Effects of UV-C Irradiation and Thermal Processing on the Microbial and Physicochemical Properties of Agave tequilana Weber var. azul Extracts at Various pH Values
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Agave Tequilana Plant
2.3. Obtaining A. tequilana Weber var. azul Extract
2.4. Extract Characterization
2.5. UV-C Irradiation Treatments
2.6. Thermal Treatment (TT)
2.7. UV-C Irradiation Measurements
2.8. Microbiological Analysis
2.9. Analytical Methods
2.10. Color Measurements
2.11. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of the Agave Extract
3.2. Microbiological Inactivation
3.3. Physicochemical Properties
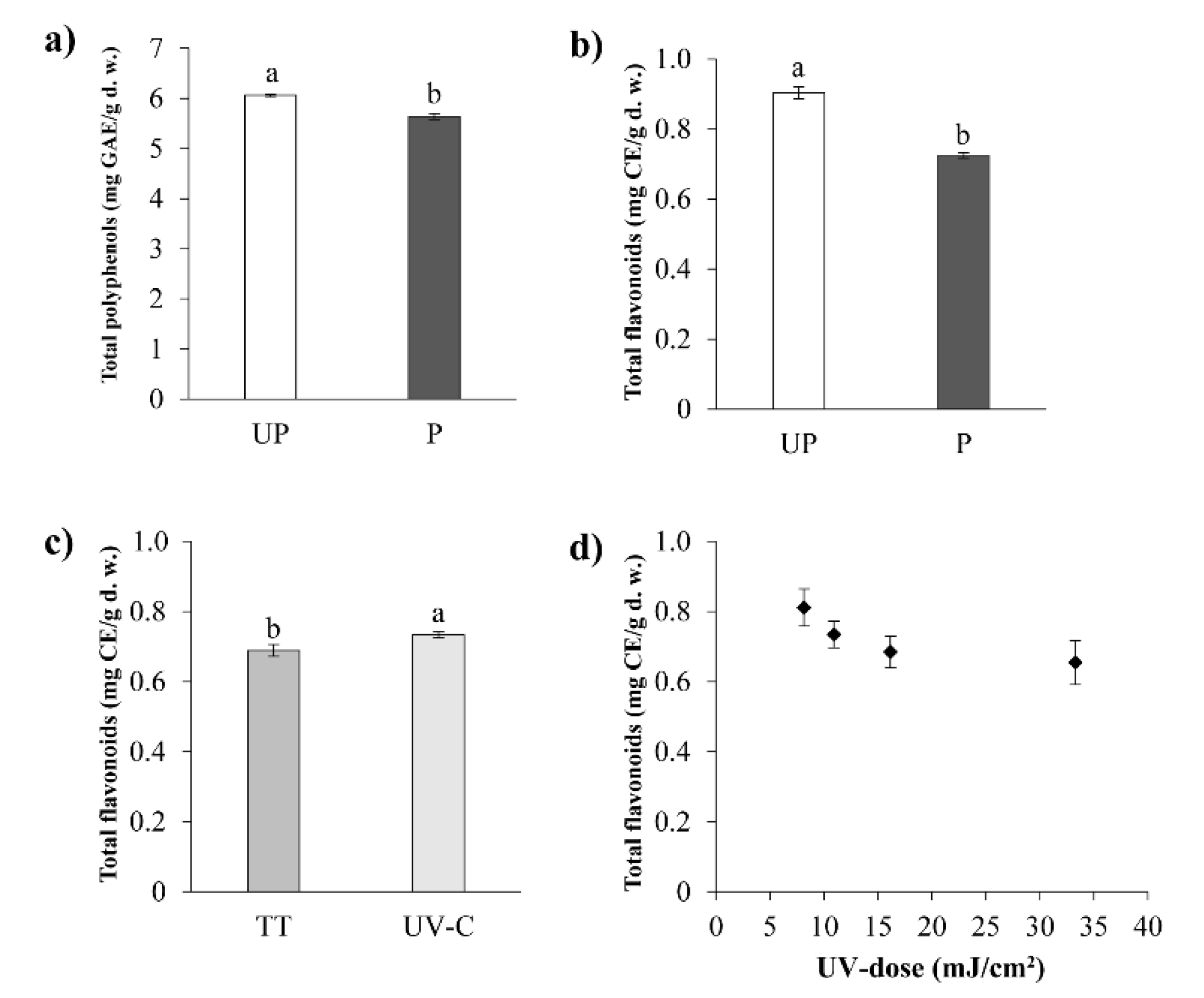
3.3.1. Total Polyphenols (TP)
3.3.2. Total Flavonoids (TF)
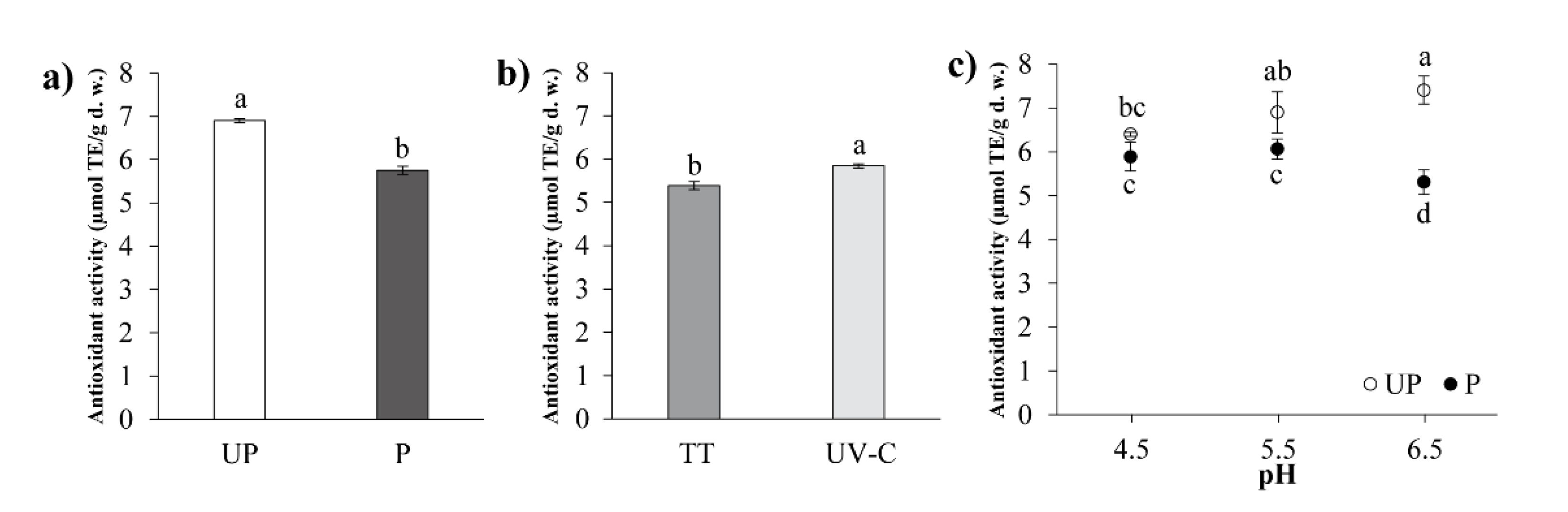
3.3.3. Antioxidant Activity (AA)
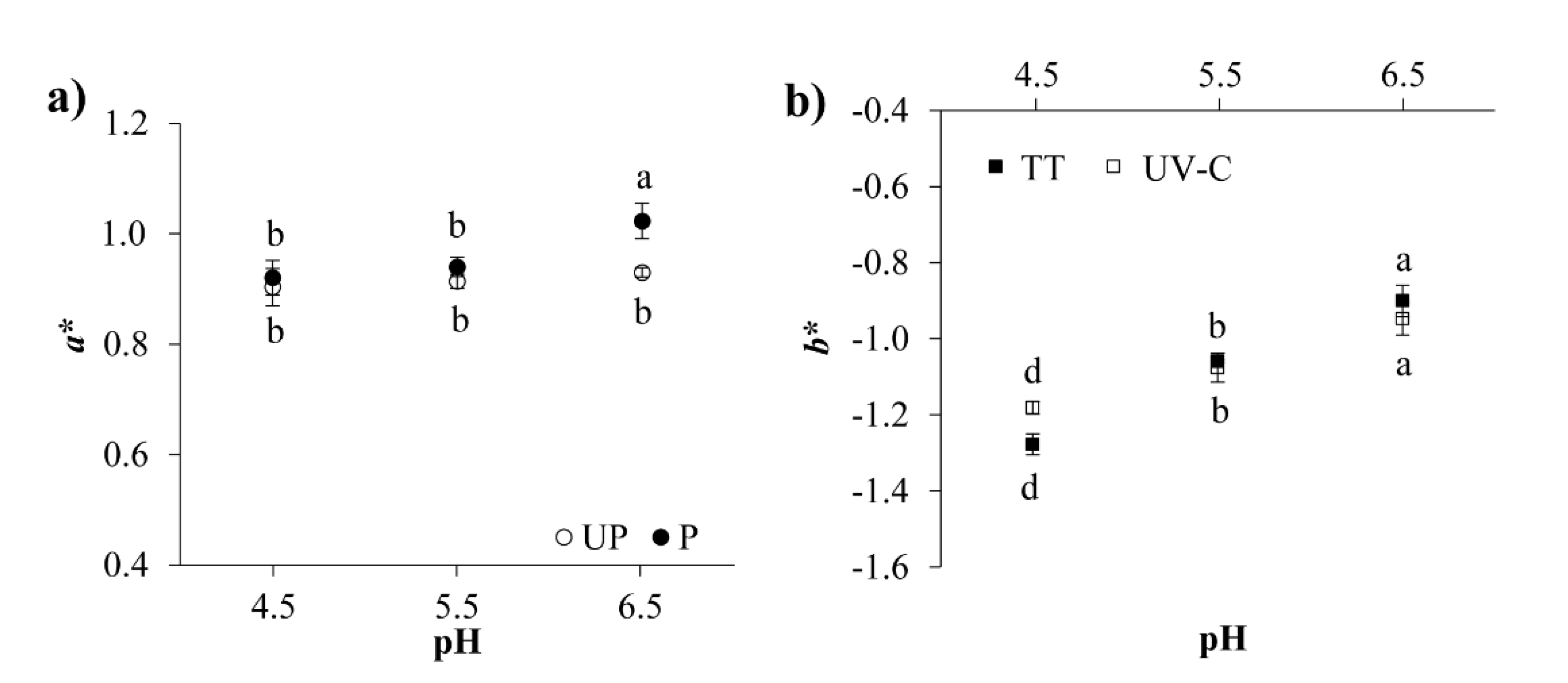
3.3.4. Color Analysis
3.3.5. Total Sugars (TS)
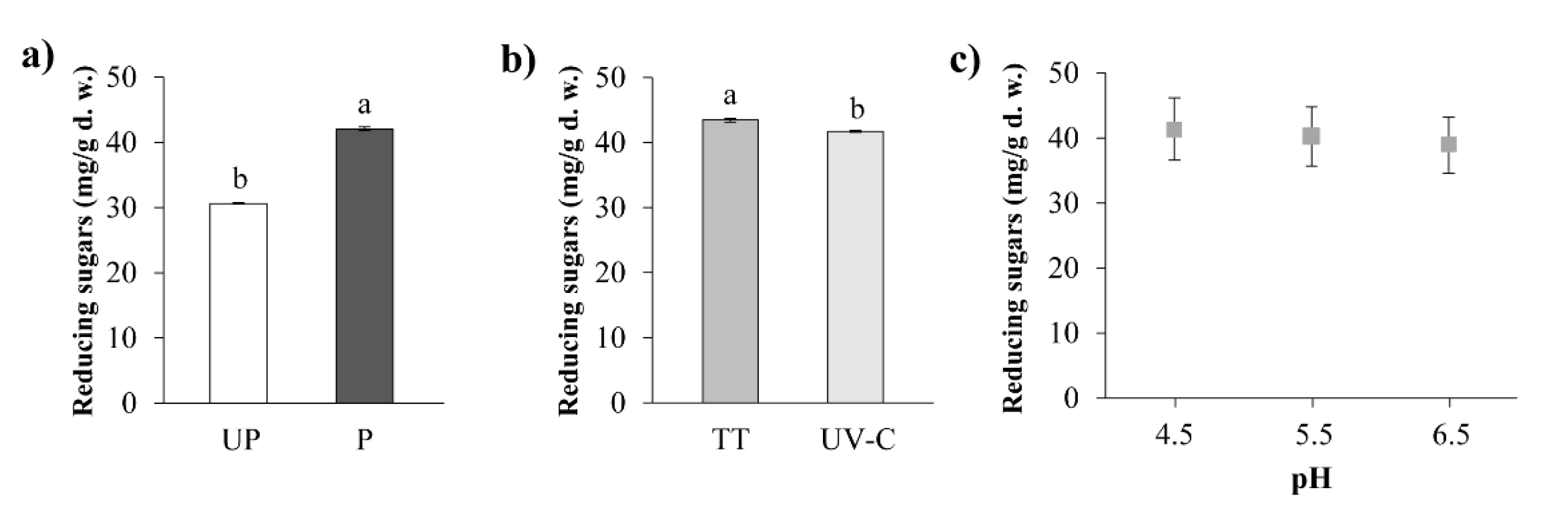
3.3.6. Reducing Sugars (RS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UV-C | Ultraviolet light of short wavelengths |
| TT | Thermal treatment |
| D | UV-C Irradiation Dose |
| UP | Unprocessed |
| P | Processed |
| LRF | Log reduction factor |
| TAM | Total aerobic mesophiles |
| TC | Total coliforms |
| YM | Yeasts and molds |
| PS | Psycrophiles |
| CFU | Colony forming units |
| TP | Total polyphenols |
| TS | Total sugars |
| TF | Total flavonoids |
| AA | Antioxidant activity |
| L* | Lightness parameter |
