Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application
Abstract
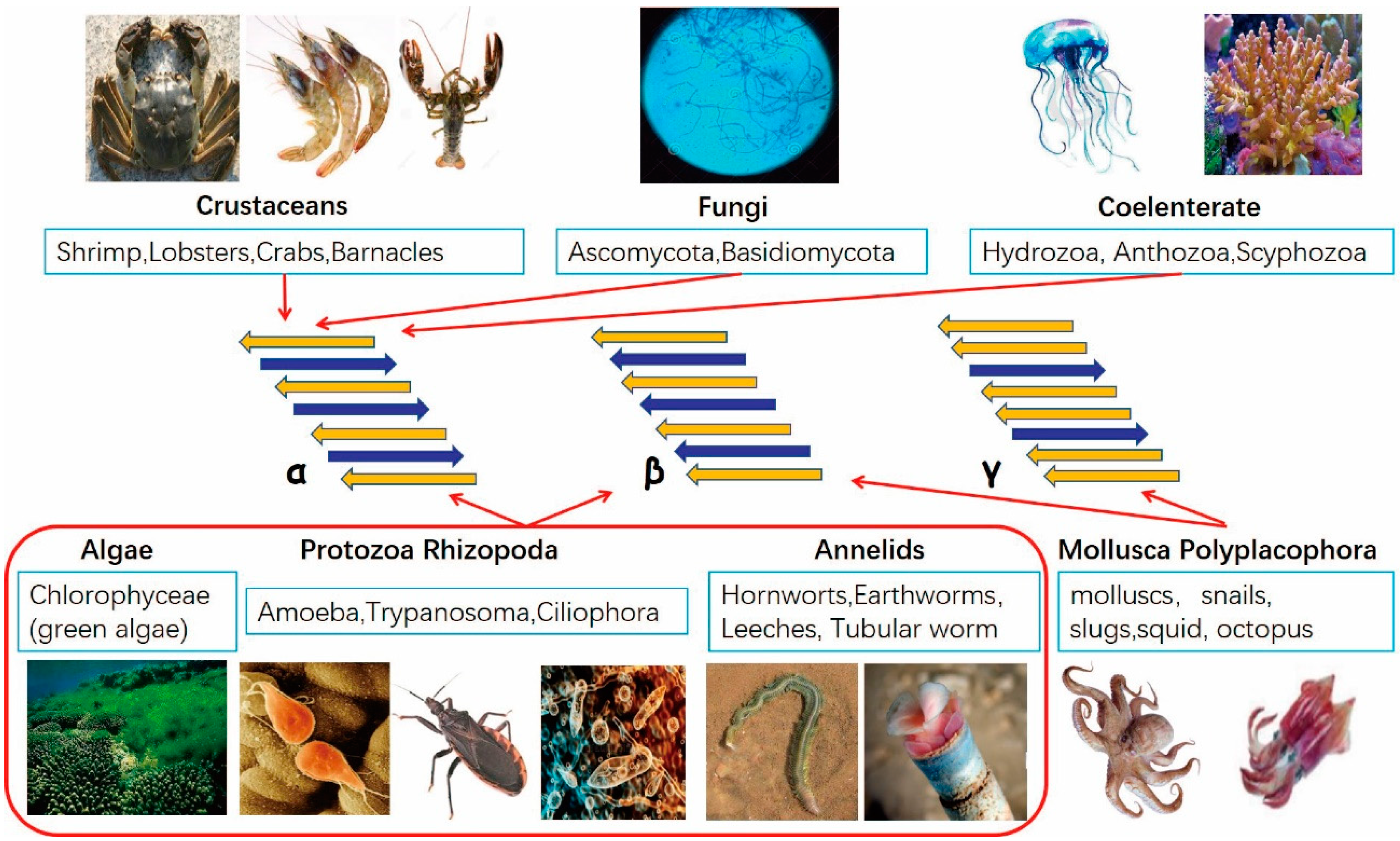
1. Introduction
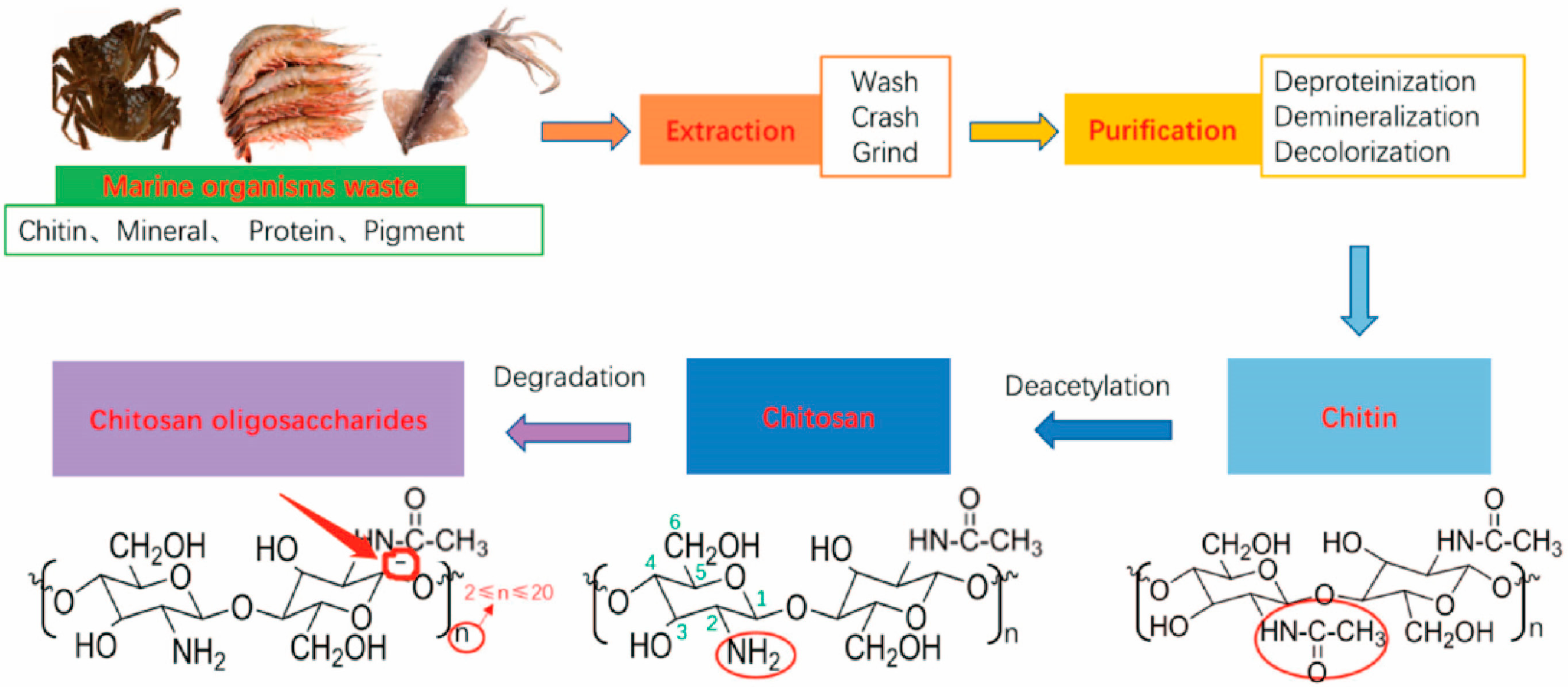
2. Production
3. Structure of Chitosan
4. Factors Influencing Chitosan Properties
4.1. Molecular Weight (MW)
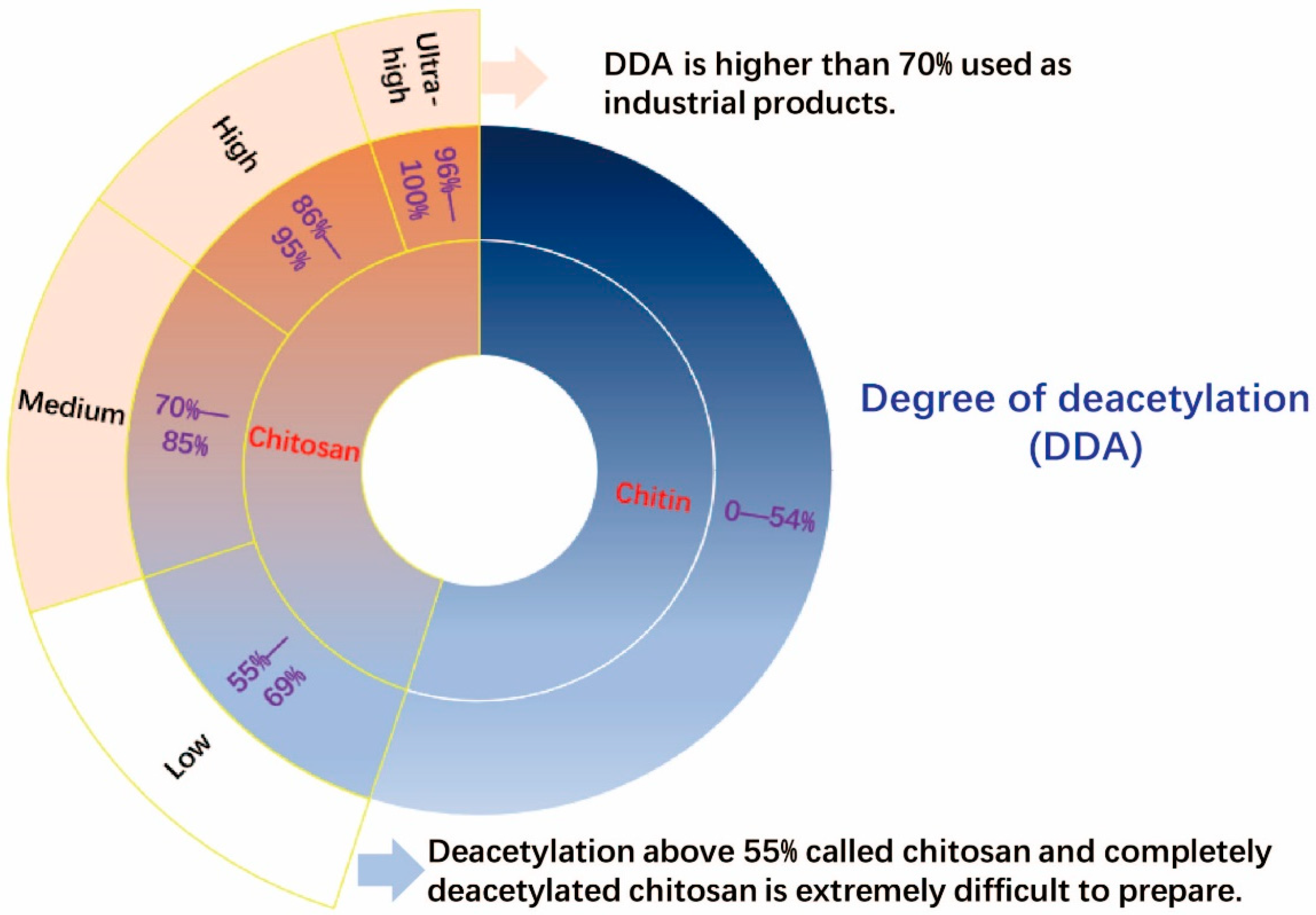
4.2. Degree of Deacetylation (DDA)
5. Solubility of Chitosan
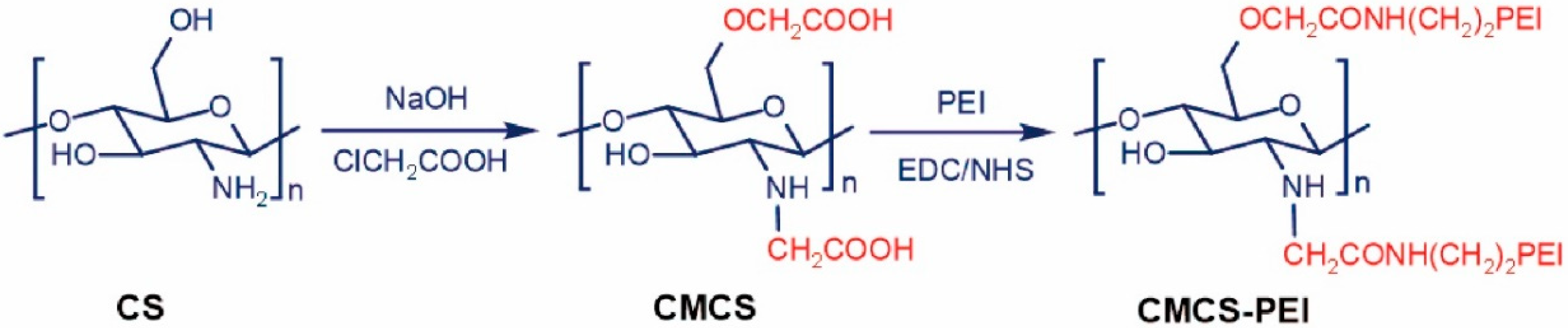
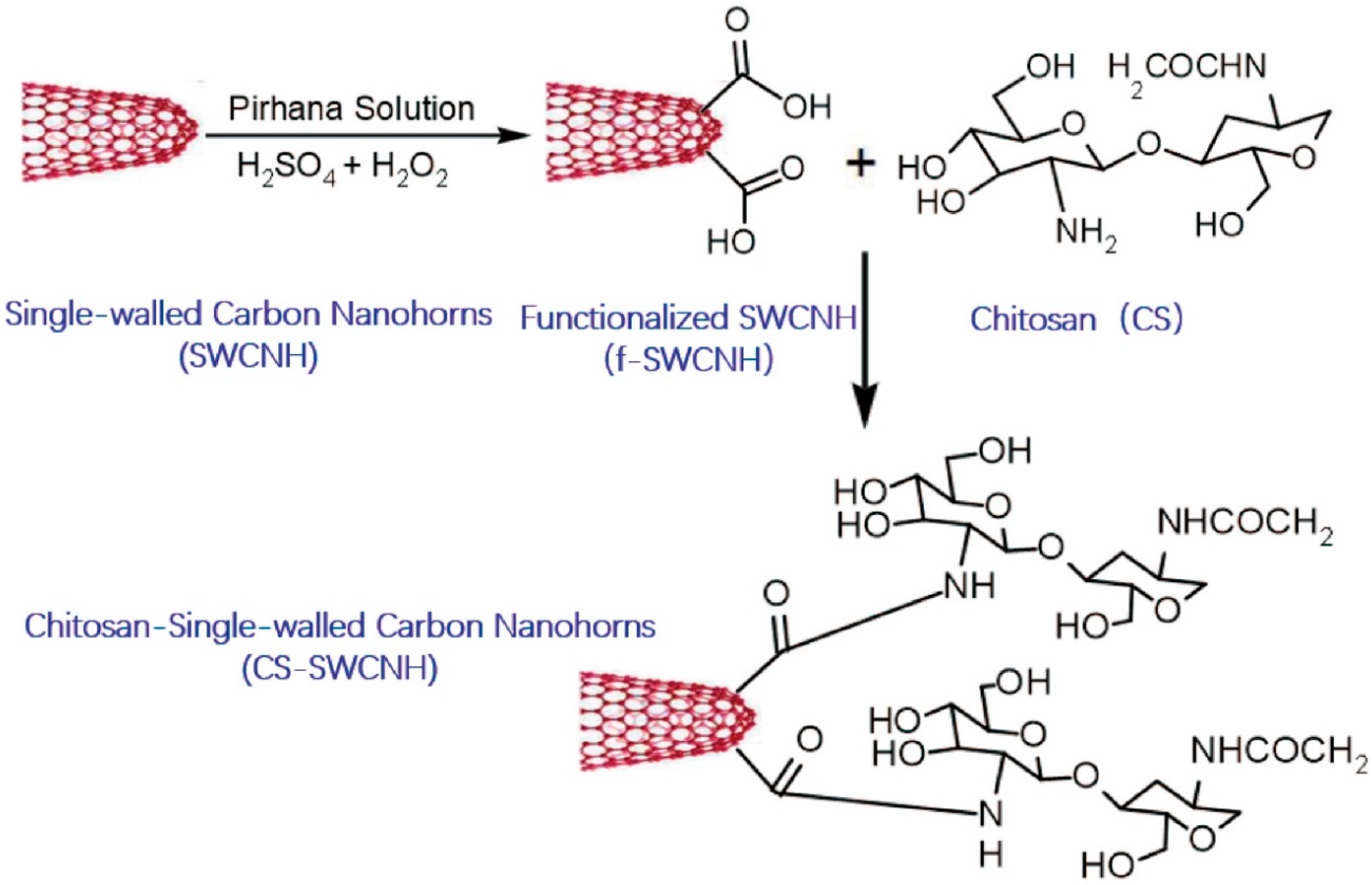
6. Modifications of Chitosan
- (1)
- Carboxylation: chitosan molecules contain more free -NH2 and -OH, introducing carboxyl functional groups, among which carboxymethyl is the most common, that replace the side chain ammonium salt, can obtain water-soluble, alcohol soluble, organic solvent-soluble, surface-active and fibrous polymer derivatives. Dumont et al. [45] suspended the chitosan powder in isopropanol, added isopropanol chloroacetate solution to the solution, reacted at room temperature and continuously stirred with magnetic force to obtain carboxymethyl chitosan with a high degree of protonation. Feng et al. [46] successfully prepared N,O-carboxymethyl chitosan from chitosan and chloroacetic acid under alkaline condition. The obtained carboxymethyl chitosan not only retains the original superior properties, but also improves the solubility more effectively, and has the function of moisturizing.
