Abstract
The genus Silene (family Caryophyllaceae) comprises more than 700 species, which are widely distributed in temperate zones of the Northern Hemisphere, but are also present in Africa and have been introduced in other continents. Silene produces a high diversity of secondary metabolites and many of them show interesting biological and pharmacological activities. More than 450 compounds have been isolated; important classes include phytoecdysteroids (which mimic insect molting hormones), triterpene saponins (with detergent properties), volatiles, other terpenoids and phenolics. This review focusses on the phytochemical diversity, distribution of Silene secondary metabolites and their biological activities.
1. Introduction
The genus Silene (family Caryophyllaceae) comprises more than 700 species (allocated in 39 sections) of annuals, biennials, and perennials which are mainly distributed in temperate zones of the Northern Hemisphere of Eurasia and America, but also in Africa [1,2]. Presently, the genus Silene includes several taxa which were formerly treated as different genera, such as Coronaria, Cucubalus, Lychnis, Melandrium, Petrocopsis, and Viscaria [1]. There are two major centers of diversity in Silene: one in the Mediterranean/Middle East and one in Central Asia. A few taxa have been introduced to other continents.
The genus consists mainly of herbaceous plants and, more rarely, small shrubs or subshrubs. The flowers have free petals, with each petal consisting of a usually visible limb that can be divided or entire, and a claw that is included within the synsepalous calyx. Silene has been placed in the tribe Sileneae and the subfamily Caryophylloideae. In molecular phylogenetic studies, the genus Silene clusters in two major clades of approximately equal size, which are tentatively classified as Silene subgenus Silene and Silene subgenus Behen (Moench) Bunge [3,4]. In the most recent taxonomic revision covering the entire genus, Silene has been divided into 44 sections, without any rank above that [5]. Common names of Silene are campion and catchfly. Red Campion (S. dioica), white Campion (S. latifolia, S. alba) and bladder Campion (S. vulgaris) are common wildflowers throughout Europe. Some species of Silene have served as important model plants for studies in ecology, genetics and evolution by famous scientists such as Charles Darwin, Gregor Mendel, Carl Correns, Herbert G. Baker, and Janis Antonovics [6]. Silene is an important model system for genetic studies on gynodioecy, dioecy, and polyploidy.
Silene also includes a number of cultivated species and widespread weeds [7]. S. acaulis, S. multifida and S. regia have been cultivated as ornamental plants because they produce beautiful flowers [8]. The roots of several species, such as S. latifolia, S. acaulis, S. kumaonensis, and S. conoidea which are rich in saponins with detergent properties, have been traditionally used as a soap substitute for washing clothes similar to other plants of the Caryophyllaceae [9,10]. The soap is obtained by simmering roots in hot water [11,12]. A few species are edible such as S. acaulis, S. cucubalis, and S. vulgaris [13,14,15,16]. Especially young shoots and the leaves of S. vulgaris are much appreciated in the traditional gastronomy of Turkey, Italy, Austria, and Spain [14]. A number of Silene species have been used in traditional medicine to treat inflammations, bronchitis, cold, and infections or as a diuretic, antipyretic, analgesic, and emetic [17,18,19,20,21,22,23,24]. Phytoecdysteroids mimic molting hormones of insects and are therefore of interest for chemical ecology and for applications of plant derived insecticides. Because of page restrictions, a thorough review of traditional uses of members of Silene or their pharmacology is out of scope of this review.
Silene produces a diversity of secondary metabolites, many of them are important for the plants as defence compounds against herbivores and microbes [25,26]. In this review, the secondary metabolites which have been isolated from the genus Silene are tabulated in detail; the review is based on an analysis of the relevant literature and data bases such as PubMed, Scifinder, and ScienceDirect. The diversity of structures of identified phytochemicals, their names and corresponding plant sources are summarized in Table 1 (below the main text).
2. Phytochemical Diversity
Phytochemical investigations of the genus Silene have led to the isolation of several phytoecdysteroids [27], triterpene saponins [28], terpenoids, benzenoids, flavonoids [29], anthocyanidins, N-containing compounds [30], sterols, and vitamins [31,32] (see Table 1). The abundance and widespread occurrence of triterpene saponins is a typical feature of the family Caryophyllaceae. Of special interest is the presence of phytoecdysteroids which mimic insect molting hormones and which strongly interfere with the metamorphosis of insects. The predominantly edysteroid positive genera of Silene, (including the former genera Coronaria, Lychnis and Petrocoptis) are in the Silenoideae [33,34,35]. Information on the phytochemistry of the genera Coronaria, Cucubalus, Lychnis, Melandrium, Petrocopsis, and Viscaria is not included, except if it was published under the merged genus Silene. A chemotaxonomical analysis of the data with view on the molecular phylogeny of Silene will be part of a subsequent publication.
3. Biological Properties
3.1. In Vitro Biological Activities
3.1.1. Antimicrobial and Antifungal Activities
Erturk et al. [8] extracted the apolar fractions from chloroform extract of S. multifida and tested for the antimicrobial activities against six bacteria (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae and Proteus vulgaris and one pathogenic fungus Candida albicans (0.5 mg/mL). All fractions of S. multifida showed activity against all tested bacteria. Only two fractions showed antifungal activity. The oil samples of S. vulgaris and S. cserei subsp. aeoniopsis were also screened against the several standard strains of bacteria and the yeast Candida using the microdilution method [36]. Both of these oils displayed the same activity profile, having notable antibacterial activity against the Gram-negative bacterium Klebsiella pneumoniae at a concentration of 4 g/mL and significant antifungal activity against Candida albicans (16 g/mL). Methanol extracts from three Silene species from Iran (S. gynodioca, S. spergulifolia and S. swertiifolia) were screened for their possible in vitro antibacterial activities by the disc diffusion method [37]. Results indicated that S. swertiifolia has a strong antibacterial activity against three Gram-positive and gram-negative bacteria, namely Haemophilus influenzae, Pseudomonas aeruginosa and Bacillus cereus, whereas S. spergulifolia showed a strong inhibition against Bacillus cereus. Bajpai et al. [38] examined the chemical composition of the essential oil isolated from S. armeria and tested the efficacy of essential oil (5 µL/mL, corresponding to 1000 ppm/disc) and different extracts (7.5 µL/mL, corresponding to 1500 ppm/disc) against a diverse range of food spoilage and food-borne pathogens (Bacillus subtilis ATCC6633, Listeria monocytogenes ATCC19166, Staphylococcus aureus KCTC1916, S. aureus ATCC6538, Pseudomonas aeruginosa KCTC2004, Salmonella typhimurium KCTC2515, Salmonella enteritidis KCTC2021, Escherichia coli O157-Human, E. coli ATCC8739, E. coli O57:H7 ATCC43888 and Enterobacter aerogenes KCTC2190). The results of this study suggested that the essential oil and leaf extracts derived from S. armeria could be used for the development of novel types of antibacterial agents to control food spoilage and food-borne pathogens.
We have studied the antimicrobial activity of different extracts and phytoecdysteroids from Silene plants towards pathogenic microorganisms [39]. Acinetobacter spec., Enterococcus faecalis, Klebsiella oxytoca, Pantoea agglomerans, Proteus rettgeri, Pseudomonas aeruginosa and Staphylococcus aureus strains were inhibited by the methanol extract of S. wallichiana (Minimal Inhibitory Concentration, MIC = 2.5 mg/mL), while Escherichia coli and Klebsiella pneumoniae were inhibited with a MIC = 1.25 mg/mL. The butanol extract of S. wallichiana showed activity against pathogenic bacteria Acinetobacter sp., E. coli, K. pneumoniae, P. agglomerans, P. aeruginosa and P. rettgeri, whereas chloroform extract inhibited only Citrobacter freundii, E. coli and P. aeruginosa (MIC = 1.25 mg/mL). The CHCl3 extract of S. brachuica inhibited growth of three Gram-negative (Enterococcus faecalis, Proteus rettgeri, and Pseudomonas aeruginosa) and one Gram-positive (Micrococcus luteus) bacterial strain. The CHCl3 extract of S. viridiflora was active against M. luteus, P. rettgeri, Klebsiella pneumoniae, and P. aeruginosa, whereas the extract of S. wallichiana exhibited activity against only pathogenic bacteria E. coli, M. luteus and P. aeruginosa [40]. Pure phytoecdysteroids (viticosterone E, 20-hydroxyecdysone-22-benzoate, 2-deoxy-20-hydroxyecdysone, 2-deoxyecdysone, 20-hydroxyecdysone and integristerone A) isolated from S. wallichiana exhibited very low activity against the bacteria [39]. Also a preliminary screening of the CHCl3 extract from the aerial part of S. guntensis exhibited antibacterial effects against Escherichia coli, P. aeruginosa, and Acinetobacter sp. [41]. From above-mentioned results we can conclude that the apolar fractions of Silene exhibit moderate activity against both Gram-positive and Gram-negative bacteria, and this activity may be attributed to a synergistic effect, due to the presence of phenols and some monoterpenoids in the apolar fraction.
3.1.3. Antioxidant Activity
Methanol extracts from three Silene species from Iran (S. gynodioca, S. spergulifolia and S. swertiifolia) were screened for their possible in vitro antioxidant activities by three complementary test systems, namely DPPH free radical-scavenging, metal chelating activity and β-carotene/linoleic acid oxidation [37]. Results showed that S. swertiifolia, which contains high amount of phenolics and flavonoids, exhibited the greatest antioxidant activity. The extracts of S. swertiifolia and S. spergulifolia showed a higher potency than ascorbic acid in scavenging of DPPH free radical. In the metal-chelating assay all extracts had a lower activity than ascorbic acid. In the β-carotene/linoleic acid system, oxidation of linoleic acid was effectively inhibited by the S. swertiifolia extract. The radical scavenging activity of the plant extracts decreased in the following order: ascorbic acid (IC50 = 0.13 mg/mL) > S. swertiifolia (IC50 = 0.13 mg/mL) > S. spergulifolia (IC50 = 0.21 mg/mL) > S. gynodioca (IC50 = 0.29 mg/mL).
Conforti et al. [44] studied the in vitro antioxidant activity of the hydroalcoholic extract from S. vulgaris. A very good correlation between radical scavenging activity and polyphenol content (for S. vulgaris 67.5 mg/g of extract) was found. Taskin and Bitis [45] reported that S. alba subsp. divaricata leaves have beneficial effects on ferrous chelating, DPPH radical-scavenging and ABTS radical cation scavenging abilities. This plant contained the highest phenolic compounds and may thus exert protection against oxidative damage. The radical scavenging ability of the extracts and phytoecdysteroids of S. guntensis were evaluated by us using the reaction with the stable DPPH radical [46]. In our experiments phytoecdysteroids were ineffective for DPPH radical scavenging activity (IC50 value > 100 µg/mL). Maximum scavenging activity of DPPH was observed with the water extract (IC50 68.90 μg/mL) of S. guntensis, followed by the activities of the butanol, methanol, and chloroform extracts with IC50 values of 69.12, 122.48, and 148.28 μg/mL, respectively. The activities of 20-hydroxyecdysone, 2-deoxy-20-hydroxyecdysone, and 2,3-diacetate-22-benzoate-20-hydroxyecdysone were 144.75, 157.29, and 291.38 μg/mL, respectively. However, we assume that the antioxidant effect of these extracts might be attributed to some co-eluting phenolic compounds and not to phytoecdysteroids, lipids etc.
3.1.4. Phagocytic Activity
Popov et al. [47] studied the effects of the polysaccharides from plants and callus of S. vulgaris (silenans) on uptake capacity and myeloperoxidase activity in the peripheral human neutrophils and monocytes and rat peritoneal macrophages in vitro. All polysaccharides (three silenans from the intact plant; pectic polysaccharides P1, P2 and P3) and two from the callus (acidic arabinogalactan C1 and pectin C2) enhanced uptake capacity at concentration of 15 mg/mL. The acidic arabinogalactan C1 was only found to stimulate lysosomal activity of the peripheral phagocytes. The effect of some polysaccharides was established in peritoneal resident macrophages. Pectins P1, P3 and C2 failed to enhance myeloperoxidase activity of the macrophages in calcium-free solution, whereas arabinogalactan C1 was independent of extracellular calcium. Polysaccharides studied failed to influence either complement receptor CR3- or scavenger receptor SR-mediated adhesion of the macrophages. The data obtained demonstrate that the S. vulgaris may be used as sources of immunoactive polysaccharides and that pectins and weakly acidic arabinogalactan seem to stimulate macrophages through different mechanisms. Complement receptor type 3 and scavenger receptor failed to mediate the cell activation induced by plant polysaccharides.
3.1.5. Inhibition of Nitric Oxide (NO) Production
Conforti et al. [44] examined whether S. vulgaris can modulate the production of NO by the RAW 264.7 mouse macrophage cell line pre-treated with a hydroalcoholic extract (10–1000 μg/mL) prior to activation by bacterial lipopolysaccharide (LPS). The treatment of RAW 264.7 macrophages with LPS (1 μg/mL) for 24 h, induced NO production which can be quantified by utilising the chromogenic Griess reaction and measuring the accumulation of nitrite, a stable metabolite of NO. The beneficial effect of extracts on the quenching of inflammatory mediators in macrophages can be mediated through oxidative degradation of phagocytosis products, such as O2− and HOCl. S. vulgaris had a weak cytotoxicity (202 ± 2.6 μg/mL), while the reference drug indomethacin showed cytotoxicity with IC50 = 58 μg/mL.
3.1.6. Antitumor Activity
In our in vitro experiments pure compounds, such as phytoecdysteroid 2,3-diacetate-22-benzoate-20-hydroxyecdysone showed a moderate inhibition against HeLa and HepG-2 cells (IC50 values (127.97 ± 11.34 μM) and (106.76 ± 7.81 μM), respectively), while 2-deoxy-20-hydroxyecdysone inhibited MCF-7 cells at a concentration IC50 = 126.54 ± 12.09 μM [46]. Conforti et al. [44] reported that a hydroalcoholic extract from S. vulgaris showed a weak cytotoxicity against the murine monocytic macrophage cell line RAW 264.7 (IC50 = 712 μg/mL). Behzad et al. [48] also informed that S. ampulata, S. peduncularis plants showed no cytotoxic activity (IC50 > 100 μL/mL) against normal and cancer cell lines.
The triterpene saponins from the roots of S. fortunei were tested in an in vitro lymphocyte proliferation assay. The saponins, jenisseensosides C and D and their deacylated derivatives stimulated the proliferation of the Jurkat tumor cell lines (human T-cell leukaemia) at low concentrations (1 nM to 5 μM). At high concentrations (>10 μM), they inhibited the proliferation of the cells probably due to the induction of apoptosis [28]. These authors [49] reported that the trans- and cis-p-methoxycinnamoyl triterpene saponins jenisseensosides A to D (from S. jenisseensis and S. fortunei) increased the accumulation and cytotoxicity of the anticancer agent cisplatin in HT 29 (human colon tumor) cells.