| a* | a* color parameter |
| b* | b* color parameter |
| ∆E | Total color difference |
| mJ/cm2 | Millijoules per square centimeter |
References
- López, M.G.; Urías-Silvas, J.E. Agave fructans as prebiotics. In Recent Advances in Fructooligosacharides Research; Shiomi, N., Benkeblia, N., Onodera, S., Eds.; Research Signpoint: Kerala, India, 2007; pp. 297–310. [Google Scholar]
- Waleckx, E.; Gschaedler, A.; Colonna-Ceccaldi, B.; Monsan, P. Hydrolysis of fructans from Agave tequilana Weber var. azul during the cooking step in a traditional tequila elaboration process. Food Chem. 2008, 108, 40–48. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López, M.G. Identification, classification, and discrimination of agave syrups from natural sweeteners by infrared spectroscopy and HPAEC-PAD. Food Chem. 2015, 167, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Márquez, D.B.; Contreras, J.C.; Rodríguez, R.; Mussatto, S.I.; Wong-Paz, J.E.; Teixeira, J.A.; Aguilar, C.N. Influence of thermal effect on sugars composition of Mexican Agave syrup. CyTA-J. Food. 2015, 13, 607–612. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Leal-Díaz, M.A.; Cortés-Ceballos, E.; Gutiérrez-Uribe, J.A. Agave (Agave spp.) and its traditional products as a source of bioactive compounds. Curr. Bioact. Compd. 2012, 8, 218–231. [Google Scholar] [CrossRef]
- Arrizon, J.; Morel, S.; Gschaedler, A.; Monsan, P. Comparison of the water-soluble carbohydrate composition and fructan structures of Agave tequilana plants of different ages. Food Chem. 2010, 122, 123–130. [Google Scholar] [CrossRef]
