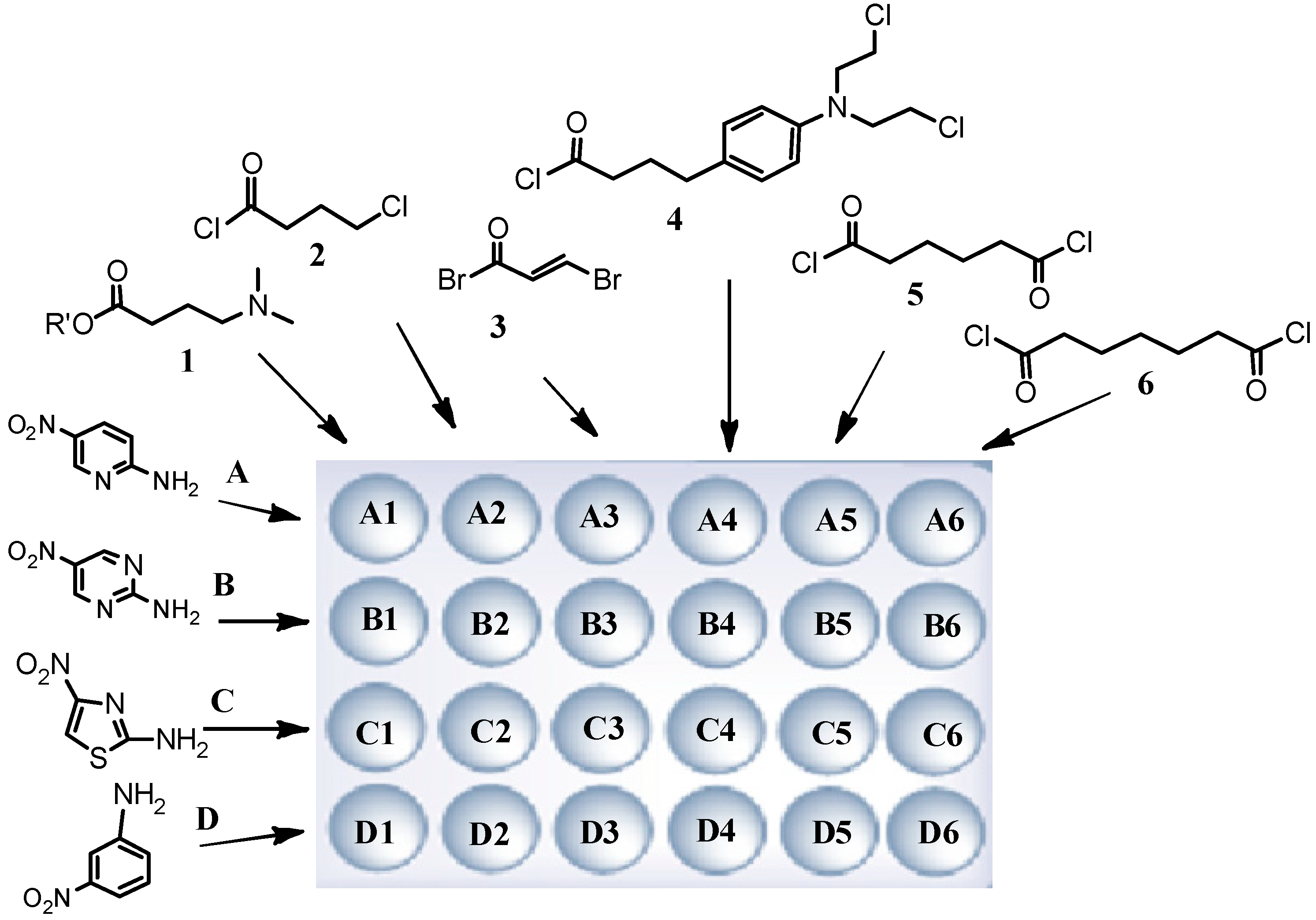
3.2.1. Library 1
To introduce the 4-(dimethylamino)butanamido- fragment, the “superactive ester”
1 was prepared as described earlier [
12]. 4-Dimethylaminobutyric acid (0.137 g; 0.82 mmol) and NMM (902 μL; 0.82 mmol) were added to a vigorously stirred solution of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium tetrafluoroborate (0.269 g; 0.82 mmol) in DMF (20 mL) and cooled to 0 °C. The stirring was continued for an additional 2 h in 0 °C, after which time these mixtures were added to the resins
V and stirred together for an additional 2h at 0 °C and overnight at room temperature.