3.2. In Vivo Biological Activities
3.2.1. Antitumor Activity
Zibareva [50] reported that the ecdysteroid-containing extract of S. viridiflora exerted antitumor activity in vivo, however investigations with individual phytoecdysteroids showed no effect [51]. Also, El-Mofti [52,53] reported that ecdysone was able to induce neoplastic lesions in toads and mice, a result which appears somewhat surprising when considering the very low doses of ecdysone used. The phytoecdysteroids cyasterone, polypodine B, and decumbesterone A showed potent antitumor activities in a mouse-skin model in vivo in a two-stage carcinogenesis trial, using 7,12-dimethylbenz[a]anthracene as initiator and 12-O-tetradecanoylphorbol-13-acetate (TPA) as promoter [54]. However, Lagova and Valueva [55] reported that 20-hydroxyecdysone was mainly ineffective in preventing tumor growth in mice, but it stimulated the growth of mammary gland carcinomas. Because ecdysteroids structurally resemble sex hormones, they might indeed bind to steroid hormone receptors in mammals and stimulate the growth of hormone-dependent tumors. Binding studies performed so far for 20-hydroxyecdysone and a set of phytoecdysteroids [56,57] do not support this hypothesis, but they were not performed with all in vivo metabolites.
3.2.2. Immunomodulatory Activity
The total ecdysteroid preparation from S. viridiflora for immunostimulation in vivo was analyzed by Shakhmurova et al. [58]. The preparation (5 mg/kg) acts as an effective immunomodulator in normal mice and in mice with secondary immunodeficiency developed under irradiation, and with acute toxic hepatitis. The immunomodulating activity of total ecdysteroids from S. viridiflora is comparable with that of the known immunity stimulator T-activin, a polypeptide preparation from cattle thymus. Furthermore, Bushneva et al. [59] showed that pectic polysaccharide named silenan which was isolated from the aerial parts of S. vulgaris, possess immunomodulatory activity. Ghonime et al. [60] confirmed the immunomodulatory activity Silene species. Extracts from S. nocturna were examined for their immunomodulatory effect in Balb/c mice. Treatment (intraperitoneal injection) with five doses of the methanol extract enhanced the total white blood cells count (up to 1.2 × 104 cells/mm3). Bone marrow cell density also increased significantly after the administration of the extract. Furthermore, spleen weight of the treated groups was significantly increased as compared to controls. Two groups of mice were immunosuppressed with cyclophosphamide; the one which was pre-treated with S. nocturna extracts significantly restored their resistance against lethal infection with the predominantly granulocyte-dependant Candida albicans.
3.2.3. Adaptogen and Actoprotection Activity
The total ecdysteroid preparation from S. viridiflora (“Siverinol”) and S. brachuica (“Silekbin”) was analyzed for actoprotector and adaptogenic activity in vivo by several researchers [61,62,63,64]. Siverinol (oral intake doses ranged between 100 and 3000 mg/kg b.w. over 14 days) increased endurance (swimming tests) and reduced the recovery time (lactic acid recycling, regeneration of glycogen stores) after a severe physical load. Chronic exposure over 7–14 days resulted in a significant stimulation of erythropoiesis and increase of muscle size. Moreover, it reduced the stress effects of an extended physical exercise. The pharmacocorrective influence of Siverinol and Silekbin to biochemical mechanisms of dis-adaptation and basal processes of bioenergetics in the muscle tissue of the experimental animals was the base of actoprotector activity.
3.2.4. Hepatoprotection Activity
The effect of an oral administration of a 50% ethanol extract from S. aprica on acute liver injury was examined in rats intoxicated with carbon tetrachloride and acetaminophen [65]. The results indicated that S. aprica protected the liver intoxication as judged by morphological and biochemical observations. An increase in both lipid peroxidation and triglyceride concentrations occurred in the liver after carbon tetrachloride injection; S. aprica administration significantly reduced these changes. Also Shin et al. [66] reported that a S. takesimensis extract or a mixed extract with Melandrium firmum relieved fibrotic liver damage induced by carbon tetrachloride through inhibition of ALT and AST enzymes in the liver. The extracts inhibited hepatic fibrosis without affecting liver stromal cells by decreasing the amount of collagen, alpha-smooth muscle actin, and TGF-β inside the liver tissues.
3.2.5. Electrical Activity of the Heart
Golovko and Bushneva [21] studied the effect of silenan (a pectin polysaccharide from S. vulgaris), during development of arrhythmia and in disorders of cell-cell interactions in the zone of contact between the venous sinus and atrial cells. Electrical activity of myocardial cells was studied with spontaneously contracting strips from the sinoatrial area of Rana temporaria heart. Silenan corrected disorders in the conduction of action potentials between cells of the sinoatrial area of frog heart forming a functional syncytium. Recovery of action potential conduction in the sinoatrial cells was recorded in long-term experiments (>8 h). The effect of silenan mainly concerned the background of arrhythmic generation and impaired propagation of action potentials.
3.2.6. Insecticidal Activity
Phytoecdysteroids are analogues of insect molting hormones and sometimes their concentration in plants can reach 0.01%–3%. Even at ultralow concentrations they can affect insect development. For example, ecdysteroid 20-hydroxyecdysone at concentrations of 10−8 to 10−9 M initiates the transformations occurring in embryogenesis and during larval development with instant metamorphosis to the adult insect [67]. The potential insect deterrent activity of several Silene species, such as S. conoidea, S. ampulata and S. peduncularis, have been reported by several authors [48,68]. Chermenskaya et al. [69] reported that ethanol extracts of the aerial parts of S. sussamyrica showed substantial insecticidal activity, especially against western flower thrips larvae Frankliniella occidentalis Perg. (Thysanoptera: Thripidae). In this case we can assume this plant probably contained phytoecdysteroids and these compounds caused death of F. occidentalis larvae.
4. Conclusions
The genus Silene is known to be a source of biological active compounds. Phytochemical analysis of Silene demonstrated their richness in various compounds (>450 compounds have been isolated) belonging to different structural types, such as phytoecdysteroids, triterpene saponins, terpenoids, benzenoids, flavonoids, N-containing compounds, sterols, vitamins and others. The most prominent compounds in Silene species are the phytoecdysteroids, which have a similar chemical structure to molting hormones of insects. From data collected in this review, it is evident that the genus Silene comprises a wide range of pharmaceutically promising, interesting, and valuable plants. Some species of the genus Silene are used as ornamental plants and in folk medicine to treat inflammations, bronchitis, cold, and infections or as a diuretic, antipyretic, analgesic, emetic, etc. Many of the traditional uses have been validated by scientific research. It would be important for future studies to include the former genera Coronaria, Cucubalus, Lychnis, Melandrium, Petrocopsis, and Viscaria, which presently are partly included in the larger genus Silene [1].
The collected data provides a means to understand the latest developments in the pharmacology and phytochemistry of the genus Silene. Current pharmacological data is in many cases limited to studies on plant extracts and, hence, efforts are needed towards the isolation of biologically active compounds. Due to their various promising activities, further studies are warranted to be carried out on the drug development of Silene extracts and their constituents.

Table 1.
Structures and Distribution of Secondary Metabolites in the Genus Silene.
| Phytoecdysteroids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
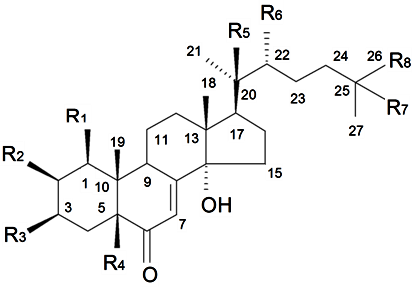 | ||||||||||
| Name | Structure | Plant Source | Reference | |||||||
| Substituents in Steroidal Core | Substituents in Side-Chain | |||||||||
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | |||
| Brahuisterone | H | H | OH | OH | H | OH | OH | CH3 | S. brahuica Boiss | [70] |
| 2-Deoxy-20,26-dihydroxyecdysone | H | H | OH | H | OH | OH | OH | CH2OH | S. pseudotites Bess ex Reichenb | [71,72] |
| 22-Deoxy-20,26-dihydroxyecdysone | H | OH | OH | H | OH | H | OH | CH2OH | S. nutans L. | [73,74] |
| 2-Deoxyecdysone | H | H | OH | H | H | OH | OH | CH3 | S. brahuica Boiss, S. claviformis Litv, S. fridvaldszkyana Hampe, S. gigantea L., S. graminifolia Otth, S. latifolia (Gilib) Aschers, S. otites (L.) Wibel, S. praemixta M Pop, S. pseudotites Bess ex Reichenb, S. repens Patrin, S. roemeri Friv, S. scabrifolia Kom, S. tomentella Schischk, S. wallichiana Klotsch | [71,72,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] |
| 2-Deoxyecdysone-3-acetate | H | H | OAc | H | H | OH | OH | CH3 | S. scabrifolia Kom | [99] |
| 2-Deoxyecdysone-22-acetate | H | H | OH | H | H | OAc | OH | CH3 | S. brahuica Boiss, S. otites (L.) Wibel | [74,100] |
| 2-Deoxyecdysone-22-benzoate | H | H | OH | H | H | OBz | OH | CH3 | S. wallichiana Klotsch | [85] |
| 2-Deoxyecdysone-22-glucoside | H | H | OH | H | H | OGlu | OH | CH3 | S. praemixta M Pop, S. pseudotites Bess ex Reichenb | [71,72,98] |
| 2-Deoxy-20-hydroxyecdysone | H | H | OH | H | OH | OH | OH | CH3 | S. antirrhina L., S. brahuica Boiss, S. chlorifolia Smith, S. claviformis Litv, S. cretica L., S. disticha Willd, S. fridvaldszkyana Hampe, S. gigantea L., S. guntensis B Fredtsch, S. italica (L.) Pers, S. italica ssp. nemoralis, S. latifolia (Gilib) Aschers, S. linicola C.C.Gmelin., S. otites (L.) Wibel, S. portensis L., S. praemixta M Pop, S. pseudotites Bess ex Reichenb, S. repens Patrin, S. roemeri Friv, S. scabrifolia Kom, S. viridiflora L., S. wallichiana Klotsch | [46,71,72,76,77,78,79,81,82,85,86,87,88,89,90,91,92,93,94,96,97,98,100,101,102,103,104,105,106,107,108,109,110,111,112,113] |
| 2-Deoxy-20-hydroxyecdysone-3-acetate | H | H | OAc | H | OH | OH | OH | CH3 | S. otites (L.) Wibel, S. praemixta M Pop | [114,115] |
| 5α-2-Deoxy-20-hydroxyecdysone-3-acetate | H | H | OAc | H (α) | OH | OH | OH | CH3 | S. otites (L.) Wibel | [114] |
| 2-Deoxy-20-hydroxyecdysone-22-acetate | H | H | OH | H | OH | OAc | OH | CH3 | S. otites (L.) Wibel | [74] |
| 2-Deoxy-20-hydroxyecdysone-25-acetate | H | H | OH | H | OH | OH | OAc | CH3 | S. wallichiana Klotsch | [116] |
| 2-Deoxy-20-hydroxyecdysone-3-benzoate | H | H | OBz | H | OH | OH | OH | CH3 | S. wallichiana Klotsch | [77] |
| 2-Deoxy-20-hydroxyecdysone-22-benzoate | H | H | OH | H | OH | OBz | OH | CH3 | S. nutans L., S. otites (L.) Wibel, S. supina Bieb, S. tatarica (L.) Wild | [74,81,91,101,117,118,119,120,121] |