- (2)
- Etherification: the hydroxyl of chitosan can react with methyl ether, ether, benzyl ether and other alkylating agents to form an ether. By the way of cellulose modification, hydroxyalkyl chitin and carboxyalkyl chitin can be obtained by the reaction of basic chitin and etherification reagent.
- (3)
- Crosslinking: chitosan can be crosslinked in or between molecules through -OH and -NH2 with aldehydes, anhydrides or epoxides with two functional groups, and grafted to form network polymers to obtain crosslinked products with improved mechanical properties, providing conditions for further grafting modification.
- (4)
- Chelation: -OH and -NH2 have coordination and chelation. They can form complexes with transition metal ions first, and then cross-linked with the cross-linking agent. Chitosan with template memory and selective adsorption can be prepared.
- (5)
- Acylation: the hydroxyl group and amino group on the sugar residue of the chitosan molecular chain can react with some derivatives of organic acids, such as anhydrides and acyl hydrides, including O-acylation to form esters and N-acylation to form amides. N-acylation products are obtained usually after the introduction of aliphatic or aromatic acyls with different molecular weights, whose solubility in organic solvents is greatly improved.
- (6)
- Oxidation: the -OH of chitosan can be oxidized, among which H2O2 is the most widely used method to degrade chitosan. The C6 hydroxyl group can be oxidized to the aldehyde or carboxyl group, and the C3 hydroxyl group can be oxidized to a carbonyl group. If CrO3 is used as an oxidant in the perchlorate suspension of chitosan, C6 hydroxy can be oxidized to carboxyl.
- (7)
- Alkylation: the hydroxyl and amino groups on chitosan form respective water-soluble derivatives through alkylation. Hydroxypropyl chitosan can be obtained by the reaction of chitosan and propylene oxide on the hydroxyl group in the basic condition, and N-alkylated chitosan can be obtained in an acid condition.
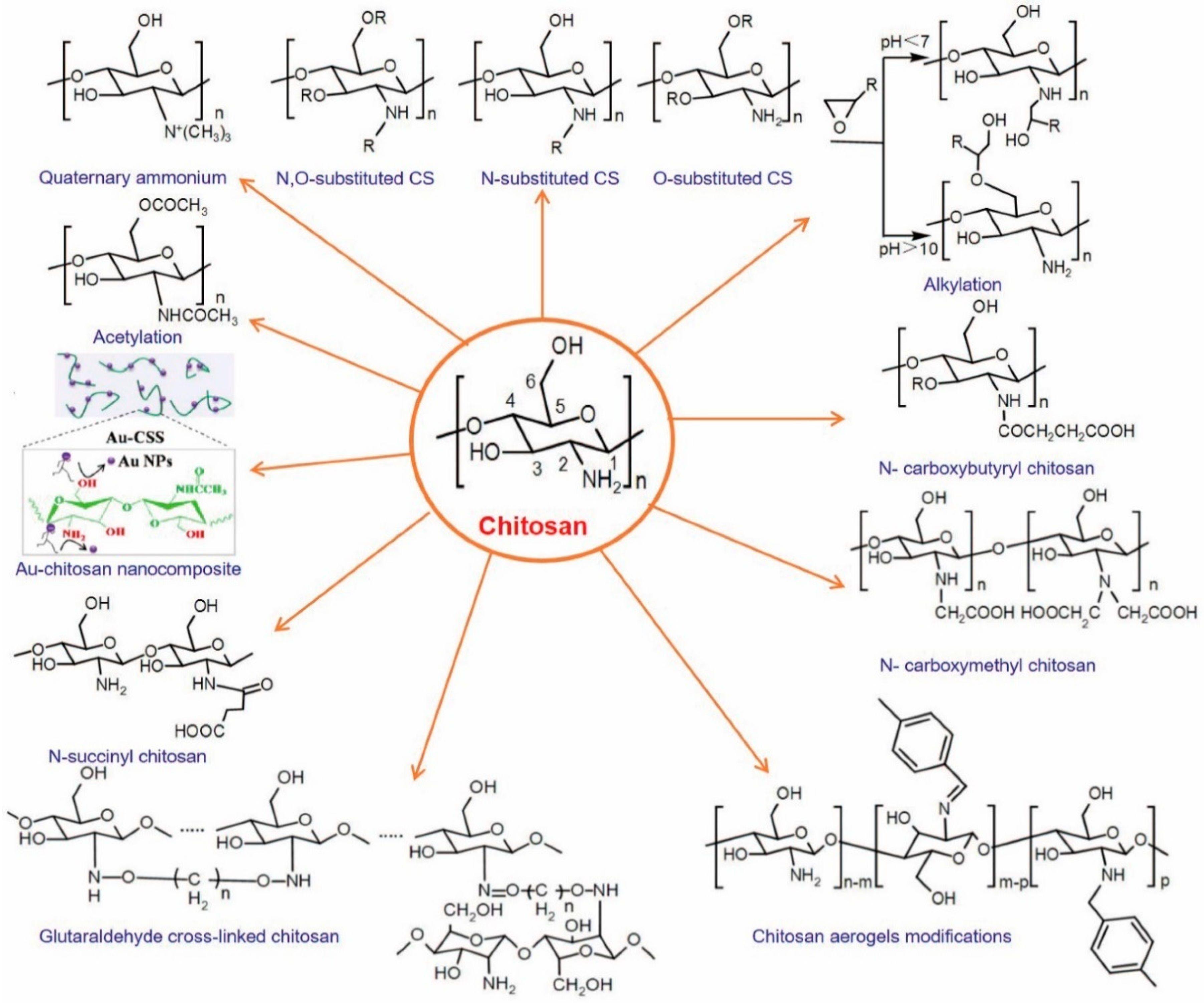
6.1. Graft Copolymerization of Chitosan
6.2. Blending Modification of Chitosan
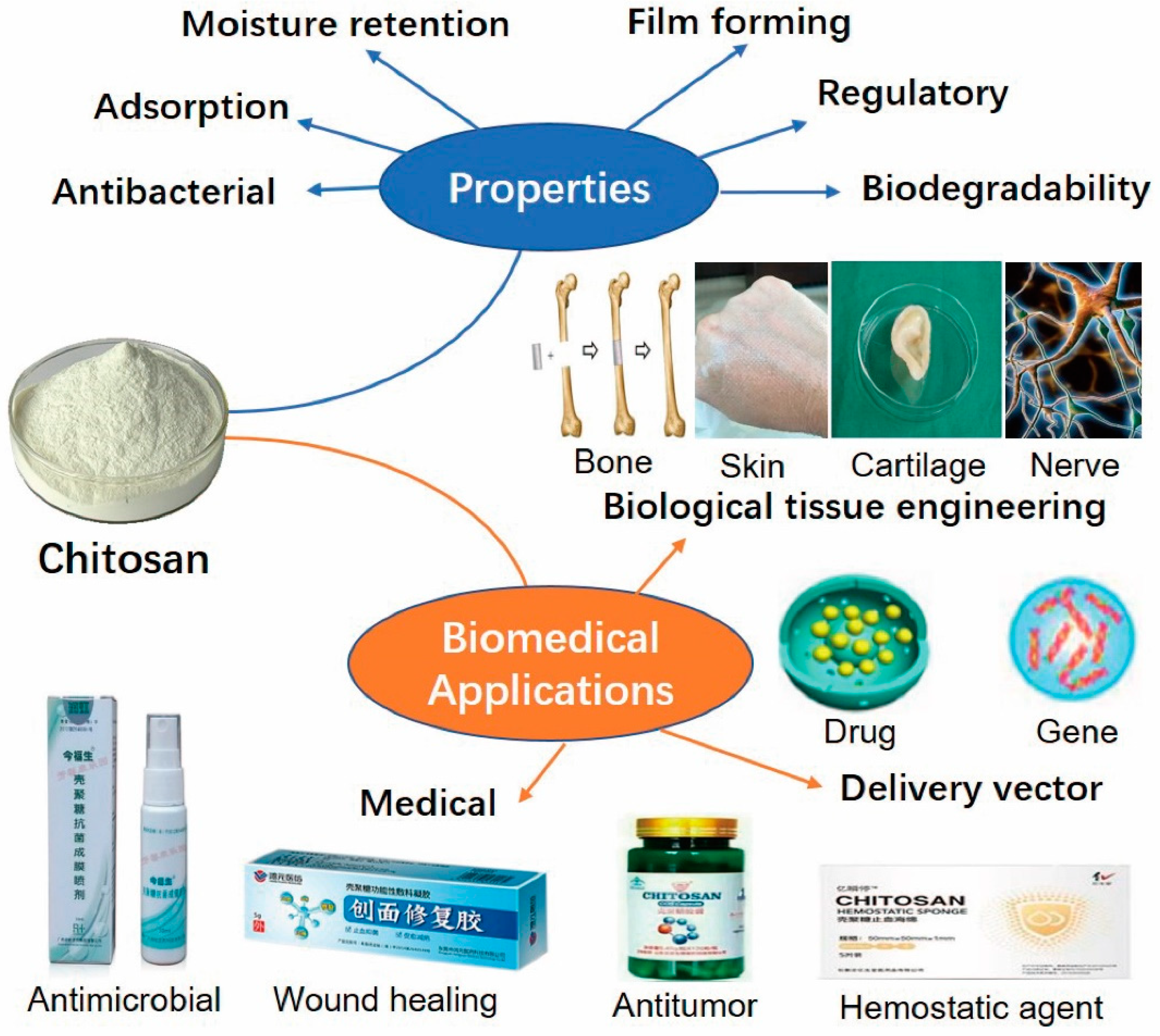
7. Biological Properties
- (1)
- Antibacterial. Chitosan is an only natural weak alkaline polysaccharide that easily dissolves in a dilute acid solvent. The dissolved chitosan contains an amino group (NH3+), which can inhibit bacteria by binding negative electrons and its antibacterial activity may enhance with concentration. Chitosan with different molecular weight can stop several bacteria growths, and has a strong inhibitory effect on Escherichia coli and Staphylococcus aureus, showing similar characteristics with antibiotics [62]. The antibacterial mechanism of chitosan can be divided into two ways: One is that chitosan forms a layer of the polymer by adsorbing on the cell surface. The membrane prevents the transport of nutrients to the cells and plays the role of bacteriostasis and decontamination; the second is that chitosan penetrates the cells through osmosis, adsorbs the anionic cytoplasm in the cells and causes flocculation, which disrupts the normal physiological activities of cells and kills bacteria [63,64,65]. When the molecular weight of chitosan is different and the bacteria that act on it are different, the antibacterial mechanism of chitosan is different, but in essence, its antibacterial property comes from the antibacterial factor NH3+ [66,67]. Chitosan has amino and acetyl groups on its molecular chain, so it is amphoteric. Its isoelectric point is pH 6.2. When the pH of the solution is higher than this value, chitosan will not have a positive charge and no bacteriostatic effect. If it is lower than this value, it will have a positive charge. The degree of deacetylation of chitosan may also affect its antibacterial effect. The content of the free amino group and the bacteriostatic rate increased with the increase of the deacetylation degree [68]. The bacterial strains, pH, temperature, salinity, molecular weight, concentration and degree of deacetylation of chitosan may be closely related to the antibacterial effect of chitosan. However, considering the high number of bacteria strains and the complexity of the bio environment generated, it is not clear the specific interaction between these factors [69,70]. The latest studies have enforced an investigation of the inhibitory activity of biofilm production of chitosan and its derivatives as prominent agents and diminish virulence properties by several pathogenic bacteria. Conjugation of chitosan with other bioactive materials can further promote its antimicrobial activity [71]. Ionic gelation was used to produce chitosan nanoparticles (CNP) and a different concentration of CNP (0–20% w/w) was used to prepare starch-based nanocomposite films. An in vitro and in vivo study proved the antimicrobial characteristics of CNP-starch films. For instance, the growth of tested pathogens such as Salmonella typhimurium, Escherichia coli, Staphylococcus aureus and Bacillus cereus were inhibited by CNP-starch films about 15–20%.