N-(6-Aminopyridin-3-yl)-3-(4-(dimethylamino)butanamido)benzamide (A1). Yield: 0.050 g (36%). 1H-NMR (DMSO-d6) δ = 10.10 (s,1H, NH), 7.22 (t,1H, NH), 6.80–7.70 (m, 7H, Ar-H), 3.95 (s, 2H, NH2), 2.99 (t, 2H, CH2), 2.72 (s, 6H, CH3), 2.32 (t, 2H, CH2); 1.85–1.88 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 173.45 (CONH), 166.93 (CONH), 159.90 (C), 157.89 (C), 149.90 (CH), 139.72(C), 134.58 (CH), 131.85 (C), 129.61 (CH), 123.36 (CH), 119.71 (CH), 117.02 (CH), 115.64 (CH), 59.87 (CH2), 42.05 (CH3), 30.46 (CH2), 19.70 (CH2).
N-(2-Aminopyrimidin-5-yl)-3-(4-(dimethylamino)butanamido)benzamide (B1). Yield: 0.045 g (32%). 1H-NMR (DMSO-d6) δ = 9.48 (s,1H, NH), 7.27 (t,1H, NH), 6.85–7.35 (m, 6H, Ar-H), 3.30–4.10 (bs, 2H, NH2), 2.99 (t, 2H, CH2), 2.77 (s, 6H, CH3), 2.31 (t, 2H, CH2); 1.78–1.88 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 173.38 (CONH), 167.48 (CONH), 159.20 (C), 157. 96 (C), 139.72(C), 134.55 (CH), 134.33 (CH), 131.52 (C), 129.10 (CH), 119.71 (CH), 116.98 (CH), 115.63 (CH), 59.85 (CH2), 42.23 (CH3), 30.35 (CH2), 19.36 (CH2).
N-(2-Aminothiazol-5-yl)-3-(4-(dimethylamino)butanamido)benzamide (C1). Yield: 0.040 g (28%). 1H-NMR (DMSO-d6) δ = 9.48 (s,1H, NH), 7.95 (t,1H, NH), 6.83–7.37 (m, 5H, Ar-H), 3.94 (s, 2H, NH2), 2.99 (t, 2H, CH2), 2.77 (s, 6H, CH3), 2.28 (t, 2H, CH2); 1.80–1.86 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 173.44 (CONH), 167.50 (CONH), 162.30 (C), 157.85 (C), 134.58 (C), 131.53 (C), 129.16 (CH), 120.04 (CH), 119.29 (CH), 116.49 (CH), 115.63 (CH), 59.87 (CH2), 42.22 (CH3), 30.36 (CH2), 19.37 (CH2).
N-(3-Aminophenyl)-3-(4-(dimethylamino)butanamido)benzamide (D1). Yield: 0.049 g (35%). 1H-NMR (DMSO-d6) δ = 9.48 (s,1H, NH), 7.22 (t,1H, NH), 6.84–7.95 (m, 8H, Ar-H), 4.54 (s, 2H, NH2), 2.99 (t, 2H, CH2), 2.73 (s, 6H, CH3), 2.31 (t, 2H, CH2); 1.85–1.88 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 173.45 (CONH), 166.93 (CONH), 159.90 (C), 157.89 (C), 149.90 (CH), 139.72(C), 134.58 (CH), 131.85 (C), 129.61 (CH), 123.36 (CH), 119.71 (CH), 117.02 (CH), 115.64 (CH), 59.87 (CH2), 42.05 (CH3), 30.46 (CH2), 19.70 (CH2).
3.2.2. Library 2
4-Chlorobutanamido derivatives were obtained by direct acylation of resins V by 4-chlorobutanoyl chloride (2, 2 mmol; 0.282 g; 0.224 mL). The above acid chloride was dissolved in DCM (20 mL), chilled to 0 °C and every portion was added to chilled resins V with DMAP (0.0025 g; 0.0205 mmol). These mixtures were stirred for 2 h in 0 °C and overnight at room temperature.