| 2-Deoxy-20-hydroxyecdysone-3-crotonate | H | H | OCOC2H2CH3 | H | OH | OH | OH | CH3 | S. otites (L.) Wibel | [114] |
| 2-Deoxy-20-hydroxyecdysone-3,22-diacetate | H | H | OAc | H | OH | OAc | OH | CH3 | S. otites (L.) Wibel | [114] |
| 2-Deoxy-20-hydroxyecdysone-22-glucoside | H | H | OH | H | OH | O-β-d-Glu | OH | CH3 | S. italica ssp. nemoralis | [104] |
| 2-Deoxy-20-hydroxyecdysone-25-glucoside | H | H | OH | H | OH | OH | O-β-d-Glu | CH3 | S. gigantea L. | [91,92,95] |
| 2-Deoxyintegristerone A | OH | OH | OH | H | OH | OH | OH | CH3 | S. italica ssp. nemoralis, S. otites (L.) Wibel, S. pseudotites Bess ex Reichenb, S. viridiflora L. | [74,102,105,112,122] |
| 5α-2-Deoxyintegristerone A | OH | OH | OH | H (α) | OH | OH | OH | CH3 | S. italica ssp. nemoralis, S. pseudotites Bess ex Reichenb | [72,110] |
| 22-Deoxyintegristerone A | OH | OH | OH | H | OH | H | OH | CH3 | S. italica ssp nemoralis, S. nutans L. | [74,105] |
| 5α-22-Deoxyintegristerone A | OH | OH | OH | H (α) | OH | H | OH | CH3 | S. nutans L. | [74] |
| 2-Deoxypolypodine B-3-glucoside | H | H | O-β-d-Glu | OH | OH | OH | OH | CH3 | S. pseudotites Bess ex Reichenb, S. viridiflora L. | [71,72,123] |
| 2-Deoxy-5,20,26-trihydroxyecdysone | H | H | OH | OH | OH | OH | OH | CH2OH | S. viridiflora L. | [122] |
| 20,26-Dihydroxyecdysone (Podecdysone C) | H | OH | OH | H | OH | OH | OH | CH2OH | S. fridvaldszkyana Hampe, S. nutans L., S. otites (L.) Wibel., S. viridiflora L. | [71,74,76,88,89,90,93,94,95,124] |
| 20,26-Dihydroxyecdysone-2,22-diacetate | H | OAc | OH | H | OH | OAc | OH | CH2OH | S. viridiflora L. | [71,125] |
| 20,26-Dihydroxyecdysone-3,22-diacetate | H | OH | OAc | H | OH | OAc | OH | CH2OH | S. viridiflora L. | [71,125] |
| Ecdysone | H | OH | OH | H | H | OH | OH | CH3 | S. cretica L., S. disticha Willd, S. echinata Otth, S. italica (L.) Pers., S. italica ssp. nemoralis, S. linicola C.C.Gmelin., S. otites (L.) Wibel, S. portensis L., S. praemixta M Pop, S. pseudotites Bess. ex Reichenb, S. radicosa Bois et Heldr | [71,72,88,89,90,92,93,96,107,109,111,112,113,114,115] |
| Ecdysone-22-sulfate | H | OH | OH | H | H | OSO3H | OH | CH3 | S. brahuica Boiss | [126] |
| Ecdysteroside | H | OH | O-α-d-Gal (1→6) α-d-Gal | H | OH | OH | OH | CH3 | S. tatarica (L.) Wild | [127] |
| 5α-20-Hydroxyecdysone | H | OH | OH | H (α) | OH | OH | OH | CH3 | S. italica ssp. nemoralis | [110] |
| 5α-20-Hydroxyecdysone-22-benzoate | H | OH | OH | H (α) | OH | OBz | OH | CH3 | S. scabrifolia Kom | [128] |
| 20-Hydroxyecdysone | H | OH | OH | H | OH | OH | OH | CH3 | S. acaulis (L.) Jacg, S. altaica Pers, S. ambigua Turcz, S. antirrhina L., S. apetala Willd, S. aprica Turel, S. armeria L., S. bashkirorum Janish, S. bellidifolia Juss. ex Jacq, S. bergiana Lindm, S. borystenica (Gruner) Walters, S. bourgeaui H. Christ, S. brachypoda Rouy, S. brahuica Boiss, S. burchelli Otth, S. campanulata, S. Watson, S. caramanica Boiss, S. catholica (L.) Aiton fil, S. caucasica Boiss, S. chamarensis Turcz, S. chlorantha Willd, S. chlorifolia Smith, S. ciliata Pourret, S. ciliata var graefteri (P), S. claviformis Litv, S. coeli-rosa (L.) Godron in Gren, S. colorata Poiret, S. colorata ssp. trichocalysina, S. coronaria (L.) Clairv, S. cretaceae Fisch, S. cretica L., S. damboldtiana Greuter et Melzh, S. densiflora (L.) Wib. Drurv, S. dioica (L.) Clairv, S. disticha Willd, S. echinata Otth, S. elegans L., S. fetissovii Lazkov, S. firma Siebold et Zucc, S. flavescens Waldst et Kit, S. foliosa Maxim, S. fridvaldszkyana Hampe, S. fruticosa L., S. fruticulosa (Pall.) Schishk, S. gallica L., S. gallica var. quiquivulnera (L.) Koch, S. gebleriana Schrenk, S. gigantea L., S. goulimyi Turrill, S. graefferi Guss, S. graminifolia Otth, S. guntensis B Fredtsch, S. hifacensis Rouy ex willk, S. holopetala Lebed, S. ichebogda Glub, S. incurvifolia Kar et Kir, S. italica (L.) Pers, S. italica | [46,71,72,75,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,95,96,97,98,101,104,106,107,108,109,111,112,113,115,117,121,129,130,131,132,133,134,135,136,137,138,139,140] |
| 20-Hydroxyecdysone | H | OH | OH | H | OH | OH | OH | CH3 | ssp. nemoralis, S. jenisseensis Willd, S. kungessana B Fedtsch, S. latifolia (Gilib) Aschers, S. linicola C.C.Gmelin, S. longicalycina Kom, S. longicilia (Brot) Otth, S. mellifera Boiss. et Reuter, S. melzheimeri Greuter, S. micropetala Lag, S. mollissima (L.) Pers, S. mongolica Maxim, S. multicaulis Guss, S. multiflora (Waldst et Kit) Pers, S. nemoralis Waldst et Kit, S. nutans L., S. obovata Schischk., S. odoratissima Bunge, S. oligantha Boiss, S. otites (L.) Wibel, S. otites var. parviflorus, S. paradoxa L., S. parnassica Boiss, S. patula Desf, S. portensis L., S. praemixta M Pop, S. pseudotites Bess. ex Reichenb, S. psevdovelutina Rothm, S. pygmaea Adams, S. quinquevulnera L., S. radicosa Bois et Heldr, S. regia, S. reichenbachii Vis, S. repens Patrin, S. roemeriFriv, S. rubella L., S. saxatilis Sims, S. saxifraga L., S. scabriflora Brot, S. scabrifolia Kom, S. schafta S.G.Gmel. ex Hohen, S. schischkinii (M Pop) Vved, S. schmuckeri Wettst, S. secundiflora Otth, S. sendtneri Boiss, S. sericea All, S. sieberi Fenzl, S. sobolevskajae Czer, S. supina Bieb, S. spergulifolia (Willd) Bieb, S. squamigera Boiss, S. stenophylla Ledeb, S. stylosa Bunge, S. sussamyrica Lazkov, S. tatarica (L.) Wild, S. thessalonica Boiss et Heldr, S. tomentella Schischk, S. turchaninova Lazkov, S. turgida L., S. uralensis (Rupr) Bocquet, S. viridiflora L., S. viscosa (L.) Pers, S. wallichiana Klotsch, S. wolgensis (Hornem) Otth, S. zawadskii Herbich | [46,71,72,75,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,95,,96,97,98,101,104,106,107,108,109,111,112,113,115,117,121,129,130,131,132,133,134,135,136,137,138,139,140] |
| 20-Hydroxyecdysone-2-acetate | H | OAc | OH | H | OH | OH | OH | CH3 | S. otites (L.) Wibel | [102,112] |
| 20-Hydroxyecdysone-3-acetate | H | OH | OAc | H | OH | OH | OH | CH3 | S. otites (L.) Wibel | [102,112] |
| 20-Hydroxyecdysone-22-acetate | H | OH | OH | H | OH | OAc | OH | CH3 | S. otites (L.) Wibel | [74] |
| 20-Hydroxyecdysone-20-benzoate | H | OH | OH | H | OBz | OH | OH | CH3 | S. tatarica (L.) Wild | [141] |
| 20-Hydroxyecdysone-22-benzoate | H | OH | OH | H | OH | OBz | OH | CH3 | S. otites (L.) Wibel, S. scabrifolia Kom, S. wallichiana Klotsch | [74,83,142] |
| 20-Hydroxyecdysone-22-benzoate-25-glucoside | H | OH | OH | H | OH | OBz | O-β-d-Glu | CH3 | S. otites (L.) Wibel | [74] |
| 20-Hydroxyecdysone -2,3-diacetate-22-benzoate | H | OAc | OAc | H | OH | OBz | OH | CH3 | S. guntensis B Fredtsch | [46] |
| 20-Hydroxyecdysone-22,25-dibenzoate | H | OH | OH | H | OH | OBz | OBz | CH3 | S. scabrifolia Kom | [142] |
| 20-Hydroxyecdysone-3-glucoside | H | OH | O-β-d-Glu | H | OH | OH | OH | CH3 | S. otites (L.) Wibel | [103] |
| 20-Hydroxyecdysone-25-glucoside | H | OH | OH | H | OH | OH | O-β-d-Glu | CH3 | S. otites (L.) Wibel | [74] |
| 26-Hydroxyintegristerone A | OH | OH | OH | H | OH | OH | OH | CH2OH | S. fridvaldszkyana Hampe | [95] |
| 26-Hydroxypolypodine B | H | OH | OH | OH | OH | OH | OH | CH2OH | S. fridvaldszkyana Hampe, S. nutans L., S. viridiflora L. | [71,74,95,108] |
| Inokosterone | H | OH | OH | H | OH | OH | H | CH2OH | S. disticha Willd, S. pseudotites Bess. ex Reichenb, S. regia Sims | [72,95,112] |
| Integristerone A | OH | OH | OH | H | OH | OH | OH | CH3 | S. brahuica Boiss., S. claviformis Litv,S. fridvaldszkyanaHampe, S. gigantea L., S. italica ssp. nemoralis, S. nutans L., S. otites (L.) Wibel, S. repens Patrin, S. scabrifolia Kom, S. supina Bieb, S. tatarica (L.) Wild, S. tomentella Schischk, S. viridiflora L., S. wallichiana Klotsch | [74,77,78,79,80,81,83,86,87,92,95,104,108,112,135,143] |
| Integristerone A-25-acetate | OH | OH | OH | H | OH | OH | OAc | CH3 | S. brahuica Boiss | [144] |
| Polypodine B | H | OH | OH | OH | OH | OH | OH | CH3 | S. altaica Pers, S. antirrhina L., S. brachypoda Rouy, S. brahuica Boiss, S. campanulata S. Watson, S. caramanica Boiss, S. catholica (L.) Aiton fil, S. caucasica Boiss, S. chlorifolia Smith, S. ciliata Pourret, S. cretica L., S. damboldtiana Greuter et Melzh, S. disticha Willd, S. echinata Otth, S. fridvaldszkyana Hampe, S. italica (L.) Pers, S. italica ssp. nemoralis, S. linicola C.C.Gmelin, S. mellifera Boiss et Reuter, S. nutans L., S. paradoxa L., S. parnassica Boiss, S. pseudotites Bess. ex Reichenb, S. radicosa Bois et Heldr, S. regia Sims, S. repens Patrin, S. roemeri Friv, S. schmuckeri Wettst, S. sendtneri Boiss, S. supina Bieb, S. tatarica (L.) Wild, S. tomentella Schischk, S. viridiflora L. | [71,72,78,79,80,81,84,86,88,89,90,91,92,93,95,96,97,101,104,106,107,108,111,112,113,118,131,135] |
| Ponasterone A | H | OH | OH | H | OH | OH | H | CH3 | S. antirrhina L., S. brahuica Boiss, S. chlorifolia Smith, S. disticha Willd, S. echinata Otth, S. italica (L.) Pers, S. portensis L., S. pseudotites Bess. ex Reichenb, S. radicosa Bois et Heldr, S. regia Sims | [50,71,72,88,89,90,92,94,96,106,111,112,113] |
| Sileneoside A | H | OH | OH | H | OH | O-α-d-Gal | OH | CH3 | S. brahuica Boiss, S. nutans L., S. scabrifolia Kom, S. supina Bieb, S. tatarica (L.) Wild, S. viridiflora L. | [95,108,112,135] |
| Sileneoside B | H | OH | O-β-d-Gal | H | OH | O-β-d-Gal | OH | CH3 | S. brahuica Boiss | [136] |
| Sileneoside C | OH | OH | OH | H | OH | O-α-d-Gal | OH | CH3 | S. brahuica Boiss | [137] |
| Sileneoside D | H | OH | O-β-d-Gal | H | OH | OH | OH | CH3 | S. brahuica Boiss, S. scabrifolia Kom, S. supina Bieb, S. tatarica (L.) Wild, S. viridiflora L. | [108,112,143,145] |
| Silenoside E (Blechnoside A) | H | H | O-β-d-Glu | H | H | OH | OH | CH3 | S. brahuica Boiss | [84] |
| 5α-Silenoside E | H | H | O-β-d-Glu | H (α) | H | OH | OH | CH3 | S. brahuica Boiss | [146] |
| Sileneoside F | H | H | O-β-d-Glu | OH | H | OH | OH | CH3 | S. brahuica Boiss | [147] |
| Sileneoside G | H | OH | O-α-d-Glu | H | OH | O-α-d-Gal | OH | CH3 | S. brahuica Boiss | [148] |
| Sileneoside H | OH | OH | OH | H | OH | O-α-d-Gal | OAc | CH3 | S. brahuica Boiss | [149] |
| Taxisterone | H | OH | OH | H | OH | H | OH | CH3 | S. italica ssp. nemoralis, S. nutans L., S. viridiflora L. | [104,150,151] |
| Tomentesterone A | H | H | OH | H (α) | H | OAc | OBz | CH3 | S. tomentella Schischk | [80] |
| Tomentesterone B | H | H | OH | H (α) | H | OH | OBz | CH3 | S. tomentella Schischk | [152] |
| Viticosterone E | H | OH | OH | H | OH | OH | OAc | CH3 | S. brahuica Boiss, S. linicola C.C.Gmelin, S. otites (L.) Wibel, S. praemixta M Pop, S. tomentella Schischk, S. wallichiana Klotsch | [80,85,102,107,112,115,135] |
| Viticosterone E-22-benzoate | H | OH | OH | H | OH | OAc | OAc | CH3 | S. wallichiana Klotsch | [104,153] |
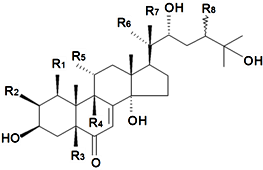 | ||||||||||
| Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Plant Source | Reference |