- (2)
- Adsorption. Chitosan has forceful adsorption function and can selectively adsorb heavy metal ions, cholic acid, cholesterol, triglyceride and grease [72,73,74,75]. It cannot be digested and absorbed in the gastrointestinal tract in a short time, on the contrary, it can absorb the fat that can significantly prevent the digestive system from absorbing cholesterol and triglycerides, avoiding the excessive accumulation of cholesterol and fatty acids. Eating 2 g of chitosan every day can effectively absorb the fat in the food, which is beneficial to losing weight [76]. Researchers try to find out the relationship between the structure of chitosan and the lowering of blood lipids and blood glucose, which is mainly due to the amino group of chitosan itself, which makes chitosan a poly ionomer. One possible way is that the chitosan catabolic compound can be adsorbed with negatively charged fatty acids and cholesterol. The simple chitosan can adsorb many times of its weight of oil, which can effectively prevent the digestive system from absorbing cholesterol and triglycerides, prevent the accumulation of cholesterol and fatty acids in the body and promote its excretion from the body. By reducing intestinal lipid absorption, the levels of cholesterol and triglycerides in plasma were reduced. Another possibility is that chitosan combines with negative bile acids to reduce the amount of bile in the liver and empty the gallbladder. There must be a certain amount of bile acid reserve in the gallbladder, so that the cholesterol in the plasma or liver can be converted into bile acid to maintain the bile acid reserve, thus reducing the cholesterol concentration in the plasma or liver [77].
- (3)
- Moisture retention. The molecular chains of chitosan and its derivatives contain numerous active hydrophilic polar groups, such as -OH, -NH2, -COOH, etc. [78]. The content of the carboxyl group in carboxylated chitosan is far more than other derivatives, and the repulsion of the negative charge on the carboxyl group makes the polymer chain space to be extended especially large, even at a lower concentration, there is a strong interaction between molecules. The force on the water molecule is strengthened due to the hydrophilicity of the carboxyl group and the large extension of the molecular chain so that it has better moisture absorption and retention performance.
- (4)
- Film forming. Chitosan has stable physicochemical properties and outstanding film-forming performance, its film-forming ability is closely related to the internal structure. The higher the deacetylation degree of chitosan, the lower the swelling and tensile strength of the membrane and the more difficult the degradation of the corresponding chitosan membrane in vitro and in vivo [79]. Since there are more crystal structures in chitosan with a high degree of deacetylation, the molecular rigidity is stronger and the water absorption is lower. The film formation and its characteristics are greatly regulated by the relative molecular weight of chitosan. The lower the molecular weight is the lower the tensile strength and the stronger the permeability of the membrane. The larger the molecular weight is, the more the crystal structure is, and the higher the molecular entanglement is. Therefore, the flexibility of the molecule is poor, the tensile strength is high and the permeability of the membrane is poor [80]. Since chitosan is degradable, the changes in the molecular weight might affect the properties of the membrane; the chitosan crosslinking degree also affects the properties of the membrane. With the increase of the crosslinking degree, the spatial network structure formed between the molecules increases, the tensile strength of the membrane increases and the water permeability decrease. Chitosan membrane biomedical materials are widely used, such as preventing postoperative abdominal adhesions, purifying drugs and serum antibodies, manufacturing artificial renal membrane, artificial skin, contact lens membrane, drug sustained-release, dental surgery and nerve repair materials, etc. [81].