N-(6-Aminopyridin-3-yl)-3-(4-chlorobutanamido)benzamide (A2). Yield: 0.037 g (27%). 1H-NMR (DMSO-d6) δ = 10.08 (s,1H, NH), 7.40 (t,1H, NH), 6.88–8.22 (m, 7H, Ar-H), 3.90–4.40 (bs, 2H, NH2), 3.70 (t, 2H, CH2), 2.36 (t, 2H, CH2); 1.88–1.98 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 170.43 (CONH), 167.13 (CONH), 162.29 (CH), 148.79 (C), 139.37 (C), 131.30 (C), 131.23 (CH), 131.23 (C), 128.90 (CH), 123.13 (CH), 117.93 (CH), 116.59 (CH), 114.41 (CH), 45.01 (CH2), 33.35 (CH2), 27.83 (CH2).
N-(2-Aminopyrimidin-5-yl)-3-(4-chlorobutanamido)benzamide (B2). Yield: 0.047 g (34%). 1H-NMR (DMSO-d6) δ = 10.09 (s,1H, NH), 7.21 (t,1H, NH), 6.82–8.35 (m, 6H, Ar-H), 3.30–4.10 (bs, 2H, NH2), 3.75 (t, 2H, CH2), 2.35 (t, 2H, CH2); 1.88–1.98 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 171.38 (CONH), 167.79 (CONH), 159.88 (CH), 157. 96 (C), 139.72(C), 136.64 (CH), 132.24 (C), 129.38 (C), 129.18 (CH), 119.81 (CH), 117.12 (CH), 114.81 (CH), 59.85 (CH2), 42.23 (CH3), 33.37 (CH2), 27.86 (CH2).
N-(2-Aminothiazol-5-yl)-3-(4-chlorobutanamido)benzamide (C2). Yield: 0.032 g (23%). 1H-NMR (DMSO-d6) δ = 10.09 (s,1H, NH), 7.41 (t,1H, NH), 6.87–8.81 (m, 5H, Ar-H), 3.20–3.70 (bs, 2H, NH2), 3.80 (t, 2H, CH2), 2.35 (t, 2H, CH2); 1.85–1.96 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 171.44 (CONH), 167.66 (CONH), 167.13 (C), 139.37 (C), 134.58 (C), 131.53 (C), 129.16 (CH), 120.04 (CH), 119.29 (CH), 115.34 (CH), 115.32(CH), 44.95 (CH2), 33.34 (CH2), 27.83 (CH2).
N-(3-Aminophenyl)-3-(4-chlorobutanamido)benzamide (D2). Yield: 0.042 g (31%). 1H-NMR (DMSO-d6) δ = 10.06 (s,1H, NH), 7.42 (t,1H, NH), 6.76–8.31 (m, 8H, Ar-H), 4.34 (s, 2H, NH2), 3.75 (t, 2H, CH2), 2.36 (t, 2H, CH2); 1.81–1.85 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.35 (CONH), 167.78 (CONH), 167.12 (C), 149.90 (C), 139.72(C), 134.58 (CH), 131.31 (C), 129.61 (CH), 128.88 (CH), 123.36 (CH), 119.71 (CH), 118.09 (CH), 117.02 (CH), 115.64 (CH), 44.93 (CH2), 33.34 (CH2), 27.82 (CH2).
3.2.3. Library 3
Bromoacrylic acid chloride (3) was used to carry out the acylation reaction of resins V. 2-Bromoacrylic acid (2 mmol; 0.302 g) was dissolved in dry THF (20 mL), then oxalyl chloride (4 mmol; 0.508 g; 0.343 mL) was added, and warming to mild reflux under a drying tube for 2 h. The excess oxalyl chloride and solvent were removed under reduced pressure and the residue co-evaporated with dry DCM (5 mL, twice). The above acid chloride was dissolved in DCM (20 mL), chilled to 0 °C and such portions were added to chilled resins V with DMAP (0.0025 g; 0.0205 mmol). The mixtures were stirred for 2 h in 0 °C and overnight at room temperature.