- Ade-Ajayi, A.F.; Hammuel, C.; Ezeayanaso, C.; Ogabiela, E.E.; Udiba, U.U.; Anyim, B.; Olabanji, O. Preliminary phytochemical and antimicrobial screening of Agave sisalana Perrine juice (waste). J. Environ. Chem. Ecotoxicol. 2011, 3, 180–183. [Google Scholar]
- Ahumada-Santos, Y.P.; Montes-Avila, J.; Uribe-Beltrán, M.J.; Díaz-Camacho, S.P.; López-Angulo, G.; Vega-Aviña, R.; López-Valenzuela, J.Á.; Heredia, J.B.; Delgado-Vargas, F. Chemical characterization, antioxidant and antibacterial activities of six Agave species from Sinaloa, Mexico. Ind. Crop. Prod. 2013, 49, 143–149. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Ávila-Reyes, J.A.; Uribe-Soto, J.N.; González-Valdez, L.S. The phenols of the genus Agave (Agavaceae). J. Biomater. Nanobiotechnol. 2013, 4, 9–16. [Google Scholar] [CrossRef]
- Olvera-García, V.; Cardador-Martínez, A.; del Campo, S.T.M. Effect of thermal process and filtration on the antioxidant activity and physicochemical parameters of Agave atrovirens extracts. J. Food Res. 2015, 4, 155–167. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Giannuzzi, L.; Zaritzkym, N. Ultraviolet treatment of orange juice to inactivate E. coli O157:H7 as affected by native microflora. Food Bioprocess Technol. 2010, 3, 603–614. [Google Scholar] [CrossRef]