- (5)
- Regulatory. Chitosan can activate lymphocytes with the immune function, distinguish normal cells from cancer cells and kill cancer cells [82]. Besides, it also has the function of regulating the endocrine system, regulating the pH value of the body to weak alkaline, improving the utilization rate of insulin, making the insulin secretion normal, inhibiting the rise of blood sugar, reducing blood lipid and helping to prevent and treat diabetes [83]. Many researchers have found that chitin, chitosan and chitosan oligosaccharide have immunomodulatory effects. The molecular weight of chitin and chitosan is more than 1 million, so its immunogenicity is very weak or almost negligible [84]. The immune response mediated by chitosan and its derivatives is closely related to its chemical structure. After deacetylation, chitin has -NH2 on the chitosan molecule, which can combine with H+, enhance affinity, chemotactic leukocyte and induce a local macrophage. Macrophages play an important role in the immune response and regulation [85]. There are receptors of bacterial polysaccharides on the surface of macrophages. Chitosan, as a bacterial polysaccharide like substance, can stimulate the activation of macrophages, thus promoting the phagocytic ability and enhancing the activity of hydrolase secreted by macrophages [86]. As a natural high molecular material, chitosan with unique structure presents natural physiological activity in vivo, which can stimulate local tissues, promote cell proliferation and then evolve into macrophages, produce inflammatory mediators and improve the body’s resistance to inflammation.
- (6)
- Biodegradability. Chitosan is a kind of natural medical polymer material with excellent biodegradability, which is determined by its chemical structure. Chitosan has obvious degradation under the action of a lysozyme in vitro or in body fluid [87]. Degradation products are methyl sugar and oligosaccharide, which are safe for the body and can be decomposed, absorbed and metabolized. N-acetylglucosamine, one of the degradation products, is very important for scar repair of tissues and is toxic to some malignant tumors in vivo, so it can be utilized as cancer chemotherapy drugs [88]. Its degradation products are generally non-toxic to the human body, no accumulation in the body, no immunogenicity, so it can be used to manufacture artificial skin, surgical suture, bone repair materials, contact glasses, artificial dialysis membrane, anticoagulant materials, etc., carried a very broad application prospect in the medical field.
8. Application of Chitosan Biomaterials
9. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Braconnot, H. Sur la nature des champignons. Ann. Chim. Paris 1811, 79, 265–304. [Google Scholar]
- Knorr, D. Use of chitinous polymers in food. A challenge for food research and development. Food Technol. 1984, 38, 85–97. [Google Scholar]
- Khan, F.I.; Rahman, S.; Queen, A.; Ahamad, S.; Ali, S.; Kim, J.; Hassan, M.I. Implications of molecular diversity of chitin and its derivatives. Appl. Microbiol. Biotechnol. 2017, 101, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs. 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of chitosan and its oligomers from shrimp shell waste, their characterization and antimicrobial effect. Vet. World 2017, 10, 170–175. [Google Scholar] [CrossRef]
- Hoppe-Seyler, F. Ueberchitin und cellulose. Chem. Ges. 1894, 27, 3329–3331. [Google Scholar] [CrossRef]
- Justino, C.I.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Graphene based sensors and biosensors. Trac Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Kuo, C.; Chen, C.; Hsiao, C.; Chen, I. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 1176, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Mincea, M.; Negrulescu, A.; Ostafe, V. Preparation, modification, and applications of chitin nanowhiskers, a review. Rev. Adv. Mater. Sci. 2012, 30, 225–242. [Google Scholar]
- Franca, E.F.; Freitas, L.C.G.; Lins, R.D. Chitosan molecular structure as a function of N -acetylation. Biopolymers 2011, 95, 448–460. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of chitosan for biomedical applications. Chitosan Based Biomater. 2017, 1, 3–30. [Google Scholar]
- Thomas, S.; Ninan, N.; Mohan, S.; Francis, E. Natural Polymers, Biopolymers, Biomaterials, and Their Composites, Blends and IPNs; Apple Academic Press: Toronto, UK, 2012. [Google Scholar]
- Deepthi, S.; Venkatesan, J.; Kim, S.K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources, structure, properties and applications. Mar. Drugs. 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Tokusoglu, O. Food by-Product Based Functional Food Powders; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Li, H. Production and application of chitosan by fermentation. Beijing Univ. Chem. Technol. 2000, 82. [Google Scholar]
- Jaworska, M.M.; Stępniak, I.; Galiński, M.; Kasprzak, D.; Biniaś, D.; Górak, A. Modification of chitin structure with tailored ionic liquids. Carbohydr. Polym. 2018, 20, 397–403. [Google Scholar] [CrossRef]
- Saad, M.A.; Mathai, T.; Kranthi, K.R.; Sujata, G.S.; Amit, A.; Ira, B. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar]
- Zhou, Y.; Shi, H.; Zhao, Y.; Men, Y.; Jiang, S.; Rottstegge, J.; Wang, D. Confined crystallization and phase transition in semirigid chitosan containing long chain alkyl groups. Cryst. Eng. Comm 2011, 13, 561–567. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Advances in Wound Healing materials, Science & Skin Engineering; Smithers, Ed.; Rapra: Shropshir, UK, 2015. [Google Scholar]
- Teng, D.; Yao, K.; Li, J.; Yao, F.; Yin, Y. Chitosan-Based Hydrogels, Functions and Applications; CRC Press: New York, NY, USA, 2012. [Google Scholar]
- Chena, M.M.; Huanga, Y.Q.; Caoa, H.; Liub, Y.; Guoa, H. Collagen/chitosan film containing biotinylated glycol chitosan nanoparticles for localized drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 339–346. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. The influence of molecular weight of quaternized chitosan on antifungal activity. Carbohydr. Polym. 2008, 71, 694–697. [Google Scholar] [CrossRef]