| 24(28)-Dehydromakisterone A | H | OH | H | H | H | CH3 | OH | CH2 | S. fridvaldszkyana Hampe, S. italica ssp.nemoralis, S. otites (L.) Wibel, S. roemeri Friv | [71,76,88,89,90,92,93,94,95,104,112] |
| 2-Deoxy-21-hydroxyecdysone | H | H | H | H | H | CH2OH | H | H | S. otites (L.) Wibel, S. pseudotites Bess. ex Reichenb | [102,112] |
| 5α-2-Deoxy-21-hydroxyecdysone | H | H | H (α) | H | H | CH2OH | H | H | S. otites (L.) Wibel | [102] |
| 9α,20-Dihydroxyecdysone | H | OH | H | OH (α) | H | CH3 | OH | H | S. italica ssp. nemoralis | [109,110] |
| 9β,20-Dihydroxyecdysone | H | OH | H | OH | H | CH3 | OH | H | S. italica ssp. nemoralis | [110] |
| Makisterone A | H | OH | H | H | H | CH3 | OH | CH3 | S. otites (L.) Wibel | [112] |
| Nusilsterone | OH | OH | H | H | H | CH3 | OH | OH | S. nutans L. | [154] |
| Turkesterone | H | OH | H | H | OH | CH3 | OH | H | S. linicola C. C. Gmelin | [107] |
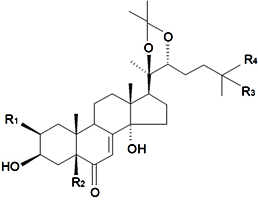 | ||||||||||
| Name | R1 | R2 | R3 | R4 | Plant Source | Reference | ||||
| 2-Deoxy-20-hydroxyecdysone-20,22-acetonide | H | H | OH | CH3 | S. viridiflora L. | [122] | ||||
| 5α-2-Deoxy-20-hydroxyecdysone-20,22-acetonide | H | H (α) | OH | CH3 | S. viridiflora L. | [155] | ||||
| 2-Deoxy-5,20,26-trihydroxyecdysone-20,22-acetonide | H | OH | OH | CH2OH | S. viridiflora L. | [122] | ||||
| 20,26-Dihydroxyecdysone-20,22-acetonide | OH | H | OH | CH2OH | S. viridiflora L. | [122] | ||||
| 20-Hydroxyecdysone-20,22-acetonide | OH | H | OH | CH3 | S. scabrifolia Kom | [156] | ||||
| 20-Hydroxyecdysone 20,22-acetonide-25-acetate | OH | H | OAc | CH3 | S. viridiflora L. | [123] | ||||
| 5,20,26-Trihydroxyecdysone-20,22-acetonide | OH | OH | OH | CH2OH | S. viridiflora L. | [122] | ||||
| 20-Hydroxyecdysone-2,3-acetonide | R1 = OH | 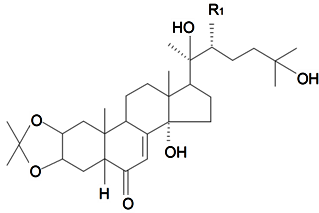 | S. scabrifolia Kom | [142] | ||||||
| 20-Hydroxyecdysone-2,3-acetonide-22-benzoate | R1 = OBz | S. scabrifolia Kom | [83,142] | |||||||
| 5α-Dihydro rubrosterone | R1 = H (α) | 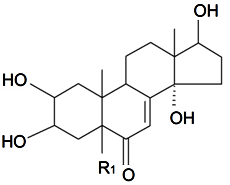 | S. otites (L.) Wibel | [103] | ||||||
| 5β-Dihydro rubrosterone | R1 = H | S. otites (L.) Wibel | [103] | |||||||
| 20, 22-Acetal isovaleric aldehyde-5β-cholest-7-en-2β,3β,14α,20R,22R,25-hexahydroxy-6-on | R1 = H (α) | 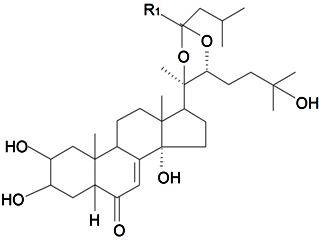 | S. claviformis Litv | [87] | ||||||
| 20,22-Acetal epiisovaleric aldehyde-5β-cholest-7-en-2β,3β,14α,20R,22R,25-hexahydroxy-6-on | R1 = H | S. claviformis Litv | [87] | |||||||
| Dihydropoststerone | 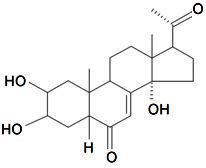 | S. otites (L.) Wibel | [74] | |||||||
| Poststerone | 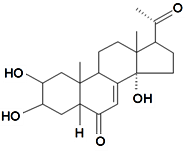 | S. otites (L.) Wibel | [74] | |||||||
| Poststerone | 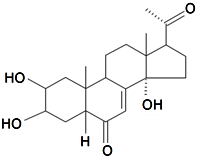 | S. otites (L.) Wibel | [74] | |||||||
| Rubrosterone | 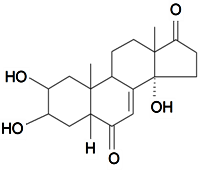 | S. otites (L.) Wibel | [74] | |||||||
| Makisterone C-2,3;20,22-diacetonide | 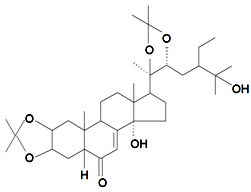 | S. viridiflora L. | [155] | |||||||
| Praemixisterone | 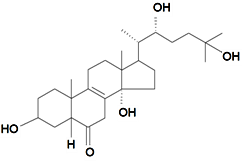 | S. praemixta M Pop | [134] | |||||||
| Sidisterone | 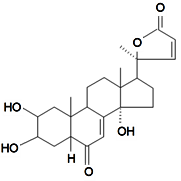 | S. dioica (L.) Clairv, S. otites (L.) Wibel, S. pseudotites Bess.ex Reichenb | [86,90,92,94,106,112,133] | |||||||
| Silenosterone | 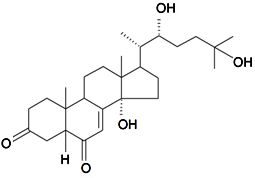 | S. praemixta M Pop | [82] | |||||||
| Triterpene Saponins | ||||||||||
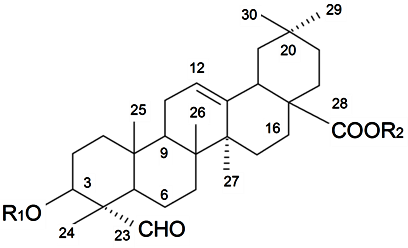 | ||||||||||
| β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl-3β-hydroxy-23-oxoolean-12-en-28-oic acid 28-O-β-d-xylopyranosyl(1→3)-β-d-xylopyranosyl(1→4)-α-l-rhamnopyranosyl (1→2)-β-d-fucopyranoside (Silenosides A) | R1 = -β-d-GlcUAp-2←1-β-d-Galp R2 = -β-d-Fucp-2←1-α-l-Rhap-4←1-β-d-Xylp-3←1-β-d-Xylp | S. vulgaris (Moench) Garcke (syn. S. inflata) | [157] | |||||||
| 3-O-{β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl]-(1→3)]-β-d- glucuronopyranosyl}-28-O-{β-d-xylopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)]-[3,4-di-O-acetyl-β-d-quinovopyranosyl-(1→4)]-β-d-fucopyranosyl] gypsogenin (Silenorubicunoside A) | R1 = -β-d-GlcUAp- | 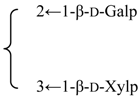 | S. rubicunda Franch | [158] | ||||||
| R2 = -β-d-Fucp- | 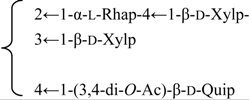 | |||||||||
| 3-O-{β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl]-(1→3)]-β-d- glucuronopyranosyl}-28-O-{β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)-3,4-di-O-acetyl-β-d- fucopyranosyl] gypsogenin (Silenorubicunoside C) | R1 = -β-d-GlcUAp- | 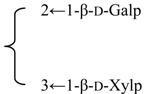 | S. rubicunda Franch | [158] | ||||||
| R2 = -(3,4-di-O-Ac)-β-d-Fucp-2←1-α-l-Rhap-4←1-β-d-Xylp | ||||||||||
| 3-O-{β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucuronopyrannosyl}-28-O-{β-d-xylopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)- α-l-rhamnopyranosyl-(l→2)-[3,4-di-O-acetyl-β-d-fucopyranosyl} gypsogenin (Glanduloside C) | R1 = -β-d-GlcUAp- | 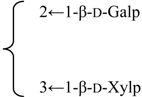 | S. rubicunda Franch | [158] | ||||||
| R2 = -(3,4-di-O-Ac)-β-d-Fucp-2←1-α-l-Rhap-4←1-β-d-Xylp-3←1-β-d-Xylp | ||||||||||
| Gypsogenin 3-O-β-xylopyranosyl-(1→3)-[β-galactopyranosyl-(1→2)]-β-glucuronopyranside | R1 = -β-d-GlcUAp- | 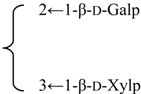 | S. cucubalus Wib | [159] | ||||||
| R2 = H | ||||||||||
| Nutanoside | R1 = -β-d-GlcUAp-3←1-β-d-Galp- |  | S. nutans L. | [160] | ||||||
| R2 = -α-l-Rhap- |  | |||||||||
| Gypsogenin 3-O-glucuronide | R1 = -β-d-GlcUAp, R2 = H | S. vulgaris (Moench) Garcke | [161] | |||||||
| Gypsogenin 3-O-glycoside | R1 = -β-d-Glcp, R2 = H | S. vulgaris (Moench) Garcke | [161] | |||||||
 | ||||||||||
| 3-O-[β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl]- 28-O-[β-d-glucopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-4-O-trans-p-methoxycinnamoyl- fucopyranosyl] quillaic acid(Jenisseensoside A) | R1 = -β-d-GlcUAp-2←1-β-d-Galp R2 = (4-O-E-p-methoxycinnamoyl-)-β-d-Fucp-2←1-α-l-Rhap-2←1-β-d-Glcp | S. jenisseensis Willd | [162,163] | |||||||
| 3-O-[β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl]- 28-O-[β-d-glucopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-4-O-cis-p-methoxycinnamoyl- fucopyranosyl] quillaic acid(Jenisseensoside B) | R1 = -β-d-GlcUAp-2←1-β-d-Galp R2 = (4-O-Z-p-methoxycinnamoyl-)-β-d-Fucp-2←1-α-l-Rhap-2←1-β-d-Glcp | S. jenisseensis Willd | [162,163] | |||||||
| 3-O-β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl- 28-O-[{α-l-rhamnopyranosyl -(1→2)}-{4-O-trans-p-methoxycinnamoyl}-β-d-fucopyranosyl] quillaic acid (Jenisseensoside C) | R1 = -β-d-GlcUAp-2←1-β-d-Galp R2 = (4-O-E-p-methoxycinnamoyl-)-β-d-Fucp-2←1-α-l-Rhap | S. fortunei Wis, S. jenisseensis Willd | [163,164] | |||||||
| 3-O-β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl- 28-O-[{α-l-rhamnopyranosyl -(1→2)}-{4-O-cis-p-methoxycinnamoyl}-β-d-fucopyranosyl] quillaic acid (Jenisseensoside D) | R1 = -β-d-GlcUAp-2←1-β-d-Galp R2 = (4-O-Z-p-methoxycinnamoyl-)-β-d-Fucp-2←1-α-l-Rhap | S. fortunei Wis, S. jenisseensis Willd | [163,164] | |||||||
| 3-O-[β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl]quillaic acid-28-O-α-l-rhamnopyranosyl-(1→2)-3-O-acetyl-4-O-trans-p-methoxycinnamoyl β-d-fucopyranoside (Jenisseensoside E) | R1 = -β-d-GlcUAp-2←1-β-d-Galp R2 = (3-O-Ac-, 4-O-E-p-methoxycinnamoyl-)-β-d-Fucp-2←1-α-l-Rhap | S. fortunei Wis | [28] | |||||||
| 3-O-[β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl]quillaic acid-28-O-α-l-rhamnopyranosyl-(1→2)-3-O-acetyl-4-O-cis-p-methoxycinnamoyl β-d-fucopyranoside (Jenisseensoside F) | R1 = -β-d-GlcUAp-2←1-β-d-Galp R2 = (3-O-Ac-, 4-O-Z-p-methoxycinnamoyl-)-β-d-Fucp-2←1-α-l-Rhap | S. fortunei Wis | [28] | |||||||
| 3-O-[β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl] quillaic acid-28-O-[α-l-arabinopyranosyl-(1→2)-α-l-arabinopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)]-[6-O-acetyl-β-d-glucopyranosyl-(1→3)]-4-O-acetyl-β-d-fucopyranoside | R1 = -β-d-GlcUAp-2←1-β-d-Galp | S. fortunei Wis | [28] | |||||||
| R2 = 4-O-Ac-β-d-Fucp- |  | |||||||||
| 3-O-[β-d-Galactopyranosyl-(1→2)-β-d-glucuronopyranosyl]-28-O-[[α-l-arabinopyranosyl-(1→2)-α-l-arabinopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)]-[β-d-glucopyranosyl-(1→3)]-4-O-acetyl-β-d-fucopyranosyl] quillaic acid | R1 = -β-d-GlcUAp-2←1-β-d-Galp | S. fortunei Wis | [164] | |||||||
| R2 = 4-O-Ac-β-d-Fucp- |  | |||||||||
| 3-O-β-d-Galactopyranosyl(1→2)-β-d-glucuronopyranosyl-3β,16α-dihydroxy-23-oxoolean-12-en-28-oic acid 28-O-β-d-xylopyranosyl(1→4)-[β-d-glucopyranosyl(1→2)]-α-l-rhamnopyranosyl (1→2)-β-d-fucopyranoside(Silenosides B) | R1 = -β-d-GlcUAp-2←1-β-d-Galp | S. vulgaris (Moench) Garcke | [157] | |||||||
| R2 = -β-d-Fucp-2←1-α-l-Rhap- | 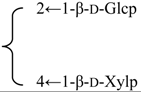 | |||||||||
| 3-O-α-l-Arabinopyranosyl(1→3)-[β-d-galactopyranosyl (1→2)]-β-d-glucuronopyranosyl-3β,16α-dihydroxy-23-oxoolean-12-en-28-oic acid 28-O-β-d-xylopyranosyl(1→4)-[β-d-glucopyranosyl(1→2)]-α-l-rhamnopyranosyl (1→2)-β-d-fucopyranoside (Silenosides C) | R1 = -β-d-GlcUAp- | 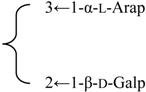 | S. vulgaris (Moench) Garcke | [157] | ||||||
| R2 = -β-d-Fucp-2←1-α-l-Rhap- | 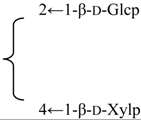 | |||||||||
| 3-O-{β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl]-(1→3)]-β-d- glucuronopyranosyl}-28-O-{β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)]-[4,6-di-O-acetyl-β-d-glycopyranosyl-(1→3)]-4-O-acetyl-β-d-fucopyranosyl] quillaic acid (Silenorubicunoside B) | R1 = -β-d-GlcUAp- | 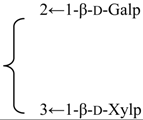 | S. rubicunda Franch | [158] | ||||||
| R2 = (4-O-Ac)-β-d-Fucp- |  | |||||||||
| 3-O-β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucuronopyrannosyl}-28-O-{β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(l→2)-[6-O-acetyl-β-d-glycopyranosyl-(1→3)]-4-O-acetyl -β-d-fucopyranosyl}quillaic acid | R1 = -β-d-GlcUAp- | 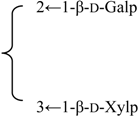 | S. rubicunda Franch | [158] | ||||||
| R2 = (4-O-Ac)-β-d-Fucp- | 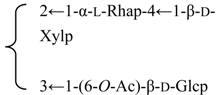 | |||||||||
| 3-O-{β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucuronopyrannosyl}-28-O-{β-d-xylopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(l→2)-[3,4-di-O-acetyl-β-d-quinovopyranosyl-(1→4)]-β-d-fucopyranosyl}quillaic acid (Pachystegioside A) | R1 = -β-d-GlcUAp- | 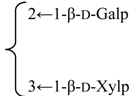 | S. rubicunda Franch | [158] | ||||||
| R2 = -β-d-Fucp- | 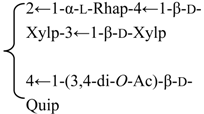 | |||||||||
| Quillaic acid 3-O-β-xylopyranosyl-(1→3)-[β-galactopyranosyl-(1→2)]-β-glucuronopyranside | R1 = -β-d-GlcUAp- | 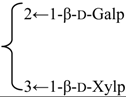 | S. cucubalus Wib | [159] | ||||||
| R2 = H | ||||||||||
| Quillaic acid 3-O-glucuronide | R1 = -β-d-GlcUAp R2 = H | S. vulgaris (Moench) Garcke | [161] | |||||||
| 3-O-β-d-Xylopyranosyl-(1→3)-β-d-galactopyranosyl-(1→2)-β-d-glucuronopyranosyl quillaic acid 28-O-β-l-rhamnopyranosyl-(1→2)-[4-methoxycinnamoyl-(3)]-4-O-acetyl-β-d-fucopyranoside(Silenoside) | R1 = -β-d-GlcUAp- | 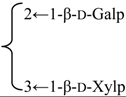 | S. szechuensis F.N. Williams | [165] | ||||||