- Koutchma, T. Advances in ultraviolet light technology for non-thermal processing of liquid foods. Food Bioprocess Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Usaga, J.; Padilla-Zakour, O.I.; Worobo, R.W. UV tolerance of spoilage microorganisms and acid-shocked and acid-adapted Escherichia coli in apple juice treated with a commercial UV juice-processing unit. J. Food Prot. 2016, 79, 294–298. [Google Scholar] [CrossRef]
- Quintero-Ramos, A.; Churey, J.J.; Hartman, P.; Barnard, J.; Worobo, R.W. Modeling of Escherichia coli inactivation by UV irradiation at different pH values in apple cider. J. Food Prot. 2004, 67, 1153–1156. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Palgan, I.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food Bioprocess Technol. 2012, 5, 680–686. [Google Scholar] [CrossRef]
- Hanes, D.E.; Worobo, R.W.; Orlandi, P.A.; Burr, D.H.; Miliotis, M.D.; Robl, M.G.; Bier, J.W.; Arrowood, M.J.; Churey, J.J.; Jackson, G.J. Inactivation of Cryptosporidium parvum oocysts in fresh apple cider by UV irradiation. Appl. Environ. Microbiol. 2002, 68, 4168–4172. [Google Scholar] [CrossRef] [PubMed]
- Gopisetty, V.V.S.; Patras, A.; Kilonzo-Nthengea, A.; Yannam, S.; Bansode, R.R.; Sasges, M.; Burns, S.M.; Vergne, M.J.; Pan, C.; Xiao, H. Impact of UV-C irradiation on the quality, safety, and cytotoxicity of cranberry-flavored water using a novel continuous flow UV system. LWT-Food Sci. Technol. 2018, 95, 230–239. [Google Scholar] [CrossRef]
- AOAC methods 932.12 and 981.12. In Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1996.