- Duan, J.F. Natural Polymer Material; Huazhong University of Science and Technology Press: Wuhan, China, 2016; pp. 179–185. [Google Scholar]
- Arnaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.J.; Chen, S.Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. 2008, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Winter, D.; Hipler, U.C. Molecular-weight-dependent toxic effects of chitosans on the human keratinocyte cell line HaCaT. Ski. Pharm. Physiol. 2010, 23, 164–170. [Google Scholar] [CrossRef]
- Wimardani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Ikeda, M.A. Cytotoxic effects of chitosan against oral cancer cell lines is molecular-weight dependent and cell-type-specific. Int. J. Oral Res. 2012, 3, 1–10. [Google Scholar]
- Yu, X.X.; Li, H. Natural Food Additives; China Light Industry Press: Beijing, China, 2014; Volume 3, pp. 141–144. [Google Scholar]
- Shaji, J.; Jain, V.; Lodha, S. Chitosan, A novel pharmaceutical excipient. Int. J. Pharm. Appl. Sci. 2010, 1, 11–28. [Google Scholar]
- Narimane, M.B.; Pierre, H.E.; Helene, B.; Guillaume, P.; Cedric, D.; Philippe, M. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques, a review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan, material characterization and in vitro evaluation via albumin adsorption and preosteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Zakaria, Z.; Izzah, Z.; Jawaid, M.; Hassan, A. Effect of degree of deacetylation of chitosan on thermal stability and compatibility of chitosan-polyamide blend. Bioresources 2012, 7, 5568–5580. [Google Scholar] [CrossRef]
- Plackett, D. Biopolymers, New Materials for Sustainable Films and Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Chen, Y.L. Preparation and Characterization of Water-Soluble Chitosan Gel for Skin Hydration. Ph.D. Thesis, University Sains Malaysia, Penang, Malaysia, 2008. [Google Scholar]
- Jeon, Y.J.; Shahidi, F.; Kim, S.K. Preparation of chitin and chitosan chitooligosacchrides and their applications in physiological functional foods. Food Rev. Int. 2000, 16, 159–176. [Google Scholar] [CrossRef]
- Chiaiba, S.; Ke, E.; Ki, M. Preparation of higher N-acetylchitooligosaceharides in high yields. Adv. Chitin Sci. 1998, 3, 89–96. [Google Scholar]
- Haske-Cornelius, O.; Bischof, S.; Beer, B.; Bartolome, M.J.; Olakanmi, E.O.; Mokoba, M.; Guebitz, G.M.; Nyanhongo, G.S. Enzymatic synthesis of highly flexible lignin cross-linked succinyl-chitosan hydrogels reinforced with reed cellulose fibres. Eur. Polym. J. 2019, 120, 109201. [Google Scholar] [CrossRef]
- Kumar, G.; Smith, P.J.; Payne, G.F. Enzymatic grafting of a natural product onto chitosan to confer water solubility under basic conditions. Biotechnol. Bioeng. 1999, 63, 154–165. [Google Scholar] [CrossRef]
- Liu, N.; Ni, S.; He, T.; Chang, Y.; Qin, M. Research progress on the enzymatic modification of chitosan. Pap. Pap. Mak. 2016, 1, 22–26. [Google Scholar]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications, Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Dumont, V.C.; Mansur, A.A.; Carvalho, S.M.; Borsagli, F.G.M.; Pereira, M.M.; Mansur, H.S. Chitosan and carboxymethyl-chitosan capping ligands, effects on the nucleation and growth of hydroxyapatite nanoparticles for producing bio-composite membranes. Mater. Sci. Eng. C. 2016, 59, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Fengyan, L.; Yan, Q. Preparation and characterization of no carboxymethyl chitosan magnetic composite microspheres. Mater. Eng. 2014, 58, 41–45. [Google Scholar]
- Yuan, W.; Yin, X.Q.; He, Y.N. Progress in the research of carboxyl-chitosan. Chem. Reag. 2007, 29, 277–286. [Google Scholar]
- Pintoa, R.V.; Gomes, P.S.; Fernandes, M.H. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Mater. Sci. Eng. C. 2020, 109, 110557. [Google Scholar] [CrossRef]
- Ai, L.F.; Wang, G.H. Progress with respect to modification and application of chitosan. China Surfactant Deterg. Cosmet. 2011, 41, 289–293. [Google Scholar]
- Fu, F.; Yang, B.; Hu, X.; Tang, H.; Zhang, Y.; Xu, X.; Zhang, Y.; Touhid, S.S.B.; Liu, X.; Zhu, Y.; et al. Biomimetic synthesis of 3D Au-decorated chitosan nanocomposite for sensitive and reliable SERS detection. Chem. Eng. J. 2019, 392, 123693. [Google Scholar] [CrossRef]
- Goff, R.L.; Mahe, O.; Coz-Botrel, R.L. Insight in chitosan aerogels derivatives—Application in catalysis. React. Funct. Polym. 2020, 146, 104393. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications, A Review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Kweon, D.; Song, S.; Park, Y. Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials 2003, 9, 1595–1601. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H.; Su, W.W. Modification of different molecular weights of chitosan by p-Coumaric acid, preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef] [PubMed]
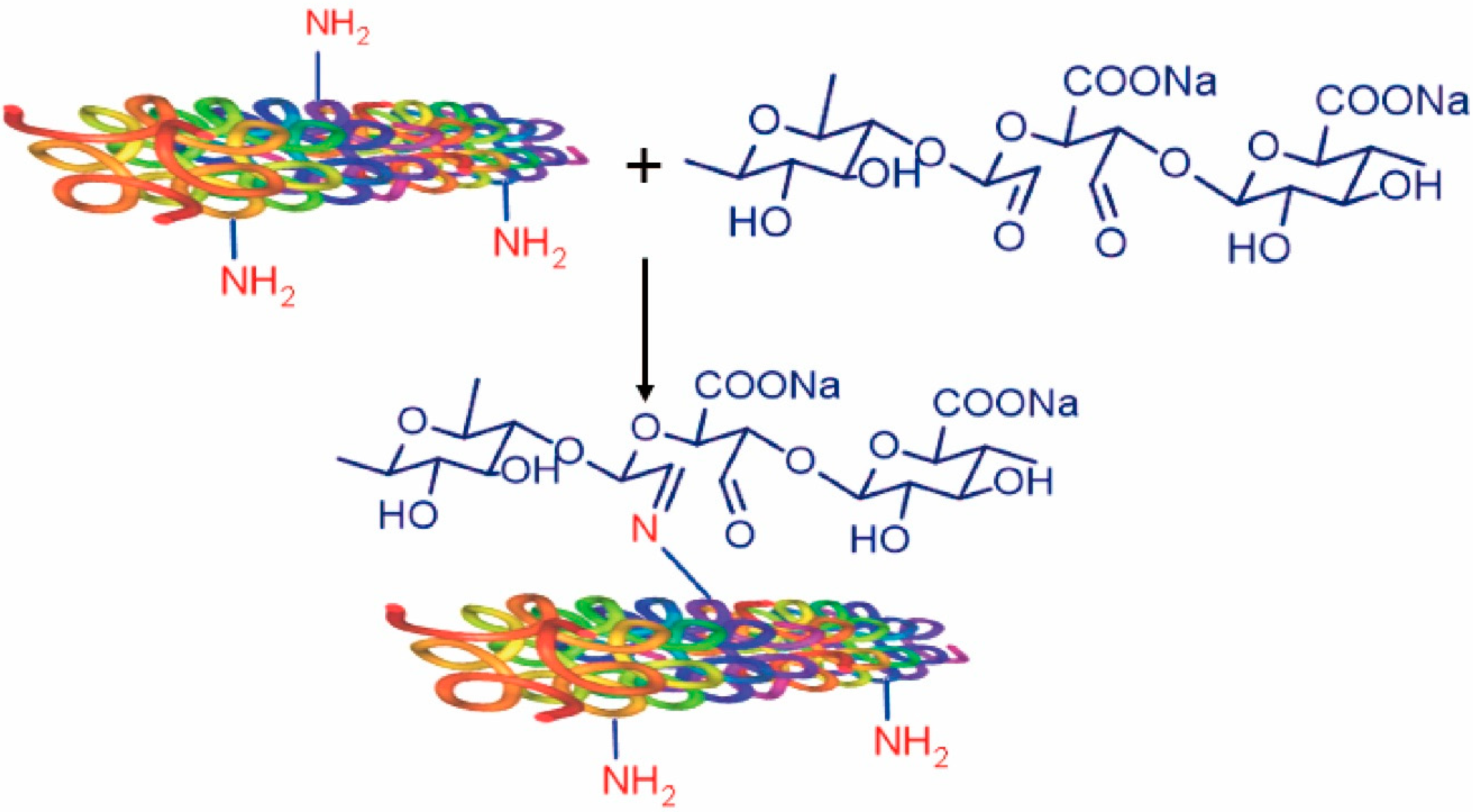
- Liu, X.; Mo, Y.; Liu, X.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y.; Wang, C.; Ramakrishna, S. Synthesis, characterisation and preliminary investigation of the haemocompatibility of polyethyleneimine-grafted carboxymethyl chitosan for gene delivery. Mater. Sci. Eng. C 2016, 62, 173–182. [Google Scholar] [CrossRef]
- Depan, D.; Misra, R.D.K. The interplay between nanostructured carbon-grafted chitosan scaffolds and protein adsorption on the cellular response of osteoblasts, structure–function property relationship. Acta Biomater. 2013, 9, 6084–6094. [Google Scholar] [CrossRef]
- Du, T.; Chen, Z.; Li, H.; Tang, X.; Li, Z.; Guan, J.; Liu, C.; Du, Z.; Wu, J. Modification of collagen–chitosan matrix by the natural crosslinker alginate dialdehyde. Int. J. Biol. Macromol. 2016, 82, 580–588. [Google Scholar] [CrossRef]
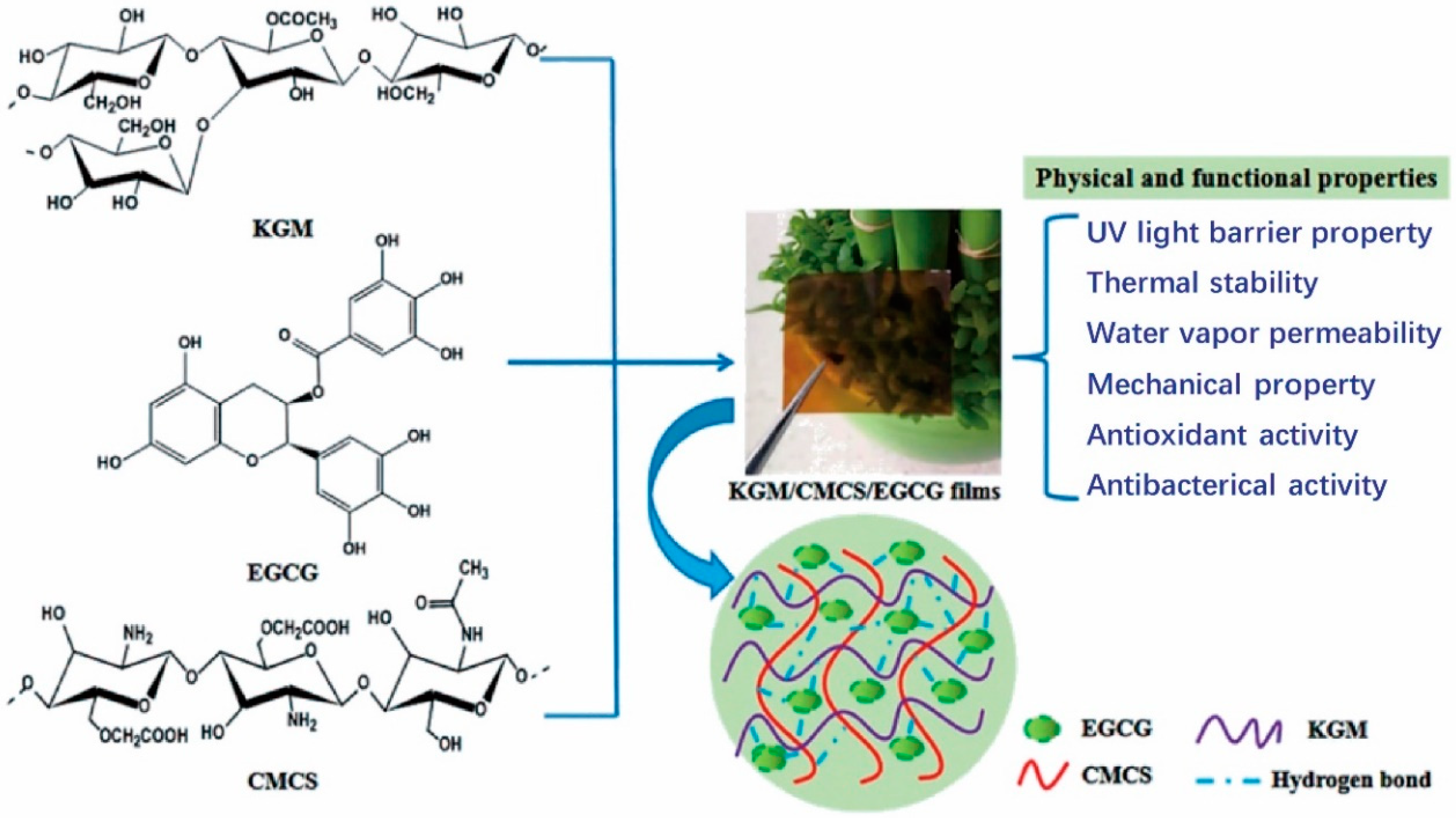
- Sun, J.; Jiang, H. Preparation and characterization of multifunctional konjac glucomannan/carboxymethyl chitosan biocomposite films incorporated with epigallocatechin gallate. Food Hydrocoll. 2020, 105, 756–766. [Google Scholar] [CrossRef]