(E)-N-(6-Aminopyridin-3-yl)-3-(3-bromoacrylamido)benzamide (A3). Yield: 0.044 g (30%). 1H-NMR (DMSO-d6) δ = 10.35 (s,1H, NH), 9.65 (s,1H, NH), 6.78–8.31 (m, 7H, Ar-H), 7.80 (d, 1H, CH), 7.65 (d, 1H, CH), 3.25–3.65 (bs, 2H, NH2). 13C-NMR (DMSO-d6) δ = 167.71 (CONH), 162.26 (CH), 160.74 (CONH), 148.79 (C), 139.37(C), 131.30 (C), 131.23 (C), 129.10 (CH), 128.86 (CH), 124.36 (CH), 123.20 (CH), 118.34 (CH), 117.93 (CH), 116.59 (CH), 114.41 (CH).
(E)-N-(2-Aminopyrimidin-5-yl)-3-(3-bromoacrylamido)benzamide (B3). Yield: 0.045 g (30%). 1H-NMR (DMSO-d6) δ = 10.36 (s,1H, NH), 9.66 (s,1H, NH), 6.62–8.31 (m, 6H, Ar-H), 7.80 (d, 1H, CH), 7.65 (d, 1H, CH), 3.20–3.45 (bs, 2H, NH2). 13C-NMR (DMSO-d6) δ = 167.78 (CONH), 162.26 (CH), 160.74 (CONH), 148.56 (C), 139.72(C), 136.64 (CH), 132.24 (C), 131.30 (C), 129.38 (CH), 128.81 (CH), 119.81 (CH), 118.05 (CH), 116.74 (CH), 114.81 (CH).
(E)-N-(2-Aminothiazol-5-yl)-3-(3-bromoacrylamido)benzamide (C3). Yield: 0.042 g (28%). 1H-NMR (DMSO-d6) δ = 10.87 (s,1H, NH), 9.66 (t,1H, NH), 6.80–8.31 (m, 5H, Ar-H), 7.80 (d, 1H, CH), 7.64 (d, 1H, CH), 3.30–3.70 (bs, 2H, NH2). 13C-NMR (DMSO-d6) δ = 167.75 (CONH), 162.28 (CH), 160.76 (CONH), 148.11 (C), 139.37 (C), 134.58 (C), 131.34 (C), 128.87 (CH), 120.04 (CH), 119.29 (CH), 118.33 (CH), 117.09 (CH), 114.79(CH).
(E)-N-(3-Aminophenyl)-3-(3-bromoacrylamido)benzamide (D3). Yield: 0.041 g (28%). 1H-NMR (DMSO-d6) δ = 10.10 (s,1H, NH), 9.12 (s,1H, NH), 6.76–8.31 (m, 8H, Ar-H), 7.80 (d, 1H, CH), 7.64 (d, 1H, CH), 3.30–3.50 (bs, 2H, NH2). 13C-NMR (DMSO-d6) δ = 167.80 (CONH), 162.30 (CH), 160.77 (CONH), 149.80 (C), 132.72 (C), 132.58 (CH), 131.32 (2C), 129.61 (CH), 128.87 (CH), 123.36 (CH), 119.71 (CH), 118.18 (CH), 116.90 (CH), 115.604 (CH), 114.64 (CH).
3.2.4. Library 4
Chlorambucil chloride (4) was used to carry out the acylation reaction of resins V. Chlorambucil (2 mmol; 0.608 g) was dissolved in dry THF (20 mL), then oxalyl chloride (4 mmol; 0.508 g; 0.343 mL) was added, and warming to mild reflux under a drying tube for 2 h. The excess oxalyl chloride and solvent were removed under reduced pressure and the residue co-evaporated with dry DCM (5 mL, twice). The above acid chloride was dissolved in DCM (20 mL), chilled to 0 °C and such portions were added to chilled resins V with DMAP (0.0025 g; 0.0205 mmol). The mixture were stirred for 2 h in 0 °C and overnight at room temperature.