- Koutchma, T.; Keller, S.; Chirtel, S.; Parisi, B. Ultraviolet disinfection of juice products in laminar and turbulent flow reactors. Innov. Food Sci. Emerg. Technol. 2004, 5, 179–189. [Google Scholar] [CrossRef]
- He, Q.; Changhong, L.; Kojo, E.; Tian, Z. Quality and safety assurance in the processing of Aloe vera gel juice. Food Control. 2005, 16, 95–104. [Google Scholar] [CrossRef]
- APHA methods. In Compendium of Methods for the Microbiological Examination of Foods; American Public Health Association: Washington, DC, USA, 1992.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 420–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; De Feo, V. Phytochemical and biological studies of Agave attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Leal-Díaz, A.M.; Jacobo-Velázquez, D.A.; Rodríguez-Rodríguez, J.; García-Lara, S.; Gutiérrez-Uribe, J.A. Characterization of concentrated agave saps and storage effects on browning, antioxidant capacity and amino acid content. J. Food Compost. Anal. 2016, 45, 113–120. [Google Scholar] [CrossRef]
- Hamissa, A.M.B.; Seffen, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Converti, A. Phenolics extraction from Agave americana (L.) leaves using high-temperature, high-pressure reactor. Food Bioprod. Process. 2012, 90, 17–21. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef]
- Gautam, D.; Umagiliyage, A.L.; Dhital, R.; Joshi, P.; Watson, D.G.; Fisher, D.J.; Choudhary, R. Nonthermal pasteurization of tender coconut water using a continuous flow coiled UV reactor. LWT-Food Sci. Technol. 2017, 83, 127–131. [Google Scholar] [CrossRef]
- Geveke, D.J. UV inactivation of bacteria in apple cider. J. Food Prot. 2005, 68, 1739–1742. [Google Scholar] [CrossRef]
- Gouma, M.; Gayán, E.; Raso, J.; Condón, S.; Álvarez, I. Inactivation of spoilage yeasts in apple juice by UV-C light and in combination with mild heat. Innov. Food Sci. Emerg. Technol. 2015, 32, 146–155. [Google Scholar] [CrossRef]
- Riganakos, K.A.; Karabagias, I.K.; Gertzou, I.; Stahl, M. Comparison of UV-C and thermal treatments for the preservation of carrot juice. Innov. Food Sci. Emerg. Technol. 2017, 42, 165–172. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.Z.; Quintero-Ramos, A.; Meléndez-Pizarro, C.O.; Meza-Velázquez, J.A.; Jiménez-Castro, J.A.; Sánchez-Madrigal, M.Á.; Ruiz-Gutiérrez, M.G.; Espinoza-Hicks, J.C. Microbial and physicochemical properties of UV-C processed Aloe vera gel blends at different pHs using a continuous flow UV system. Rev. Mex. Ing. Química 2020, 19, 175–188. [Google Scholar] [CrossRef]
- Klimczak, I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Chethan, S.; Malleshi, N.G. Finger millet polyphenols: Optimization of extraction and the effect of pH on their stability. Food Chem. 2007, 105, 862–870. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.L.; Wang, C.H.; Feng, Y.M.; Liu, Z.P. Effects of heat treatments on the stabilities of polysaccharides substances and barbaloin in gel juice from Aloe vera Miller. J. Food Eng. 2006, 75, 245–251. [Google Scholar] [CrossRef]