| R2 = (4-O-Ac)-β-d-Fucp- |  | |||||||||
| 3-O-β-d-Galactopyranosyl(1→2)] [β-d-xylopyranosyl (1→3)]-6-O-butyl-β-d-glucuronopyranosyl quillaic acid 28-O-[α-l-rhamnopyranosyl(1→2)]-3-O-acetyl-4-O-[(E)-4-methoxycinnamoyl]-β-d- fucopyranosyl ester (Visciduloside A) | R1 = 6-O-Bu-β-d-GlcUAp- | 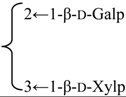 | S. viscidula Franch | [166] | ||||||
| R2 = 3-O-Ac-4-O-(E)-methoxycynnamoyl-β-d-Fucp-2←1-α-l-Rhap | ||||||||||
| 3-O-β-d-Galactopyranosyl(1→2)] [β-d-xylopyranosyl (1→3)]-6-O-butyl-β-d-glucuronopyranosyl quillaic acid 28-O-[α-l-rhamnopyranosyl(1→2)]-3-O-acetyl-4-O-[(Z)-4-methoxycinnamoyl]-β-d- fucopyranosyl ester (Visciduloside B) | R1 = 6-O-Bu-β-d-GlcUAp- | 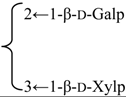 | S. viscidula Franch | [166] | ||||||
| R2 = 3-O-Ac-4-O-(Z)-4-methoxycynnamoyl-β-d-Fucp-2←1-α-l-Rhap | ||||||||||
| 3β,16α-Dihydroxyolean-12-en-23α,28β-dioic acid 28-O-{[α-d-mannopyranosyl-(1→4)][α-d-galactopyranosyl-(1→6)]-β-d-glycopyranosyl-(1→3)}[β-d-6-O-((3R)-3-hydroxy-3-methylglutaryl)glucopyranosyl-(1→6)-β-d-glucopyranoside (Silenoviscoside D) | R1 = H | S. viscidula Franch | [166] | |||||||
| R2 = -β-d-Glcp- | 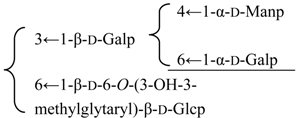 | |||||||||
| 3-O-[β-d-Galactopyranosyl(1→2)] [β-d-xylopyranosyl (1→3)]-[6-O-methyl-β-d-glucuronopyranosyl] quillaic acid 28-O-[α-l-rhamnopyranosyl(1→2)]-[3-O-acetyl-4-O-(E)-para-methoxycinnamoyl-β-d-fucopyranosyl]ester (Sinocrassuloside VIII) | R1 = 6-O-Me-β-d-GlcUAp- | 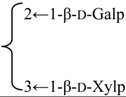 | S. viscidula Franch | [166] | ||||||
| R2 = 3-O-Ac-4-O-(E)-p-methoxycynnamoyl-β-d-Fucp-2←1-α-l-Rhap | ||||||||||
| 3-O-[β-d-Galactopyranosyl(1→2)] [β-d-xylopyranosyl (1→3)]-[6-O-methyl-β-d-glucuronopyranosyl] quillaic acid 28-O-[α-l-rhamnopyranosyl(1→2)]-[3-O-acetyl-4-O-(Z)-para-methoxycinnamoyl-β-d-fucopyranosyl]ester (Sinocrassuloside IX) | R1 = 6-O-Me-β-d-GlcUAp- | 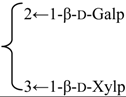 | S. viscidula Franch | [166] | ||||||
| R2 = 3-O-Ac-4-O-(Z)-p-methoxycynnamoyl-β-d-Fucp-2←1-α-l-Rhap | ||||||||||
| 3-O-{β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucuronopyrannosyl}-28-O-{α-l-rhamnopyranosyl-(l→2)-4-O-(E)-p-methoxycinnamoyl-β-d-fucopyranosyl}quillaic acid (Sinocrassuloside X) | R1 = β-d-GlcUAp- | 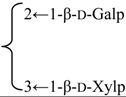 | S. rubicunda Franch | [158] | ||||||
| R2 = 4-O-(E)-p-methoxycynnamoyl-β-d-Fucp-2←1-α-l-Rhap | ||||||||||
| 3-O-β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucuronopyranosyl quillaic acid 28-O-β-d-xylopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→4)-[2′′-O-acetyl-β-d-quinovopyranosyl-(1→2)]-3′-O-acetyl-β-d-fucopyranoside (Rubicunoside A) | R1 = β-d-GlcUAp- | 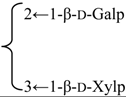 | S. rubicunda Franch | [167] | ||||||
| R2 = (3-O-Ac)-β-d-Fucp- | 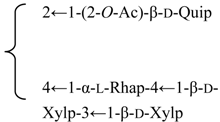 | |||||||||
| 3-O-[β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucuronopyranosyl quillaic acid 28-O-β-d-xylopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→4)-[β-d-glucopyranosyl-(1→4′)-β-d-quinovopyranosyl-(1→2)]-3′-O-acetyl-β-d-fucopyranoside (Rubicunoside B) | R1 = -β-d-GlcUAp- | 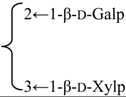 | S. rubicunda Franch | [167] | ||||||
| R2 = -(3-O-Ac)-β-d-Fucp- | 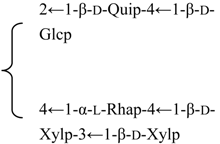 | |||||||||
| 3-O-β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucuronopyranosyl quillaic acid 28-O-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→4)-[4′′-O-acetyl-β-d-glucopyranosyl-(1→2)]-β-d-fucopyranoside (Rubicunoside C) | R1 = -β-d-GlcUAp- | 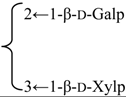 | S. rubicunda Franch | [167] | ||||||
| R2 = -β-d-Fucp- | 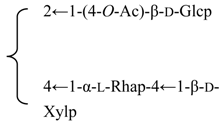 | |||||||||
| 3-O-β-d-Galactopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-[6′-O-n-butyl]-β-d-glucuronopyranosyl quillaic acid 28-O-β-d-xylopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→4)-[2′′-O-acetyl-β-d-quinovopyranosyl-(1→2)]-3′-O-acetyl-β-d-fucopyranoside (Rubicunoside D) | R1 = -(6-O-n-Bu)-β-d-GlcUAp- | 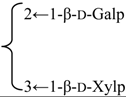 | S. rubicunda Franch | [167] | ||||||
| R2 =-(3-O-Ac)-β-d-Fucp- | 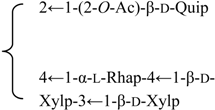 | |||||||||
| 3-O-{β-d-Galactopyranosyl(1→2) [β-d-xylopyranosyl (1→3)]-β-d-glucuronopyranosyl} quillaic acid 28-O-{β-d-xylopyranosyl- (1→3)- β-d-xylopyranosyl- (1→4)-α-l-rhamnopyranosyl(1→2)-[ β-d-quinovopyranosyl- (1→4)]-β-d-fucopyranosyl} ester | R1 = -β-d-GlcUAp- | 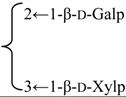 | S. rubicunda Franch | [168] | ||||||
| R2 = -β-d-Fucp- | 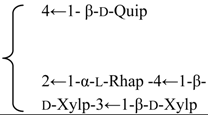 | |||||||||
| 3-O-[β-d-Galactopyranosyl(1→2)] [β-d-xylopyranosyl (1→3)]-β-d-glucuronopyranosyl quillaic acid 28-O-{[β-d-xylopyranosyl- (1→4)- α-l-rhamnopyranosyl(1→2)} [β-d-glycopyranosyl- (1→3)]-4-O-acetyl-β-d-fucopyranosyl} ester | R1 = -β-d-GlcUAp- | 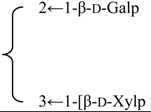 | S. rubicunda Franch | [168] | ||||||
| R2 = -4-O-acetyl- β-d-Fucp- |  | |||||||||
 | ||||||||||
| 23-O-[β-d-Glucuronopyranosyl-(1→2)-β-d-glycopyranosyl]-28-O-{β-d-glucopyranosyl-(1→3)-[α-d-galactopyranosyl-(1→6)-β-d-glycopyranosyl-(1→6)]-β-d-glucopyranosyl} gypsogenic acid (Silenorubicunoside D) | R1 = -β-d-Glcp-2←1-β-d-GlcUAp | S. rubicunda Franch | [158] | |||||||
| R2 = -β-d-Glcp- | 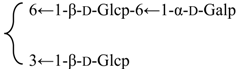 | |||||||||
| 3β-Hydroxy-16,23-dioxo-28-nor-17α-18β-olean-12-ene (Villosagenin I) | 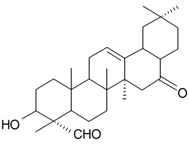 | S. villosa Forssk | [169] | |||||||
| 3β-Hydroxy-16,23-dioxo-28-norolean-17-ene (Villosagenin II) | 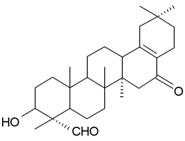 | S. villosa Forssk | [169] | |||||||
| Oleanolic acid | 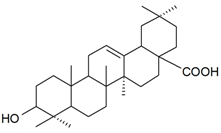 | S. succulenta Forssk | [170] | |||||||
| Sterols | ||||||||||
| Campesterol | 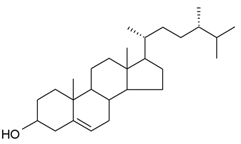 | S. brahuica Boiss | [171] | |||||||
| Cycloartenol | 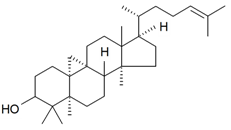 | S. cucubalus Wibel | [172] | |||||||
| 22-Dihydrospinasterol | 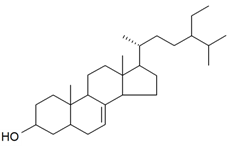 | S. cucubalus Wibel | [172] | |||||||
| Sitosterol | 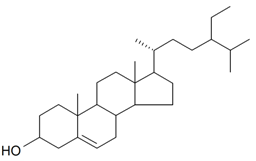 | S. brahuica Boiss, S. viridiflora L. | [31,171] | |||||||
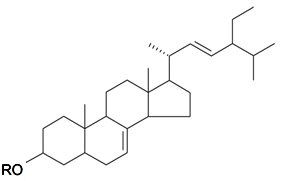 | ||||||||||
| α-Spinasterol | R = H | S. conoidea L., S. cucubalus Wibel | [9,172] | |||||||
| α-Spinasterolglucoside | R = O-β-d-Glu | S. conoidea L., S. jenisseensis Willd | [9,140] | |||||||
| Stigmasterol | 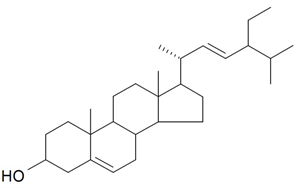 | S. brahuica Boiss, S. viridiflora L. | [31,171] | |||||||
| Phenolic Phytochemicals | ||||||||||
| Flavonoids | ||||||||||
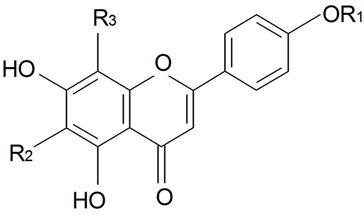 | ||||||||||
| Apigenin | R1 = R2 = R3 = H | S. saxatilis Sims | [173] | |||||||
| Apigenin-6,8-di-C-glucopyranoside (vicenin 2) | R1 = H R2 = R3 = β-d-Glcp | S. boissieri Panjut, S. chlorantha Willd, S. commutata Guss, S. cyri Schischk, S. foliosa Maxim, S. graminifolia Otth, S. jenissensis Willd, S. italic (L.) Pers, S. macrostyla, S. nutans L., S. saxatilis Sims, S. wolgensis (Hornem) Otth | [29,173] | |||||||
| Schaftoside (8-α-l-Arabinopyranosyl-6-β-d-glucopyranosylapigenin) | R1 = H R2 = β-d-Glcp R3 = α-l-Arap | S. schafta S.G.Gmel. ex Hohen | [174] | |||||||
| Vitexin (8-Glucosylapigenin) | R1 = R2 = H R3 = β-d-Glcp | S. alba (Miller) Krause, S. armeria L., S. boissieri Panjut, S. brachuica Boiss, S. bupleuroides L., S. chlorantha Willd, S. chlorifolia Smith, S. commutata Guss, S. compacta Fisch. ex Hornem, S. cretacea Fisch. ex Spreng, S. cubanensis, S. cyri Schischk, S. diclinis (Lag) M. Lainz, S. foliosa Maxim, S. graminifolia Otth, S. jenissensis Willd, S. italica (L.) Pers, S. macrostyla, S. multifida (Adams) Rohrb, S. nutans L., S. polaris (Kleopow) Holub, S. repens Patrin, S.saxatilis Sims, S. supina M. Bieb, S. turgida L., S. wolgensis (Hornem) Otth | [29,173,175,176] | |||||||
| Vitexin-2-O"-glucoside | R1 = R2 = H R3 = β-d-Glcp-2←1-β-d-Glcp | S. alba (Miller) Krause | [175] | |||||||
| Isovitexin-2-O"-glucoside | R1 = R3 = H R2 = β-d-Glcp-2←1-β-d-Glcp | S. alba (Miller) Krause | [175] | |||||||
| Isovitexin (saponaretin, homovitexin) | R1 = R3 = H R2 = β-d-Glcp | S. alba (Miller) Krause, S. armeria L., S. boissieri Panjut, S. brachuica Boiss, S. bupleuroides L., S. chlorantha Willd, S. chlorifolia Smith, S. commutata Guss, S. compacta Fisch. ex Hornem, S. cretacea Fisch. ex Spreng, S. cubanensis, S. cyri Schischk, S. diclinis (Lag) M.Lainz, S. dioica (L.) Clairv, S. foliosa Maxim, S. graminifolia Otth, S. jenissensis Willd, S. italica (L.) Pers, S. macrostyla, S. multifida (Adams) Rohrb, S. nutans L., S. polaris (Kleopow) Holub, S. repens Patrin, S. supina M. Bieb, S. turgida L., S. wolgensis (Hornem) Otth | [29,175,176,177] | |||||||
| Vitexin 4''-α-l-Rhamnopyranosyl | R1 = R2 = H R3 = β-d-Glcp-4←1-α-l-Rhap | S. conoidea L. | [17] | |||||||
| Isosaponarin (Isovitexin 4'-β-d-glucopyranoside) | R1 = R2 = β-d-Glcp R3 = H | S. armeria L., S. bupleuroides L., S. chlorifolia Smith, S. compacta Fisch. ex Hornem, S. cretacea Fisch. ex Spreng, S. cubanensis, S. polaris (Kleopow) Holub | [29] | |||||||
| Vicenin 1 | R1 = H R2 = β-d-Xylp R3 = β-d-Glcp | S. boissieri Panjut, S. chlorantha Willd, S. commutata Guss, S. cyri Schischk, S. foliosa Maxim, S. graminifolia Otth, S. jenissensis Willd, S. italica (L.) Pers, S. macrostyla, S. nutans L., S. wolgensis (Hornem) Otth | [29] | |||||||
| Vicenin 3 | R1 = H R2 = β-d-Glcp R3 = β-d-Xylp | S. boissieri Panjut, S. chlorantha Willd, S. commutata Guss, S. cyri Schischk, S. foliosa Maxim, S. graminifolia Otth, S. jenissensis Willd, S. italica (L.) Pers, S. macrostyla, S. nutans L., S. wolgensis (Hornem) Otth | [29] | |||||||
| Neovitexin | R1 = R2 = H R3 = α-l-Glcp | Silene sp. | [29] | |||||||
| Isoneovitexin | (tautomer of neovitexin) | Silene sp. | [29] | |||||||
| Vicenin their mono-, di-glucosides | S. boissieri Panjut, S.chlorantha Willd, S.commutata Guss, S.cyri Schischk, S.foliosa Maxim, S.graminifolia Otth, S.jenissensis Willd, S.italica (L.) Pers, S. macrostyla, S.nutans L., S.wolgensis (Hornem) Otth | [29] | ||||||||
| Vitexin their mono-, di-glucosides | S. brachuica Boiss, S. multifida (Adams) Rohrb, S. repens Patrin, S. supina M. Bieb, S. turgida L. | [29] | ||||||||
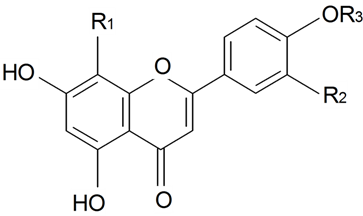 | ||||||||||
| 8(4''-O-α-l-rhamnopyranosyl)-C-β-d-glucopyranosyldiosmetin | R1 = β-d-Glcp-4←1-α-l-Rhap R2 = OH, R3 = Me | S. conoidea L. | [9] | |||||||
| 8(4''-O-α-l-rhamnopyranosyl)-C-β-d-glucopyranosylapigenin | R1 = β-d-Glcp-4←1-α-l-Rhap R2 = H, R3 = H | S. conoidea L. | [9] | |||||||
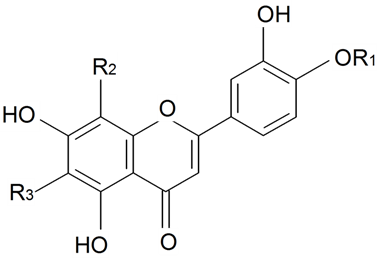 | ||||||||||
| Orientin | R1 = R3 = H R2 = β-d-Glcp | S. armeria L., S. boissieri Panjut, S. bupleuroides L., S. chlorantha Willd, S. chlorifolia Smith, S. commutata Guss, S. compacta Fisch. ex Hornem, S. cretacea Fisch. ex Spreng, S. cubanensis, S. cyri Schischk, S. foliosa Maxim, S. graminifolia Otth, S. jenissensis Willd, S. italica (L.) Pers, S. macrostyla, S. nutans L., S. polaris (Kleopow) Holub, S. saxatilis Sims, S. vulgaris (Moench) Garcke, S. wolgensis (Hornem) Otth | [29,173,176] | |||||||