N-(6-Aminopyridin-3-yl)-3-(4-(4-(bis(2-chloroethyl)amino)phenyl)butanamido)benzamide (A4). Yield: 0.060 g (28%). 1H-NMR (DMSO-d6) δ = 10.08 (s,1H, NH), 7.40 (t,1H, NH), 6.65–8.42 (m, 11H, Ar-H), 3.70–4.30 (bs, 2H, NH2), 3.69 (t, 8H, CH2), 2.47 (t, 2H, CH2), 2.17 (t, 2H, CH2), 1.65–1.88 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.31 (CONH), 167.17 (CONH), 161.89 (CH), 157.10 (C), 144.46 (C), 139.52 (C), 137.91 (C), 131.35 (C), 131.23 (CH), 131.20 (C), 129.54 (CH), 129.28 (CH), 129.00 (CH), 128.84 (CH), 125.32 (C), 124.53 (C), 123.13 (CH), 123.09 (CH), 121.08 (CH), 119.81 (CH), 111.97 (CH), 52.24 (2CH2), 41.17 (2CH2), 33.60 (CH2), 33.08 (CH2), 26.57 (CH2).
N-(2-Aminopyrimidin-5-yl)-3-(4-(4-(bis(2-chloroethyl)amino)phenyl)butanamido)benzamide (B4). Yield: 0.056 g (26%). 1H-NMR (DMSO-d6) δ = 10.09 (s,1H, NH), 7.30 (t,1H, NH), 6.65–8.43 (m, 10H, Ar-H), 3.30–4.10 (bs, 2H, NH2), 3.70 (t, 8H, CH2), 2.47 (t, 2H, CH2), 2.19 (t, 2H, CH2), 1.64–1.80 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.26 (CONH), 167.14 (CONH), 161.87 (CH), 157. 08 (C), 144.44 (C), 139.72 (C), 137.89 (C), 132.24 (C), 131.18 (C), 129.52 (CH), 129.26 (CH), 128.82 (CH), 125.29 (CH), 124.51 (CH), 123.11 (CH), 121.07 (CH), 119.79 (CH), 111.95 (CH), 51.99 (2CH2), 40.88 (2CH2), 33.58 (CH2), 33.06 (CH2), 26.54 (CH2).
N-(2-Aminothiazol-5-yl)-3-(4-(4-(bis(2-chloroethyl)amino)phenyl)butanamido)benzamide (C4). Yield: 0.061 g (29%). 1H-NMR (DMSO-d6) δ = 10.08 (s,1H, NH), 7.40 (t,1H, NH), 6.65–8.83 (m, 9H, Ar-H), 3.20–3.80 (bs, 2H, NH2), 3.65 (t, 2H, CH2), 2.45 (t, 2H, CH2), 2.18 (t, 2H, CH2), 1.65–1.80 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 171.44 (CONH), 171.26 (CONH), 167.16 (C), 162.30 (C), 144.45 (C), 139.37 (C), 134.58 (C), 131.53 (C), 129.69 (CH), 129.16 (CH), 128.93 (CH), 128.84 (CH), 120.04 (CH), 119.29 (CH), 111.94 (2CH), 111.66 (CH), 52.23 (2CH2), 40.94 (2CH2), 33.33 (CH2), 33.07 (CH2), 26.56 (CH2).
N-(3-Aminophenyl)-3-(4-(4-(bis(2-chloroethyl)amino)phenyl)butanamido)benzamide (D4). Yield: 0.059 g (28%). 1H-NMR (DMSO-d6) δ = 10.06 (s,1H, NH), 7.42 (t,1H, NH), 6.64–8.24 (m, 12H, Ar-H), 4.20–4.90 (bs, 2H, NH2), 3.69 (t, 2H, CH2), 2.35 (t, 2H, CH2); 1.64–1.85 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.32 (CONH), 171.32 (CONH), 162.30 (CH), 158.42 (C), 157.74 (C), 145.21 (C), 144.46 (C), 139.72 (C), 131.20 (C), 129.77 (CH), 129.61 (CH), 129.11 (CH), 128.86 (CH), 123.72 (CH), 123.12 (CH), 119.79 (CH), 119.16 (CH), 113.30 (CH), 111.93 (CH), 111.54 (CH), 52.22 (2CH2), 39.52 (2CH2), 33.34 (CH2), 33.07 (CH2), 26.57 (CH2).