- Liu, L.; Wen, H.; Rao, Z.; Zhu, C.; Liu, M.; Min, L.; Fan, L.; Tao, S. Preparation and characterization of chitosan–collagen peptide / oxidized konjac glucomannan hydrogel. Int. J. Biol. Macromol. 2018, 108, 376–382. [Google Scholar] [CrossRef]
- Patel, G.B.N.; Singh, L.; Singh, F.; Kulriya, P.K. Effects of MeV ions on physicochemical and dielectric properties of chitosan/PEO polymeric blend. Nucl. Instrum. Methods Phys. Res. Sec. B Beam Interact. Mater. Atoms. 2019, 3, 68–78. [Google Scholar] [CrossRef]
- Singh, D.K.; Ray, A.R. Biomedical applications of chitin, chitosan and their derivatives. Macromol. J. Sci. Part C 2007, 40, 69–83. [Google Scholar] [CrossRef]
- Li, K.J.; Guan, G.L.; Zhu, J.X.; Wu, H.; Sun, Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan–gallic acid derivative against Escherichia coli and Staphylococcusaureus. Food Control 2019, 96, 234–243. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Omura, Y.; Shigemoto, M.; Akiyama, T.; Saimoto, H.; Shigemasa, Y.; Nakamura, I.; Tsuchido, T. Antimicrobial activity of chitosan with different degrees of acetylation and molecular weights. Biocontrol. Sci. 2003, 8, 25–30. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.F. Xavier Malcata. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef]
- Feng, X.Q.; Yang, S. Antibacterial activity of chitosan with different molecular weight on Escherichia coli and the possible mechanism. China Brew. 2007, 167, 16–19. [Google Scholar]
- Hu, Y.; Du, Y.M.; Liu, H. Relationship between Antimicrobial Activity of Chitosan and Its Molecular Weight or Environmental Medium. J. Anal. Sci. 2003, 19, 305–308. [Google Scholar]
- Wang, H.; Shen, Y.X. The antibiotic activities of chitosan with different deacetyl degrees. J. Shanghai Fish. Univ. 2001, 10, 380–382. [Google Scholar]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. Food Sci. Technol. 2016, 71, 347–355. [Google Scholar] [CrossRef]
- Kumar, M.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 225, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Z.; Shen, Y.X. The study on the sporophyte stage of Monostroma latissimum. Shanghai Fish. Univ. 2000, 9, 138–146. [Google Scholar]
- Fu, X.; Shen, Y.; Jiang, X.; Huang, D.; Yan, Y. Chitosan derivatives with dual-antibacterial functional groups for antimicrobial finishing of cotton fabrics. Carbohydr. Polym. 2011, 85, 221–227. [Google Scholar] [CrossRef]
- Wu, S.P.; Wang, F.; Yuan, H.K.; Zhang, L.J.; Lu, J. Fabrication of xanthate-modified chitosan/poly (N-isopropylacrylamide) composite hydrogel for the selective adsorption of Cu(II), Pb(II) and Ni(II) metal ions. Chem. Eng. Res. Design. 2018, 139, 197–210. [Google Scholar] [CrossRef]
- Tahira, I.; Aslam, Z.; Abbas, A.; Monim-ul-Mehboob, M.; Ali, S.; Asghar, A. Adsorptive removal of acidic dye onto grafted chitosan, A plausible grafting and adsorption mechanism. and adsorption mechanism. Int. J. Biol. Macromol. 2019, 1362, 1209–1218. [Google Scholar] [CrossRef]
- Li, Q.P.; Gooneratne, S.R.; Wang, R.L.; Zhang, R.; Pan, W. Effect of different molecular weight of chitosans on performance and lipid metabolism in chicken. Anim. Feed Sci. Technol. 2016, 211, 174–180. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shiazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Zeng, X. Relationships between the molecular structure and moisture-absorption and moisture-retention abilities of carboxymethyl chitosan, II. Effect of degree of deacetylation and carboxymethylation. Carbohydr. Res. 2003, 47, 333–340. [Google Scholar] [CrossRef]
- Si, J.Z.; Yang, Y.H.; Xing, X.L.; Yang, F.; Shan, P.Y. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. Polym. Degrad. Stab. 2019, 166, 73–85. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Jarunee, S.; Srisagul, S.; Satit, P. Preparation and characterization of chitosan aqueous dispersion as a pharmaceutical film forming material. Drug Deliv. Sci. Technol. 2019, 54, 101230. [Google Scholar]
- Sunaina, S.; Ananta, K.; Ghosh, S. Non-immunogenic, porous and antibacterial chitosan and Antheraea mylitta silk sericin hydrogels as potential dermal substitute. Carbohydr. Polym. 2017, 167, 196–209. [Google Scholar]
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs. 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Wu, N.; Wen, Z.S.; Xiang, X.W.; Huang, Y.N.; Gao, Y.; Qu, Y.L. Immunostimulative Activity of Low Molecular Weight Chitosans in RAW264.7 Macrophages. Mar. Drugs. 2015, 13, 6210–6225. [Google Scholar] [CrossRef]
- Zheng, B.; Wen, Z.S.; Huang, Y.J.; Xia, M.S.; Xiang, X.W.; Qu, Y.L. Molecular Weight-Dependent Immunostimulative Activity of Low Molecular Weight Chitosan via Regulating NF-kappaB and AP-1 Signaling Pathways in RAW264.7 Macrophages. Mar. Drugs. 2016, 14, 169. [Google Scholar] [CrossRef]
- Wei, L.; Cai, C.; Lin, J.; Wang, L.; Zhang, X. Degradation controllable biomaterials constructed from lysozyme-loaded Ca-alginate microparticle/chitosan composites. Polymer 2011, 52, 5139–5148. [Google Scholar] [CrossRef]
- Amidi, M.; Romeijn, S.G.; Verhoef, J.C.; Junginger, H.E.; Bungener, L.; Huckriede, A.; Crommelin, D.J.; Jiskoot, W. N-Trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination, Biological properties and immunoge -nicity in a mouse model. Vaccine 2007, 25, 144–153. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China, Part 4; China Medical Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- Zhang, S. Hydroxyapatite Coatings for Biomedical Applicationns; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Aamna, S.; Manal, A.B. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Pharmaceutics 2019, 564, 22–38. [Google Scholar]