| Homoorientin (isoorientin)(their 8a, 6a, 6b isomers) | R1 = R2 = H R3 = β-d-Glcp | S. armeria L., S. boissieri Panjut, S. bupleuroides L., S. chlorantha Willd, S. chlorifolia Smith, S. commutata Guss, S. compacta Fisch. ex Hornem, S. cretacea Fisch. ex Spreng, S. cubanensis, S. cyri Schischk, S. italic (L.) Pers, S. littorea Brot, S. foliosa Maxim, S. graminifolia Otth, S. jenissensis Willd, S. italica (L.) Pers, S. macrostyla, S. nutans L., S. polaris (Kleopow) Holub, S. saxatilis Sims, S. viscariopsis Bornm, S. vulgaris (Moench) Garcke, S. wolgensis (Hornem) Otth | [29,173,176,177] | |||||||
| Orientin- 4'-Me ether, 4''-α-l-rhamnopyranosyl | R1 = Me R2 = β-d-Glcp-4←1-α-l-Rha R3 = H | S. conoidea L. | [17] | |||||||
| Adonivernite | R1 = R3 = H R2 = β-d-Glcp-2←1-β-d-Xylp | S. armeria L., S. bupleuroides L., S. chlorifolia Smith, S. compacta Fisch. ex Hornem, S. cretacea Fisch. ex Spreng, S. cubanensis, S. polaris (Kleopow) Holub | [29] | |||||||
| Homoadonivernite | R1 = R2 = H R3 = β-d-Glcp-2←1-β-d-Xylp | S. armeria L., S. bupleuroides L., S. chlorifolia Smith, S. compacta Fisch. ex Hornem, S. cretacea Fisch. ex Spreng, S. cubanensis, S. polaris (Kleopow) Holub | [29] | |||||||
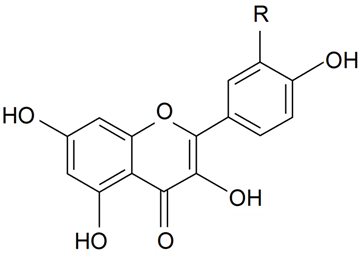 | ||||||||||
| Kaempferol | R = H | S. diclinis (Lag) M Lainz, S. littorea Brot | [176] | |||||||
| Quercetin | R = OH | S. littorea Brot | [176] | |||||||
| Anthocyanins | ||||||||||
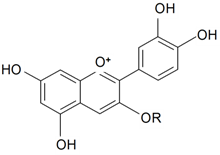 | ||||||||||
| Cyanidin-3-O-rhamnosyl glucoside | R = -β-d-Glcp-Rhap | S. armeria L. | [177] | |||||||
| Cyanidin-3-O-glucoside | R = -β-d-Glcp | S. armeria L. | [177] | |||||||
| Cyanidin-3-rhamnosyl(1→6)-glucoside-5-glucoside | R = -Glcp-5←1-Glcp-6←1-Rhap | S. dioica (L.) Clairv | [178] | |||||||
| Cyanidin-3-(4-caffeoylrhamnosyl(1→6)-glucoside)-5-glucoside | R = -Glcp-5←1-Glcp-6←1-Rhap-4←O-caffeoyl | S. dioica (L.) Clairv | [178] | |||||||
| Phenols, Phenolic Acids and Phenylpropanoids | ||||||||||
| Acetophenone |  | S. armeria L., S. otites (L.) Wibel | [179,180,181] | |||||||
| Benzaldehyde |  | S. alpestris Jacq, S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. maritima (Homem) With, S. nutans L., S. otites (L.) Wib, S. pendula L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,180,181,182,183,184,185] | |||||||
| Benzenacetaldehyde | 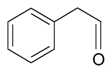 | S. chlorantha (Willd) Ehrh, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. otites (L.) Wib, S. sericea All, S. subconica Friv, S. succulenta Forssk, S. viscosa (L.) Pers | [183] | |||||||
| Benzene acetic acid |  | S. armeria L. | [179] | |||||||
| Benzenepropanal | 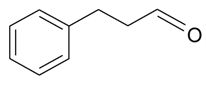 | S. latifolia Poiret | [30] | |||||||
| Benzenepropanol | 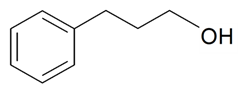 | S. latifolia Poiret, S. nutans L., S. vulgaris (Moench) Garcke | [30,185] | |||||||
| Benzenepropyl acetate |  | S. latifolia Poiret | [30] | |||||||
| Benzoin acid | 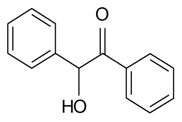 | S. armeria L. | [38,179] | |||||||
| Benzyl acetate | 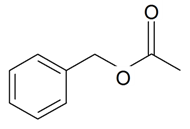 | S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib, S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,180,183,184,185] | |||||||
| Benzyl alcohol |  | S. armeria L., S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. italica (L.) Pers, S. latifolia Poiret, S. maritima (Homem) With, S. nutans L., S. otites (L.) Wib, S. sericea All, S. subconica Friv, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,179,180,181,183,184,185] | |||||||
| Benzyl benzoate |  | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. flos-jovis (L.) Greut and Burd, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill.) Greut. and Burd, S. maritima (Homem) With, S. nutans L., S. otites (L.) Wib, S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,183,184,185] | |||||||
| Benzyl isobutanoate |  | S. latifolia Poiret | [30] | |||||||
| Benzyl 3-methylbutanoate | 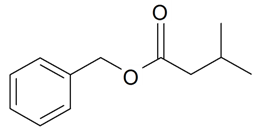 | S. flos-jovis (L.) Greut and Burd, S. viscaria (L.) Jessen | [184] | |||||||
| n-Butyl benzoate |  | S. flos-jovis (L.) Greut and Burd | [184] | |||||||
| (E)-Cinnamaldehyde | 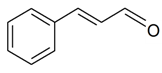 | S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L. | [30,183,185] | |||||||
| (E)-Cinnamic acetate |  | S. latifolia ssp. alba (Mill) Greut and Burd, S. nutans L. | [30,185] | |||||||
| (E)-Cinnamyl alcohol | 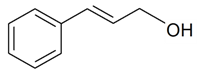 | S. latifolia ssp. alba (Mill) Greut and Burd, S. nutans L. | [30,185] | |||||||
| Coumaran |  | S. armeria L. | [38] | |||||||
| Coumarin |  | S. armeria L. | [179] | |||||||
| p-Cresol | 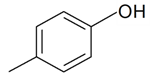 | S. dichotoma Ehrh. ssp. racemosa Chowdh (Otth) Graeb | [183] | |||||||
| p-Cymene | 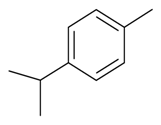 | S. gallica L. | [184] | |||||||
| 1,2-Dimethoxybenzene | 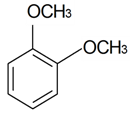 | S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. subconica Friv, S. succulenta Forssk, S. viscaria (L.) Jessen, S. viscosa (L.) Pers | [30,183,184] | |||||||
| 1,4-Dimethoxybenzene | 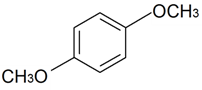 | S. rupestris L. | [184] | |||||||
| 1,4-Diethylbenzene | 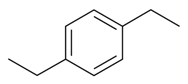 | S. flos-jovis (L.) Greut and Burd, S. gallica L., S. pendula L. | [184] | |||||||
| 1,2-Dimethylbenzene | 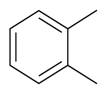 | S. alpestris Jacq, S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. pendula L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184] | |||||||
| Dimethyl salicylate | 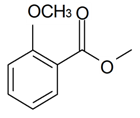 | S. alpestris Jacq, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. latifolia Poiret | [30,184] | |||||||
| Ethenyl benzene | 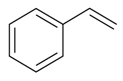 | S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib., S. pendula L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184] | |||||||
| Ethyltoluene | 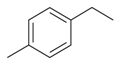 | S. dioica (L.) Clairv | [184] | |||||||
| Eugenol | 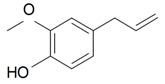 | S. armeria L. | [38,179] | |||||||
| (Z)-3-Hexenyl benzoate |  | S. nutans L., S. rupestris L. | [184,185] | |||||||
| (E)-Isoeugenol | 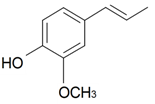 | S. latifolia Poiret | [30] | |||||||
| 2-Methoxyphenol | 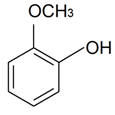 | S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. sericea All, S. subconica Friv, S. succulenta Forssk, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,183] | |||||||
| 4-Methoxyphenol |  | S. flos-jovis (L.) Greut and Burd | [184] | |||||||
| 2-Methyl benzaldehyde |  | S. latifolia Poiret | [30] | |||||||
| Methyl benzoate | 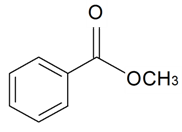 | S. alpestris Jacq, S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib, S. pendula L., S. rupestris L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,183,184] | |||||||
| Methyl eugenol | 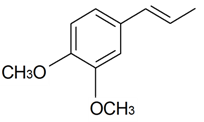 | S. latifolia Poiret | [30] | |||||||
| Methyl 2-methoxybenzoate | 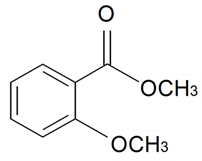 | S. maritima (Homem) With | [185] | |||||||
| Methyl salicylate | 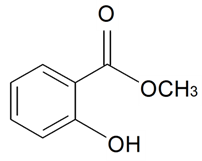 | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. maritima (Homem) With, S. nutans L., S. otites (L.) Wibel, S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscosa (L.) Pers | [30,180,181,183,184,185] | |||||||
| Phenol |  | S. armeria L. | [38] | |||||||
| Phenyl acetaldehyde | 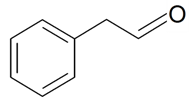 | S. armeria L., S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. latifolia Poiret, S. otites (L.) Wibel | [30,180,181,184] | |||||||
| Phenyl acetate | 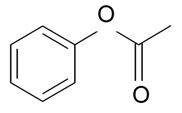 | S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. pendula L., S. viscaria (L.) Jessen | [183,184] | |||||||
| Phenyl benzoate | 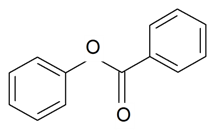 | S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. viscaria (L.) Jessen | [184] | |||||||
| 2-Phenylethanol |  | S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. maritima (Homem) With, S. otites (L.) Wibel, S. saxifraga L., S. subconica Friv, S. vulgaris (Moench) Garcke | [30,180,181,183,185] | |||||||
| 2-Phenylethyl acetate |  | S. viscaria (L.) Jessen | [184] | |||||||
| 3-Phenylpropanal | 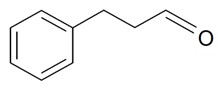 | S. armeria L. | [179] | |||||||
| 3-Phenylpropyl acetate |  | S. nutans L. | [185] | |||||||
| Propylbenzene | 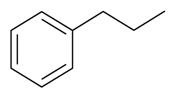 | S. alpestris Jacq, S. armeria L., S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. pendula L., S. viscaria (L.) Jessen | [184] | |||||||
| 1,2,3-Trimethylbenzene | 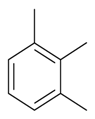 | S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. pendula L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184] | |||||||
| Terpenoids | ||||||||||
| Camphene | 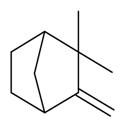 | S. alpestris Jacq, S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. gallica L. | [184] | |||||||
| Camphor |  | S. alpestris Jacq, S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. gallica L., S. pendula L. | [183,184] | |||||||
| δ-3-Carene | 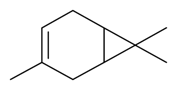 | S. otites (L.) Wibel | [180] | |||||||
| (Z)-Carveole | 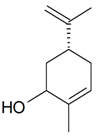 | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb | [183] | |||||||
| Carvone |  | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. vulgaris (Moench) Garcke ssp. vulgaris | [183] | |||||||
| 1,8-Cineole |  | S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. sericea All, S. subconica Friv, S. vallesia L., S. vulgaris (Moench) Garcke ssp. vulgaris | [183] | |||||||
| 5-Ethenyldihydro-5-methyl-2(3H)-furanone |  | S. maritima (Homem) With | [185] | |||||||
| Eucalyptol |  | S. alpestris Jacq, S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. gallica L., S. pendula L., S. viscaria (L.) Jessen | [184] | |||||||
| Fenchyl acetate | 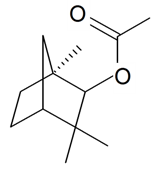 | S. chlorantha (Willd) Ehrh | [183] | |||||||
| Geranyl acetone | 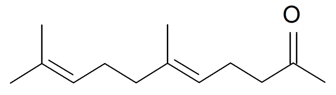 | S. nutans L. | [185] | |||||||
| 1-Hydroxylinalool | 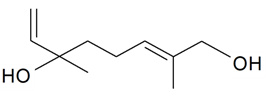 | S. otites (L.) Wibel | [181] | |||||||
| Hotrienol | 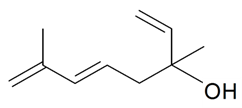 | S. otites (L.) Wibel | [181] | |||||||
| Lilac acetate A, C |  | S. maritima Withering, S. vulgaris (Moench) Garcke | [185] | |||||||
| Lilac alcohol (2R, 2'S, 5'S) | 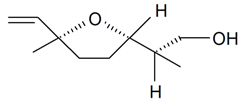 | S. alba L., S. latifolia Poiret, S. otites (L.) Wibel, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac alcohol (2S, 2'S, 5'S) | 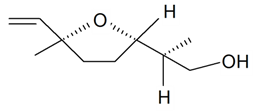 | S. alba L., S. latifolia Poiret, S. otites (L.) Wibel, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac alcohol (2S, 2'R, 5'R) | 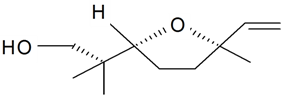 | S. latifolia Poiret, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac alcohol (2R, 2'R, 5'R) | 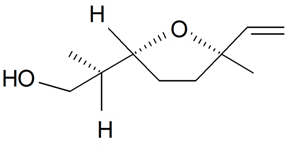 | S. latifolia Poiret | [30,181] | |||||||