3.2.5. Library 5
Bis-netropsin derivatives in library 5 were prepared by direct acylation of resins V by adipoyl chloride (5, 2 mmol; 0.366 g; 0.293 mL). The above acid chloride was dissolved in DCM (20 mL), chilled to 0 °C and this solution was added to chilled resins V with DMAP (0.0025 g; 0.0205 mmol). The mixtures were stirred for 2 h in 0 °C and overnight at room temperature.
N1,N6-bis(3-((6-Aminopyridin-3-yl)carbamoyl)phenyl)adipamide (A5). Yield: 0.042 g (36%). 1H-NMR (DMSO-d6) δ = 10.08 (s, 2H, NH), 7.62 (t, 2H, NH), 6.71–8.06 (m, 14H, Ar-H), 5.20–5.50 (bs, 4H, NH2), 2.37 (t, 4H, CH2); 1.66 (t, 4H, CH2). 13C-NMR (DMSO-d6) δ = 174.32 (2CONH), 171.29 (2CONH), 164.99 (2C), 153.67 (2C), 139.38(2C), 135.93 (2CH), 130.69 (2C), 128.58 (2CH), 122.18 (2CH), 121.72 (CH), 120.84 (2CH), 118.49 (2CH), 115.15 (2CH), 114.95 (CH), 36.25 (2CH2), 24.83 (2CH2).
N1,N6-bis(3-((2-Aminopyrimidin-5-yl)carbamoyl)phenyl)adipamide (B5). Yield: 0.047 g (40%). 1H-NMR (DMSO-d6) δ = 10.09 (s, 2H, NH), 7,60 (t, 2H, NH), 6.71–8.15 (m, 12H, Ar-H), 4.10–5.00 (bs, 4H, NH2), 2.37 (t, 4H, CH2); 1.66 (t, 4H, CH2). 13C-NMR (DMSO-d6) δ = 174.32 (2CONH), 171.29 (2CONH), 164.99 (2C), 153.676 (2C), 139.37 (2C), 135.93 (CH), 130.68 (2C), 128.59 (2CH), 122.17 (2CH), 121.73 (2CH), 118.49 (2CH), 115.14 (2CH), 36.26 (2CH2), 24.83 (2CH2).
N1,N6-bis(3-((2-Aminothiazol-4-yl)carbamoyl)phenyl)adipamide (C5). Yield: 0.034 g (29%). 1H-NMR (DMSO-d6) δ = 10.08 (s, 2H, NH), 7.60 (t, 2H, NH), 6.81–8.22 (m, 10H, Ar-H), 3.30–3.90 (bs, 4H, NH2), 2.36 (t, 4H, CH2); 1.51 (t, 4H, CH2). 13C-NMR (DMSO-d6) δ = 174.30 (2CONH), 171.30 (2CONH), 167.16 (2C), 162.30 (2C), 139.47 (2C), 131.53 (2C), 128.88 (2CH), 123.11 (2CH), 119.79 (2CH), 115.82 (2CH), 115.63 (2CH), 36.23 (2CH2), 24.75 (2CH2).
N1,N6-bis(3-((4-Aminophenyl)carbamoyl)phenyl)adipamide (D5). Yield: 0.048 g (41%). 1H-NMR (DMSO-d6) δ = 10.08 (s, 2H, NH), 7.60 (t, 2H, NH), 6.71–8.05 (m, 16H, Ar-H), 3.90–4.50 (bs, 4H, NH2), 2.37 (t, 4H, CH2); 1.65 (t, 4H, CH2). 13C-NMR (DMSO-d6) δ = 174.33 (2CONH), 171.29 (2CONH), 164.98 (2C), 153.66 (2C), 139.38 (2C), 135.93 (2CH), 130.68 (2C), 128.59 (2CH), 122.17 (2CH), 121.73 (2CH), 120.83 (2CH), 120.75 (2CH), 118.49 (2CH), 114.95 (2CH), 36.26 (2CH2), 24.82 (2CH2).