- Ouyang, Q.Q.; Hou, T.T. Construction of a composite sponge containing tilapia peptides andh improved hemostatic performance. Biol. Macromol. 2019, 139, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Romer, F.; Cornnell, L.; Walter, C.; Saiz, E.; Yue, S.; Lee, P.D.; McPhail, S.D. Highly flexible silica/chitosan hybrid scaffolds with oriented pores for tissue regeneration. Mater. J. Chem. B 2015, 38, 7560–7576. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Marti, J.; Cinca, N.; Punset, M.; Cano, I.G.; Dosta, S. Porous titanium-hydroxyapatite composite coating obtained on titanium by cold gas spray with high bond strength for biomedical applications. Colloids Surf. B Biointerfaces 2019, 1801, 245–253. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Sun, J.; McKee, M.D.; Chevrier, A.; Rossomacha, E.; Rivard, G.E.; Hurtig, M.; Buschmann, M.D. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthr. Cart. 2007, 15, 78–89. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Farrugia, B.L.; Dargaville, T.R.; Dhara, S. Chitosan-collagen scaffolds with nano/microfibrous architecture for skin tissue engineering. Biomed. J. Mater. Res. 2013, 101, 3482–3492. [Google Scholar] [CrossRef]
- Romanova, O.A.; Grigor’ev, T.E.; Goncharov, M.E.; Rudyak, S.G.; Solov’yova, E.V.; Krasheninnikov, S.T.; Saprykin, V.P.; Sytina, E.V.; Chvalun, S.N.; Pal’tsev, M.A.; et al. Chitosan as a modifying component of artificial scaffold for human skin tissue engineering. Bull. Exp. Biol. Med. 2015, 159, 557–566. [Google Scholar] [CrossRef]
- Amin, S.; Alaa, E.A.; Azam, A.; Zhifa, S. Bio-mimetic composite scaffold from mussel shells, squid penand crab chitosan for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 445–454. [Google Scholar]
- Kim, S.K. Chitin and Chitosan Derivatives, Advances in Drug Discovery and Developments; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Yeh, C.H.; Lin, P.W.; Lin, Y.C. Chitosan microfiber fabrication using a microfluidic chip and its application to cell cultures. Microfluid. Nanofluid. 2010, 8, 115. [Google Scholar] [CrossRef]
- Shaltookia, M.; Dini, G.; Mehdikhani, M. Fabrication of chitosan-coated porous polycaprolactone/strontium substituted bioactive glass nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. 2019, 105, 110138. [Google Scholar] [CrossRef]
- Keong, L.C.; Halim, A.S. In vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. Int. Mol. J. Sci. 2009, 10, 1300–1313. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials—A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Ali, A.; Hasanzadehb, M. Conductive nanofibrous chitosan/PEDOT: PSS tissue engineering scaffolds. Mater. Chem. Phys. 2019, 237, 121882. [Google Scholar]
- Pauletto, P.S.; Gonçalves, J.O.; Pinto, L.A.A.; Dotto, G.L.; Salau, N.P.G. Single and competitive dye adsorption onto chitosan–based hybrid hydrogels using artificial neural network modeling. J. Colloid Interface Sci. 2020, 56015, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Ahmeda, S.; Alic, A.A.; Javed, S. A review on chitosan centred scaffolds and their ap-plications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment, In vitro and in vivo evaluation. Int. Pharm. J. 2019, 57030, 118662. [Google Scholar] [CrossRef]
- Pacho, M.N.; Manzano, V.E.; Norma, B. Synthesis of micro- and nanoparticles of alginate and chitosan for controlled release of drugs. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 363–398. [Google Scholar]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery, a review. Carbohydr. Polym. 2020, 2311, 115744. [Google Scholar] [CrossRef]
- Luo, T.T.; Han, J.T.; Zhao, F.; Pan, X.H.; Zhang, J. Redox-sensitive micelles based on retinoic acid modified chitosan conjugate for intracellular drug delivery and smart drug release in cancer therapy. Carbohydr. Polym. 2019, 215, 8–19. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 12315, 441–451. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.G.; Rodríguez-Berna, G.; Bermejo, M.; Alvarez, I.G.; Merino, V. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar]
- Seyfoddina, A.; Chanb, A.; Chen, W.T.; Rupenthal, I.D.; Waterhouse, G.I.N.; Svirskisa, D. Electro-responsive macroporous polypyrrole scaffolds for triggered dexamethasone delivery. Eur. J. Pharm. Biopharm. 2015, 94, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Cossu, M.; Gavini, E.; Giunchedi, P.; Dalpiaz, A. Solid microparticles based on chitosan or methyl-β-cyclodextrin, A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kwon, S.; Park, J.H. Physicochemical characterizations of self-assembled nanoparticles of glycol chitosan-deoxycholic acid conjugates. Biomacromlecules 2005, 6, 1154–1158. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chielline, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Li, T.S.; Yawata, T.; Honke, K. Efficient SIRNA delivery and tumoraccumulation mediated by ionically cross-linked folic acid-polyethylene glycol)-thitosan oligosaccharide lactate nanoparticlesfor the potential targeted ovarian caneer gene therapy. Eur. J. Pharm. Sci. 2014, 52, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, A.; McGrath, M.G.; Carey, J.B.; Draper, S.J.; Hill, A.V.; O’Mahony, C.; Crean, A.M.; Moore, A.C. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Control. Release 2012, 159, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Migneco, L. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2017, 179, 273–281. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; de Melo Guedes, G.M.; da Silva, M.L.Q.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; de Aguiar Cordeiro, R.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Effect of the molecular weight of chitosan on its antifungal activity against Candida spp. in planktonic cells and biofilm. Carbohydr. Polym. 2018, 195, 662–669. [Google Scholar] [CrossRef]
- Song, X.X.; Chen, Y.Y.; Zhao, G.H.; Sun, H.B. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydr. Polym. 2020, 2311, 115689. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Elango, J.; Wu, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719. https://doi.org/10.3390/app10144719
Li B, Elango J, Wu W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Applied Sciences. 2020; 10(14):4719. https://doi.org/10.3390/app10144719
Chicago/Turabian StyleLi, Bailei, Jeevithan Elango, and Wenhui Wu. 2020. "Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application" Applied Sciences 10, no. 14: 4719. https://doi.org/10.3390/app10144719
APA StyleLi, B., Elango, J., & Wu, W. (2020). Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Applied Sciences, 10(14), 4719. https://doi.org/10.3390/app10144719