| Lilac alcohol (2R, 2'S, 5'R) | 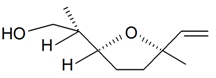 | S. latifolia Poiret, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac alcohol (2S, 2'S, 5'R) |  | S. latifolia Poiret | [30,181] | |||||||
| Lilac alcohol (2R, 2'R, 5'S) |  | S. alba L., S. latifolia Poiret, S. otites (L.) Wibel | [30,181,186] | |||||||
| Lilac alcohol (2S, 2'R, 5'S) | 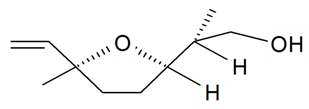 | S. alba L., S. latifolia Poiret, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac alcohol A, B, C, D |  | S. maritima (Homem) With, S. vulgaris (Moench) Garcke | [185] | |||||||
| Lilac alcohol formate |  | S. latifolia Poiret | [30] | |||||||
| Lilac aldehyde A | S. flos-cuculi (L.) Greut and Burd, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. maritima (Homem) With, S. otites (L.) Wib, S. subconica Friv, S. vallesia L., S. viscaria (L.) Jessen, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184,185] | ||||||||
| Lilac aldehyde B | S. flos-cuculi (L.) Greut and Burd, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. maritima (Homem) With, S. nutans L., S. otites (L.) Wib, S. subconica Friv, S. vallesia L., S. viscaria (L.) Jessen, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184,185] | ||||||||
| Lilac aldehyde C | S. maritima (Homem) With, S. vulgaris (Moench) Garcke | [185] | ||||||||
| Lilac aldehyde D | S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. maritima (Homem) With, S. otites (L.) Wib, S. sericea All, S. subconica Friv, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,185] | ||||||||
| Lilac aldehyde (2S, 2'S, 5'S) | 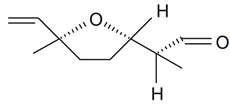 | S. alba L., S. latifolia Poiret, S. otites (L.) Wibel, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac aldehyde (2R, 2'S, 5'S) | 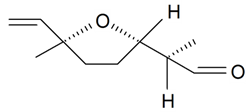 | S. alba L., S. latifolia Poiret, S. otites (L.) Wibel, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac aldehyde (2R, 2'R, 5'R) | 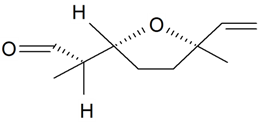 | S. latifolia Poiret, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac aldehyde (2S, 2'R, 5'R) | 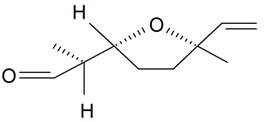 | S. latifolia Poiret, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac aldehyde (2S, 2'S, 5'R) |  | S. latifolia Poiret, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac aldehyde (2R, 2'S, 5'R) |  | S. latifolia Poiret, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac aldehyde (2S, 2'R, 5'S) |  | S. alba L., S. latifolia Poiret, S. otites (L.) Wibel, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| Lilac aldehyde (2R, 2'R, 5'S) |  | S. alba L., S. latifolia Poiret, S. otites (L.) Wibel, S. vulgaris (Moench) Garcke | [30,181,186] | |||||||
| D-Limonene | 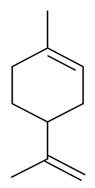 | S. alpestris Jacq, S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. maritima (Homem) With, S. nutans L., S. otites (L.) Wib, S. pendula L., S. rupestris L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [181,182,183,184,185] | |||||||
| Linalool | 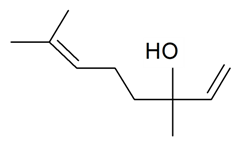 | S. armeria L., S. chlorantha (Willd) Ehrh, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. flos-cuculi (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib, S. rupestris L., S. sericea All, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers | [38,179,181,182,183,184,186] | |||||||
| (E)-Linalool oxide furanoid |  | S. maritima (Homem) With, S. otites (L.) Wibel | [181,185] | |||||||
| (Z)-Linalool oxide furanoid |  | S. chlorantha (Willd) Ehrh, S. italica (L.) Pers, S. otites (L.) Wibel, S. viscaria (L.) Jessen | [181,183,184] | |||||||
| (E)-Linalool oxide pyranoid |  | S. otites (L.) Wibel | [180,181] | |||||||
| (Z)-Linalool oxide pyranoid |  | S. otites (L.) Wibel | [180,181] | |||||||
| Linalyl acetate |  | S. flos-cuculi (L.) Greut and Burd | [182] | |||||||
| 6-Methyl-5-hepten-2-one | 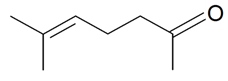 | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. vallesia L. | [183] | |||||||
| β-Myrcene | 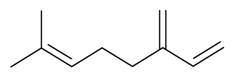 | S. armeria L., S. chlorantha (Willd) Ehrh, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. italica (L.) Pers, S. latifolia Poiret, S. nutans L., S. sericea All, S. vallesia L., S. viscosa (L.) Pers | [30,38,179,183] | |||||||
| Myrtenol |  | S. armeria L., S. otites (L.) Wibel | [38,179,180] | |||||||
| (E)-β-Ocimene | 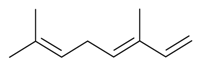 | S. alpestris Jacq, S. chlorantha (Willd) Ehrh, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib, S. pendula L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,181,183,184,185] | |||||||
| (Z)-β-Ocimene | 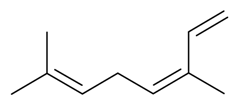 | S. nutans L., S. otites (L.) Wibel, S. vulgaris (Moench) Garcke ssp. vulgaris | [180,185] | |||||||
| (E)-Ocimene epoxide | 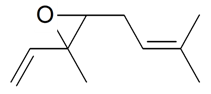 | S. nutans L. | [185] | |||||||
| (E)-Ocimenol | 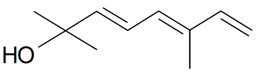 | S. nutans L. | [185] | |||||||
| (Z)-Ocimenol |  | S. nutans L. | [185] | |||||||
| α-Phellandrene | 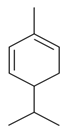 | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. subconica Friv, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,185] | |||||||
| α-Pinene | 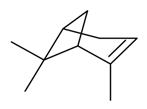 | S. alpestris Jacq, S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd., S. gallica L., S. italica (L.) Pers, S. latifolia Poiret, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wibel, S. pendula L., S. rupestris L., S. saxifraga L., S. sericea All, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [30,180,181,183,184] | |||||||
| β-Pinene | 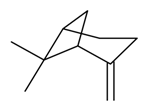 | S. alpestris Jacq, S. armeria L., S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. otites (L.) Wibel, S. pendula L., S. viscaria (L.) Jessen | [30,180,181,183,184] | |||||||
| γ-Terpinene | 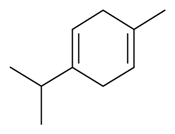 | S. coeli-rosa (L.) Godron, S. gallica L. | [184] | |||||||
| α-Terpineole |  | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. maritima (Homem) With, S. saxifraga L., S. vallesia L., S. vulgaris (Moench) Garcke | [183,185] | |||||||
| α-Terpinyl acetate | 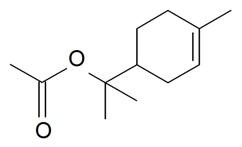 | S. flos-cuculi (L.) Greut and Burd | [182] | |||||||
| α-Thujene | 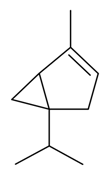 | S. coeli-rosa (L.) Godron, S. gallica L. | [184] | |||||||
| Thujone | 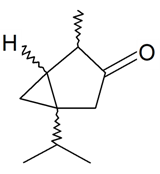 | S. alpestris Jacq | [184] | |||||||
| 2,2,6-Trimethyl-2-vinyl-5-ketotetrahydropyran |  | S. otites (L.) Wibel | [181] | |||||||
| Sesquiterpenes | ||||||||||
| α-(Z)-Bergamotene | 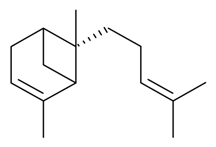 | S. latifolia Poiret | [30] | |||||||
| α-(E)-Bergamotene | 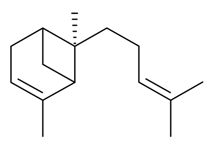 | S. latifolia Poiret | [30] | |||||||
| β-Bourbonene | 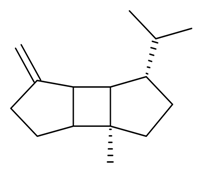 | S. flos-cuculi (L.) Greut and Burd, S. rupestris L., S. vallesia L. | [183,184] | |||||||
| δ-Cadinene | 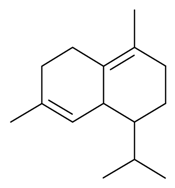 | S. latifolia Poiret, S. vallesia L. | [30,183] | |||||||
| γ-Cadinene | 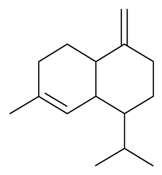 | S. vallesia L. | [183] | |||||||
| α-Caryophyllene | 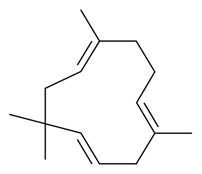 | S. vallesia L. | [183] | |||||||
| β-Caryophyllene | 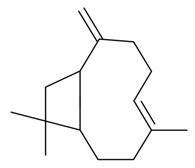 | S. chlorantha (Willd) Ehrh, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. gallica L., S. latifolia Poiret, S. otites (L.) Wibel, S. pendula L., S. rupestris L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L. | [30,181,183,184] | |||||||
| Caryophyllene oxide | 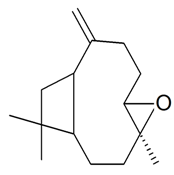 | S. armeria L. | [179] | |||||||
| β-Cedrene |  | S. vallesia L. | [183] | |||||||
| α-Copaene |  | S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. gallica L., S. pendula L., S. vallesia L. | [183,184] | |||||||
| Dendrolasin |  | S. latifolia Poiret | [30] | |||||||
| 7-epi-α-Selinene |  | S. latifolia Poiret | [30] | |||||||
| α-Farnesene |  | S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. italica (L.) Pers, S. latifolia Poiret, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. sericea All, S. succulenta Forssk, S. vallesia L. | [30,183,184,185] | |||||||
| β-Farnesene |  | S. gallica L. | [184] | |||||||
| Farnesol |  | S. armeria L. | [38,179] | |||||||
| Geranyl isovalerate |  | S. otites (L.) Wibel | [181] | |||||||
| Germacrene D |  | S. rupestris L. | [184] | |||||||
| α-Humelene |  | S. armeria L. | [38,179] | |||||||
| Longicyclene |  | S. latifolia Poiret | [30] | |||||||
| α-Longipinene |  | S. latifolia Poiret | [30] | |||||||
| 6-Methyl-5-hepten-2-one |  | S. dioica (L.) Clairv, S. nutans L., S. rupestris L. | [184,185] | |||||||
| α-Muurolene |  | S. vallesia L. | [183] | |||||||
| γ-Muurolene |  | S. italica (L.) Pers, S. sericea All, S. vallesia L., S. vulgaris (Moench) Garcke ssp. vulgaris | [183] | |||||||
| (E)-Nerolidol |  | S. latifolia Poiret | [30] | |||||||
| α-Selinene |  | S. latifolia Poiret | [30] | |||||||
| Other Volatiles | ||||||||||
| Acetamide |  | S. armeria L. | [38] | |||||||
| Acrylamide |  | S. armeria L. | [38] | |||||||
| Butanoic acid |  | S. armeria L. | [179] | |||||||
| 2-Butanone |  | S. armeria L. | [179] | |||||||
| α-Butene | | S. armeria L. | [38,179] | |||||||
| β-Butene | | S. armeria L. | [38,179] | |||||||
| (Z)-Jasmone |  | S. armeria L. | [38,179] | |||||||
| Cyclopentane |  | S. armeria L. | [38] | |||||||
| Cyclopentane oxide |  | S. armeria L. | [179] | |||||||
| 5,6-Dihydro-4H-cyclopenta-furan |  | S. armeria L. | [38] | |||||||
| Isovaleric acid |  | S. armeria L. | [38,179] | |||||||
| Methylamine | | S. armeria L. | [38] | |||||||
| Methylcyclopropane |  | S. armeria L. | [179] | |||||||
| Naphthalene |  | S. armeria L., S. alpestris Jacq, S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. viscaria (L.) Jessen | [179,184] | |||||||
| 1-Nitroso-pyrrlidine |  | S. armeria L. | [38] | |||||||
| O-Nitrostyrene |  | S. armeria L. | [38] | |||||||
| 1,8-Nonadiene | | S. armeria L. | [38,179] | |||||||
| Pentylfuran |  | S. armeria L. | [179] | |||||||
| Propanoic acid |  | S. armeria L. | [38,179] | |||||||
| Pyrrolidine |  | S. armeria L. | [179] | |||||||
| Tetrazole |  | S. armeria L. | [38,179] | |||||||
| Tropilidin |  | S. armeria L. | [38] | |||||||
| Free Fatty Acids and Their Derivatives (*-traces) | ||||||||||
 | ||||||||||
| Caprylic (8:0) | n = 4 | S. cserei Baumg subsp. aeoniopsis*, S. vulgaris (Moench) Garcke | [36] | |||||||
| Capric (10:0) | n = 6 | S. brahuica Boiss, S. guntensis B Fedtsch, S. viridiflora L., S. wallichiana Klotsch | [40,41,171] | |||||||