3.2.6. Library 6
Derivatives in library 6 were obtained using pimeloyl chloride (6, 2 mmol; 0.394 g; 0.327 mL), by direct acylation of resin V. The above acid chloride was dissolved in DCM (20 mL), chilled to 0 °C and then added to a chilled resin V with DMAP (0.0025 g; 0.0205 mmol). The mixtures were stirred for 2 h in 0 °C and overnight at room temperature.
N1,N7-bis(3-((6-Aminopyridin-3-yl)carbamoyl)phenyl)heptanediamide (A6). Yield: 0.045 g (38%). 1H-NMR (DMSO-d6) δ = 10.08 (s, 2H, NH), 7.95 (t, 2H, NH), 6.97–8.23 (m, 14H, Ar-H), 3.10–3.80 (bs, 4H, NH2), 2.33 (t, 4H, CH2); 1.48–1.56 (m, 4H, CH2), 1.15–1.45 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.41 (2CONH), 171.46 (2CONH), 167.20 (2C), 156.96 (2C), 139.53 (2C), 139.13 (2CH), 131.24 (2C), 128.88 (2CH), 123.75 (2CH), 123.10 (4CH), 119.79 (2CH), 106.95 (2CH), 36.26 (CH2), 33.56 (CH2), 28.12 (CH2), 24.83 (CH2), 24.23 (CH2).
N1,N7-bis(3-((2-Aminopyrimidin-5-yl)carbamoyl)phenyl)heptanediamide (B6). Yield: 0.056 g (47%). 1H-NMR (DMSO-d6) δ = 10.07 (s, 2H, NH), 7,60 (t, 2H, NH), 6.95–8.23 (m, 12H, Ar-H), 3.10–3.70 (bs, 4H, NH2), 2.34 (t, 4H, CH2); 1.38–1.66 (m, 4H, CH2), 1.15–1.40 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.40 (2CONH), 171.46 (2CONH), 167.19 (2C), 156.97 (2C), 139.37 (2C), 139.14 (2CH), 131.25 (2C), 128.88 (2CH), 123.75 (2CH), 123.11 (2CH), 119.80 (2CH), 106.96 (2CH), 36.26 (CH2), 33.56 (CH2), 28.11 (CH2), 24.80 (CH2), 24.23 (CH2).
N1,N7-bis(3-((2-Aminothiazol-4-yl)carbamoyl)phenyl)heptanediamide (C6). Yield: 0.052 g (43%). 1H-NMR (DMSO-d6) δ = 10.10 (s, 2H, NH), 7.60 (t, 2H, NH), 6.95–8.23 (m, 10H, Ar-H), 3.10–3.20 (bs, 4H, NH2), 2.33 (t, 4H, CH2); 1.40–1.70 (m, 4H, CH2), 1.15–1.40 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.44 (2CONH), 171.52 (2CONH), 167.24 (2C), 157.01 (2C), 139.59 (2C), 139.17 (2CH), 131.59 (2C), 128.88 (2CH), 123.14 (2CH), 119.85 (2CH), 115.68 (2CH), 106.99 (2CH), 36.30 (CH2), 33.61 (CH2), 28.16 (CH2), 24.85 (CH2), 24.28 (CH2).
N1,N7-bis(3-((4-Aminophenyl)carbamoyl)phenyl)heptanediamide (D6). Yield: 0.044 g (37%). 1H-NMR (DMSO-d6) δ = 10.11 (s, 2H, NH), 7.40 (t, 2H, NH), 6.55–8.23 (m, 16H, Ar-H), 3.00–3.50 (bs, 4H, NH2), 2.34 (t, 4H, CH2); 1.40–1.70 (m, 4H, CH2), 1.15–1.40 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ = 174.38 (2CONH), 171.46 (2CONH), 167.18 (2C), 156.94 (2C), 149.73 (2C), 139.53 (2CH), 139.07 (2CH), 131.22 (2C), 128.86 (2CH), 123.72 (2CH), 123.09 (2CH), 119.77 (2CH), 115.63 (2CH), 106.95 (2CH), 36.23 (CH2), 33.55 (CH2), 28.17 (CH2), 24.79 (CH2), 24.22 (CH2).