- Ding, W.J.; Wu, X.F.; Zhong, J.S.; Wan, J.Z. Effects of temperature, pH, and light on the stability of aloin A and characterisation of its major degradation products. Int. J. Food Sci. 2014, 49, 1773–1779. [Google Scholar] [CrossRef]
- Pala, C.U.; Toklucu, A.K. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar] [CrossRef]
- Islam, M.S.; Patras, A.; Pokharel, B.; Wu, Y.; Vergne, M.J.; Shade, L.; Xiao, H.; Sasges, M. UV-C irradiation as an alternative disinfection technique: Study of its effect on polyphenols and antioxidant activity of apple juice. Innov. Food Sci. Emerg. Technol. 2016, 34, 344–351. [Google Scholar] [CrossRef]
- Ferrario, M.; Schenk, M.; García-Carrillo, M.; Guerrero, S. Development and quality assessment of a turbid carrot-orange juice blend processed by UV-C light assisted by mild heat and addition of Yerba Mate (Ilex paraguariensis) extract. Food Chem. 2018, 269, 567–576. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; George, D.S.; Somasundram, C. Comparison of UV-C treatment and thermal pasteurization on quality of Chokanan mango (Mangifera indica L.) juice. Food Bioprod. Process. 2015, 94, 313–321. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Changes in flavonoid content of grapefruit juice caused by thermal treatment and storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 153–162. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Cvetkovic, D.; Markovic, D.; Cvetkovic, D.; Radovanovic, B. Effects of continuous UV-irradiation on the antioxidant activities of quercetin and rutin in solution in the presence of lecithin as the protective target. J. Serb. Chem. Soc. 2011, 76, 973–985. [Google Scholar] [CrossRef]
- L’homme, C.; Arbelot, M.; Puigserver, A.; Biagini, A. Kinetics of hydrolysis of fructooligosaccharides in mineral-buffered aqueous solutions: Influence of pH and temperature. J. Agric. Food Chem. 2003, 51, 224–228. [Google Scholar] [CrossRef]

| Treatments | pH | TAM | TC | YM |
|---|---|---|---|---|
| Log CFU/mL | ||||
| UP | 4.5 | 2.64 ± 0.03 | 2.70 ± 0.05 | 3.74 ± 0.05 |
| 5.5 | 4.79 ± 0.02 | 4.34 ± 0.06 | 3.00 ± 0.01 | |
| 6.5 | 5.38 ± 0.12 | 3.60 ± 0.02 | 2.70 ± 0.03 | |
| LRF | ||||
| D1 | 4.5 | 1.61 ± 0.12 f | 2.70 ± 0.05 c | 3.74 ± 0.05 ª |
| D2 | 2.64 ± 0.03 e | 2.70 ± 0.05 c | 3.74 ± 0.05 ª | |
| D3 | 2.64 ± 0.03 e | 2.70 ± 0.05 c | 3.74 ± 0.05 ª | |
| D4 | 2.64 ± 0.03 e | 2.70 ± 0.05 c | 3.74 ± 0.05 ª | |
| D1 | 5.5 | 3.59 ± 0.39 b | 3.44 ± 0.09 b | 3.00 ± 0.01 b |
| D2 | 3.52 ± 0.16 d | 4.34 ± 0.06 a | 3.00 ± 0.01 b | |
| D3 | 3.67 ± 0.10 d | 4.34 ± 0.06 a | 3.00 ± 0.01 b | |
| D4 | 4.79 ± 0.02 b | 4.34 ± 0.06 a | 3.00 ± 0.01 b | |
| D1 | 6.5 | 4.37 ± 0.11 b,c | 2.16 ± 0.05 d | 2.70 ± 0.03 c |
| D2 | 4.31 ± 0.15 c | 3.60 ± 0.02 b | 2.70 ± 0.03 c | |
| D3 | 5.38 ± 0.12 a | 3.60 ± 0.02 b | 2.70 ± 0.03 c | |
| D4 | 5.38 ± 0.12 a | 3.60 ± 0.02 b | 2.70 ± 0.03 c | |
| TT | 4.5 | 2.64 ± 0.03 c | 2.70 ± 0.05 c | 3.74 ± 0.05 ª |
| 5.5 | 4.79 ± 0.02 b | 4.34 ± 0.06 a | 3.00 ± 0.01 b | |
| 6.5 | 5.38 ± 0.12 a | 3.60 ± 0.02 b | 2.70 ± 0.03 c | |
| Treatments | pH | TP (mg GAE/g d.w.) | TF (mg CE/g d.w.) | AA (μmol TE/g d.w.) |
|---|---|---|---|---|
| UP | 4.5 | 5.87 ± 0.029 a,b,c | 0.906 ± 0.007 a | 6.40 ± 0.04 b,c |
| 5.5 | 6.17 ± 0.409 a | 0.897 ± 0.008 a | 6.89 ± 0.47 a,b | |
| 6.5 | 6.15 ± 0.377 a,b | 0.906 ± 0.028 a | 7.40 ± 0.31 a | |
| D1 | 4.5 | 5.72 ± 0.009 a,b,c | 0.826 ± 0.051 a,b,c | 6.11 ± 0.04 b,c,d,e |
| D2 | 5.61 ± 0.007 a,b,c | 0.726 ± 0.074 b,c,d,e | 6.06 ± 0.16 bc,d,e | |
| D3 | 5.61 ± 0.017 a,b,c | 0.655 ± 0.020 d,e | 5.79 ± 0.29 c,d,e,f | |
| D4 | 5.62 ± 0.055 a,b,c | 0.578 ± 0.011 e | 6.10 ± 0.08 b,c,d,e | |
| D1 | 5.5 | 5.59 ± 0.072 a,b,c | 0.766 ± 0.055 a,b,c,d | 6.21 ± 0.25 b,c,d |
| D2 | 5.64 ± 0.044 a,b,c | 0.744 ± 0.034 b,c,d | 6.16 ± 0.09 b,c,d,e | |
| D3 | 5.62 ± 0.165 a,b,c | 0.698 ± 0.063 b,c,d,e | 5.88 ± 0.07 c,d,e,f | |
| D4 | 5.62 ± 0.139 a,b,c | 0.696 ± 0.042 b,c,d,e | 6.26 ± 0.14 b,c,d | |
| D1 | 6.5 | 5.81 ± 0.006 a,b,c | 0.842 ± 0.042 a,,b | 5.35 ± 0.18 d,e,f |
| D2 | 5.72 ± 0.021 a,b,c | 0.733 ± 0.003 b,c,d | 5.55 ± 0.18 c,d,e,f | |
| D3 | 5.70 ± 0.018 a,b,c | 0.701 ± 0.055 b,c,d,e | 5.35 ± 0.11 d,e,f | |
| D4 | 5.67 ± 0.083 a,b,c | 0.688 ± 0.006 c,d,e | 5.26 ± 0.38 e,f | |
| TT | 4.5 | 5.44 ± 0.001 c | 0.635 ± 0.003 d,e | 5.35 ± 0.11 d,e,f |
| 5.5 | 5.58 ± 0.059 b,c | 0.662 ± 0.006 d,e | 5.78 ± 0.09 c,d,e,f | |
| 6.5 | 5.60 ± 0.010 a,b,c | 0.736 ± 0.007 b,c,d | 5.02 ± 0.42 f |
| Source | DF | Sum of Square | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | TF | AA | L* | a* | b* | TS | RS | ||
| Model | 17 | 1.2076 * | 0.3129 * | 12.2699 * | 1.3714 * | 0.0865 * | 0.4601 * | 60936.76 * | 712.32 * |
| UP vs. P | 1 | 0.8962 * | 0.1812 * | 6.5956 * | 0.9127 * | 0.0102 * | 0.0799 * | 52536.01 * | 652.71 * |
| TT vs. UV-C | 1 | 0.0700 | 9.0 × 10−3 * | 1.0129 * | 1.7 × 10−4 | 0.0129 * | 5.7 × 10−4 | 3991.95 * | 13.95 * |
| pH | 1 | 0.1029 * | 0.0130 * | 0.5989 * | 0.0742 * | 0.0482 * | 0.2876 * | 538.53 | 37.03 * |
| pH2 | 1 | 4.1 × 10−4 | 1.2 × 10−6 | 1.2044 * | 2.9 × 10−3 | 5.8 × 10−3 * | 6.4 × 10−5 | 538.63 | 0.0805 |
| pH × UP vs. P | 1 | 0.0260 | 2.6 × 10−3 | 2.0973 * | 0.1965 * | 4.8 × 10−3 * | 0.0576 * | 24.81 | 1.6546 |
| pH2 × UP vs. P | 1 | 0.0464 | 1.2 × 10−4 | 0.2460 * | 0.0121 | 9.2 × 10−4 | 3.1 × 10−5 | 616.41 | 1.1 × 10−3 |
| pH × TT vs. UV-C | 1 | 5.6 × 10−3 | 2.4 × 10−3 | 0.0767 | 0.0315 | 7.8 × 10−5 | 0.0163 * | 77.67 | 0.2707 |
| pH2 × TT vs. UV-C | 1 | 0.0174 | 1.0 × 10−3 | 0.0291 | 0.0178 | 4.7 × 10−4 | 1.8 × 10−3 | 292.65 | 0.0119 |
| D | 3 | 0.0181 | 0.0847 * | 0.2345 | 0.0530 | 7.7 × 10−4 | 0.0113 * | 1574.72 | 4.4460 |
| pH × D | 6 | 0.0242 | 0.0185 | 0.1741 | 0.0703 | 2.2 × 10−3 | 4.7 × 10−3 | 745.34 | 2.1390 |
| Error | 18 | 0.3806 | 0.0249 | 0.9844 | 0.1734 | 5.8 × 10−3 | 0.0172 | 2918.35 | 8.6462 |
| Treatments | pH | Color | |||
|---|---|---|---|---|---|
| L* | a* | b* | ∆E | ||
| UP | 4.5 | 35.46 ± 0.010 a,b,c | 0.903 ± 0.033 e | −1.197 ± 0.042 f,g | |
| 5.5 | 35.54 ± 0.132 a,b | 0.913 ± 0.012 e | −1.196 ± 0.065 f,g | ||
| 6.5 | 35.76 ± 0.009 a | 0.930 ± 0.008 d,e | −1.198 ± 0.008 f,g | ||
| D1 | 4.5 | 35.18 ± 0.178 b,c,d | 0.908 ± 0.012 e | −1.175 ± 0.024 e,f,g | 0.28 ± 0.17 c,d |
| D2 | 35.18 ± 0.061 b,c,d | 0.932 ± 0.001 d,e | −1.175 ± 0.007 e,f,g | 0.31 ± 0.10 b,c,d | |
| D3 | 35.29 ± 0.066 b,c,d | 0.899 ± 0.041 e | −1.181 ± 0.015 e,f,g | 0.17 ± 0.06 d | |
| D4 | 35.30 ± 0.049 b,c,d | 0.901 ± 0.001 e | −1.196 ± 0.019 f,g | 0.16 ± 0.04 d | |
| D1 | 5.5 | 35.20 ± 0.146 b,c,d | 0.931 ± 0.015 de | −1.046 ± 0.001 b,c,d | 0.37 ± 0.13 b,c,d |
| D2 | 35.17 ± 0.154 b,c,d | 0.935 ± 0.005 d,e | −1.066 ± 0.037 b,c,d,e | 0.39 ± 0.03 b,c,d | |
| D3 | 35.14 ± 0.005 c,d | 0.924 ± 0.002 d,e | −1.092 ± 0.063 c,d,e,f | 0.41 ± 0.02 b,c,d | |
| D4 | 35.15 ± 0.008 c,d | 0.937 ± 0.001 c,d,e | −1.101 ± 0.026 d,e,f | 0.40 ± 0.01 b,c,d | |
| D1 | 6.5 | 34.95 ± 0.035 d | 1.015 ± 0.007 a,b | −0.884 ± 0.011 a | 0.86 ± 0.03 a |
| D2 | 35.02 ± 0.149 d | 1.009 ± 0.007 a,b,c | −0.951 ± 0.021 a,b | 0.78 ± 0.13 a | |
| D3 | 35.22 ± 0.077 b,c,d | 1.027 ± 0.007 a,b | −0.980 ± 0.010 a,b,c,d | 0.58 ± 0.07 a,b,c | |
| D4 | 35.13 ± 0.061 c,d | 0.988 ± 0.023 b,c,d | −0.975 ± 0.021 a,b,c | 0.67 ± 0.04 a,b | |
| TT | 4.5 | 35.29 ± 0.211 b,c,d | 0.963 ± 0.033 b,c,d,e | −1.278 ± 0.027 g | 0.23 ± 0.13 d |
| 5.5 | 35.26 ± 0.028 b,c,d | 0.970 ± 0.003 b,c,d,e | −1.060 ± 0.003 b,c,d,e | 0.32 ± 0.02 b,c,d | |
| 6.5 | 34.94 ± 0.055 d | 1.074 ± 0.022 a | −0.901 ± 0.041 a | 0.87 ± 0.06 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Jiménez, A.Z.; Quintero-Ramos, A.; Sánchez-Madrigal, M.Á.; Meléndez-Pizarro, C.O.; Valdez-Cárdenas, M.d.C.; Orizaga-Heredia, M.d.R.; Méndez-Zamora, G.; Talamás-Abbud, R. Effects of UV-C Irradiation and Thermal Processing on the Microbial and Physicochemical Properties of Agave tequilana Weber var. azul Extracts at Various pH Values. Processes 2020, 8, 841. https://doi.org/10.3390/pr8070841
Santana-Jiménez AZ, Quintero-Ramos A, Sánchez-Madrigal MÁ, Meléndez-Pizarro CO, Valdez-Cárdenas MdC, Orizaga-Heredia MdR, Méndez-Zamora G, Talamás-Abbud R. Effects of UV-C Irradiation and Thermal Processing on the Microbial and Physicochemical Properties of Agave tequilana Weber var. azul Extracts at Various pH Values. Processes. 2020; 8(7):841. https://doi.org/10.3390/pr8070841
Chicago/Turabian StyleSantana-Jiménez, Aracely Z., Armando Quintero-Ramos, Miguel Á. Sánchez-Madrigal, Carmen O. Meléndez-Pizarro, María del Cielo Valdez-Cárdenas, Magdalena del Rosario Orizaga-Heredia, Gerardo Méndez-Zamora, and Ricardo Talamás-Abbud. 2020. "Effects of UV-C Irradiation and Thermal Processing on the Microbial and Physicochemical Properties of Agave tequilana Weber var. azul Extracts at Various pH Values" Processes 8, no. 7: 841. https://doi.org/10.3390/pr8070841
APA StyleSantana-Jiménez, A. Z., Quintero-Ramos, A., Sánchez-Madrigal, M. Á., Meléndez-Pizarro, C. O., Valdez-Cárdenas, M. d. C., Orizaga-Heredia, M. d. R., Méndez-Zamora, G., & Talamás-Abbud, R. (2020). Effects of UV-C Irradiation and Thermal Processing on the Microbial and Physicochemical Properties of Agave tequilana Weber var. azul Extracts at Various pH Values. Processes, 8(7), 841. https://doi.org/10.3390/pr8070841