| Lauric (12:0) | n = 8 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis*, S. guntensis B Fedtsch., S. viridiflora L., S. vulgaris (Moench) Garcke*, S. wallichiana Klotsch | [40,41,171] | |||||||
| Myristic (14:0) | n = 10 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [36,40,41,171,187] | |||||||
| Pentadecylic (15:0) | n = 11 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [36,40,41,187] | |||||||
| Palmitic (16:0) | n = 12 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [16,36,40,41,42,171,187] | |||||||
| Margaric (17:0) | n = 13 | S. guntensis B Fedtsch, S. vulgaris subsp. Macrocarpa | [41,187] | |||||||
| Stearic (18:0) | n = 14 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [16,36,40,41,42,171,187] | |||||||
| Arachidic (20:0) | n = 16 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch., S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [36,40,41,42,187] | |||||||
| Heneicosylic (21:0) | n = 17 | S. brahuica Boiss, S. guntensis B Fedtsch., S. viridiflora L., S. wallichiana Klotsch | [40,41] | |||||||
| Behenic (22:0) | n = 18 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. wallichiana Klotsch | [36,40,41] | |||||||
| Tricosylic (23:0) | n = 19 | S. brahuica Boiss, S. guntensis B Fedtsch, S. vulgaris subsp. Macrocarpa, S. viridiflora L., S. wallichiana Klotsch | [40,41,187] | |||||||
| Lignoceric (24:0) | n = 20 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis*, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke*, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [36,40,41,42,187] | |||||||
| Pentacosylic (25:0) | n = 21 | S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. vulgaris (Moench) Garcke* | [36,41] | |||||||
| Cerotic (26:0) | n = 22 | S. guntensis B Fedtsch, S. vulgaris (Moench) Garcke | [41,42] | |||||||
 | ||||||||||
| Palmitoleic (16:1) | n = 2 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch., S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [36,40,41,42,171,187] | |||||||
| Oleic (18:1) | n = 4 | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [16,36,40,41,171,187] | |||||||
| Sapienic (16:1) |  | S. cserei Baumg subsp. aeoniopsis, S. vulgaris (Moench) Garcke* | [36] | |||||||
| Heptadecenoic (17:1) |  | S. vulgaris subsp. Macrocarpa | [187] | |||||||
| Erucic (22:1) |  | S. vulgaris (Moench) Garcke | [16] | |||||||
| Eicosenoic (20:1) |  | S. cserei Baumg subsp. aeoniopsis*, S. vulgaris (Moench) Garcke | [36] | |||||||
| Elaidic (18:1) |  | S. cserei Baumg subsp. aeoniopsis*, S. vulgaris (Moench) Garcke* | [36] | |||||||
| Linoleic (18:2) |  | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [16,36,40,41,42,44,171,187] | |||||||
| Linolenic (18:3) |  | S. brahuica Boiss, S. cserei Baumg subsp. aeoniopsis, S. guntensis B Fedtsch, S. viridiflora L., S. vulgaris (Moench) Garcke, S. vulgaris subsp. Macrocarpa, S. wallichiana Klotsch | [16,36,40,41,44,171,187] | |||||||
| 3,6-Octadecadiynoic | 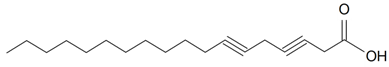 | S. armeria L. | [38] | |||||||
| Parinaric (18:4) | 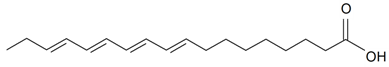 | S. vulgaris subsp. Macrocarpa | [187] | |||||||
 | ||||||||||
| n-Decanal | n = 6 | S. alpestris Jacq, S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib, S. pendula L., S. rupestris L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184] | |||||||
| n-Heptanal | n = 3 | S. alpestris Jacq, S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184] | |||||||
| n-Hexanal | n = 2 | S. alpestris Jacq, S. armeria L., S. coeli-rosa (L.) Godron, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd., S. gallica L., S. pendula L., S. viscaria (L.) Jessen | [184] | |||||||
| n-Nonanal | n = 5 | S. alpestris Jacq, S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd., S. nutans L., S. otites (L.) Wib, S. pendula L., S. rupestris L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. vulgaris | [183,184] | |||||||
| n-Octanal | n = 4 | S. alpestris Jacq, S. armeria L., S. chlorantha (Willd) Ehrh, S. coeli-rosa (L.) Godron, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. flos-jovis (L.) Greut and Burd, S. gallica L., S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib, S. pendula L., S. rupestris L., S. saxifraga L., S. sericea All, S. subconica Friv, S. succulenta Forssk, S. vallesia L., S. viscaria (L.) Jessen, S. viscosa (L.) Pers, S. vulgaris (Moench) Garcke ssp. Vulgaris | [183,184] | |||||||
 | ||||||||||
| Heptadecane | n = 15 | S. flos-cuculi (L.) Greut and Burd | [182] | |||||||
| n-Octane | n = 6 | S. alpestris Jacq, S. dioica (L.) Clairv, S. viscaria (L.) Jessen | [184] | |||||||
| Pentadecane | n = 13 | S. flos-cuculi (L.) Greut and Burd | [182] | |||||||
| (E)-4,8-Dimethyl 1,3,7 nonatriene | 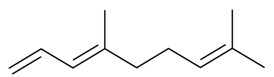 | S. otites (L.) Wibel | [181] | |||||||
| 2-Heptanone |  | S. flos-cuculi (L.) Greut and Burd, S. viscaria (L.) Jessen | [184] | |||||||
| n-Heptyl acetate |  | S. alpestris Jacq, S. viscaria (L.) Jessen | [184] | |||||||
| Hexanol |  | S. otites (L.) Wibel | [181] | |||||||
| 2-Hexenol acetate |  | S. latifolia Poiret | [30] | |||||||
| (E)-3-Hexen-1-ol | | S. otites (L.) Wibel | [181] | |||||||
| (Z)-3-Hexen-1-ol |  | S. armeria L., S. latifolia Poiret, S. nutans L., S. otites (L.) Wibel, S. rupestris L., S. sericea All, S. vallesia L., S. vulgaris (Moench) Garcke | [30,181,183,184,185] | |||||||
| (Z)-3-Hexen-1-ol acetate |  | S. alpestris Jacq, S. armeria L., S. coeli-rosa (L.) Godron, S. flos-cuculi (L.) Greut and Burd, S. gallica L., S. pendula L., S. viscaria (L.) Jessen | [184] | |||||||
| (E)-2-Hexenyl acetate |  | S. otites (L.) Wibel | [181] | |||||||
| (Z)-3-Hexenyl acetate |  | S. chlorantha (Willd) Ehrh, S. dichotoma Ehrh ssp. racemosa Chowdh (Otth) Graeb, S. flos-cuculi (L.) Greut and Burd, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. otites (L.) Wib, S. saxifraga L., S. sericea All, S. subconica Friv, S. vallesia L., S. viscosa (L.) Pers | [30,181,182,183] | |||||||
| (Z)-3-Hexenyl butyrate |  | S. otites (L.) Wibel | [181] | |||||||
| n-Hexyl acetate |  | S. alpestris Jacq, S. armeria L., S. coeli-rosa (L.) Godron, S. otites (L.) Wibel, S. rupestris L., S. viscaria (L.) Jessen | [181,184] | |||||||
| 3-Methylbutyl acetate |  | S. nutans L. | [185] | |||||||
| 4-Oxoisophorone |  | S. latifolia Poiret | [30] | |||||||
| n-Pentylacetate |  | S. viscaria (L.) Jessen | [184] | |||||||
| 1,14-Tetradecanediol |  n = 12 n = 12 | S. flos-cuculi (L.) Greut and Burd | [182] | |||||||
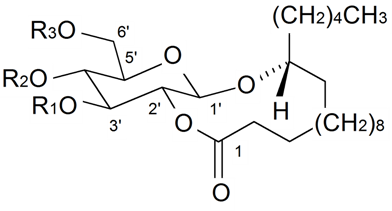 | ||||||||||
| Gallicaside A | R1 = R2 = Ac, R3 = H | S. gallica L. | [188] | |||||||
| Gallicaside C | R1 = R3 = Ac, R2 = H | S. gallica L. | [188] | |||||||
| Gallicaside F | R1 = R2 = H, R3 = Ac | S. gallica L. | [188] | |||||||
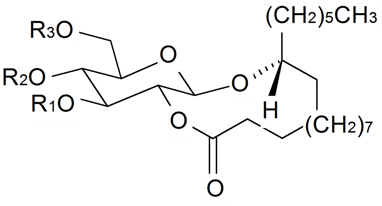 | ||||||||||
| Gallicaside B | R1 = R2 = Ac, R3 = H | S. gallica L. | [188] | |||||||
| Gallicaside D | R1 = R3 = Ac, R2 = H | S. gallica L. | [188] | |||||||
| Gallicaside E | R1 = Ac, R2 = R3 = H | S. gallica L. | [188] | |||||||
| Gallicaside G | R1 = R2 = H, R3 = Ac | S. gallica L. | [188] | |||||||
| Gallicaside H | R1 = R2 = R3 = H | S. gallica L. | [188] | |||||||
| Gallicaside I | 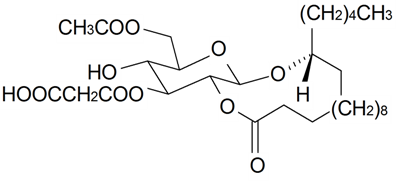 | S. gallica L. | [188] | |||||||
| Gallicaside J | 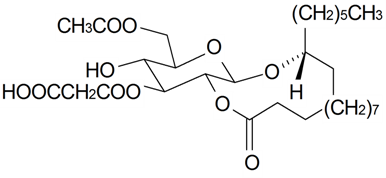 | S. gallica L. | [188] | |||||||
| Various Structures | ||||||||||
| Vitamin C (ascorbic acid) |  | S. vulgaris subsp. macrocarpa | [189] | |||||||
| Vitamin K1 (pylloquinone) |  n = 3 n = 3 | S. vulgaris subsp. macrocarpa | [189] | |||||||
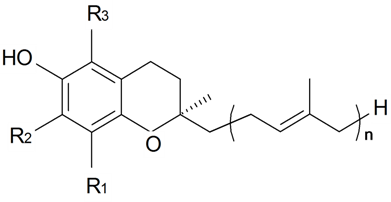 | ||||||||||
| α-Tocopherol | R1 = R2 = R3 = CH3 n = 3 | S. viridiflora L., S. vulgaris subsp. macrocarpa, S. vulgaris (Moench) Garcke | [31,189,190] | |||||||
| β-Tocopherol | R1 = R3 = CH3 R2 = H n = 3 | S. vulgaris (Moench) Garcke | [190] | |||||||
| γ-Tocopherol | R1 = R2 = CH3 R3 = H n = 3 | S. vulgaris (Moench) Garcke | [190] | |||||||
| δ-Tocopherol | R1 = R2 = R3 = H n = 3 | S. vulgaris subsp. macrocarpa, S. vulgaris (Moench) Garcke | [189,190] | |||||||
| n-Acetyl-4(H)-pyridine |  | S. alpestris Jacq, S. armeria L., S. dioica (L.) Clairv, S. flos-cuculi (L.) Greut and Burd, S. viscaria (L.) Jessen | [184] | |||||||
| Benzonitrile |  | S. armeria L., S. dioica (L.) Clairv | [184] | |||||||
| 2-Methylbutyraldoxime |  | S. chlorantha (Willd) Ehrh, S. italica (L.) Pers, S. latifolia Poiret ssp. alba (Mill) Greut and Burd, S. nutans L., S. vallesia L., S. vulgaris (Moench) Garcke ssp. vulgaris | [183,185] | |||||||
| 3-Methylbutyraldoxime |  | S. chlorantha (Willd) Ehrh, S. italica (L.) Pers, S. latifolia Poiret, S. nutans L., S. otites L., S. vallesia L. | [30,180,181,183,185] | |||||||
| Indole |  | S. latifolia Poiret, S. nutans L. | [30,185] | |||||||
| Silenin A(Cyclo-(Pro-Leu-Ser-Phe-Pro-Tyr-Leu-Val)) |  | S. szechuensis Williams | [19] | |||||||
| Silenin B(Cyclo-(Pro-Leu-Ser-Phe-Pro-Tyr-Leu-Val)) |  | S. szechuensis Williams | [19] | |||||||
| Silenin C (Cyclo-(Pro-Leu-Ser-Phe-Pro-Tyr-Leu-Val)) |  | S. szechuensis Williams | [19] | |||||||
| Silenan | A (1→4)-α-d-galacturonan | S. vulgaris (Moench) Garcke | [191] | |||||||
| 2-[6'-(O-trans-cinnamoyl)-β-d-glucopyranosyloxy)]-3-methyl-4H-pyran-4-one |  | S.vulgaris (Moench) Garcke | [192] | |||||||
| Conoidene (2,2'-(1,3-butadiene-1,4-diyl) bis[3-methoxy-5-(2-propen-1-yl) furan) |  | S. conoidea L. | [9] | |||||||
| Lutein |  | S. vulgaris subsp. macrocarpa | [189] | |||||||
| β-Carotene |  | S. vulgaris subsp. macrocarpa | [189] | |||||||
Acknowledgements
We would particularly like to express our gratitude to Mohamed L. Ashour for his help in collecting references for this paper.
Author Contributions
Nilufar Mamadalieva collected the information and drafted the manuscript which was interpreted and edited by Michael Wink. Rene Lafont conducted the biological part of the manuscript. All authors read and finally approved the final review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mabberley, D. The Plant-Book; Cambridge University Press: Cambridge, New York, NY, USA, 2008; p. 1040. [Google Scholar]
- Greuter, W. Silene (Caryophyllaceae) in Greece: A subgeneric and sectional classification. Taxon 1995, 44, 543–581. [Google Scholar] [CrossRef]
- Oxelman, B.; Lide’n, M.; Rabeler, R.K.; Popp, M. A revised generic classification of the tribe Sileneae (Caryophyllaceae). Nord. J. Bot. 2001, 20, 515–518. [Google Scholar]
- Popp, M.; Oxelman, B. Evolution of an RNA polymerase gene family in Silene (Caryophyllaceae)-incomplete concerted evolution and topological congruence among paralogues. Syst. Biol. 2004, 53, 914–932. [Google Scholar] [CrossRef]
- Chowdhuri, P.K. Studies in the genus Silene. Notes Royal Bot. Gard. Edinburgh 1957, 22, 221–279. [Google Scholar]
- Bernasconi, G.; Antonovics, J.; Biere, A.; Charlesworth, D.; Delph, L.; Filatov, D.; Giraud, T.; Hood, M.; Marais, G.; McCauley, D. Silene as a model system in ecology and evolution. Heredity 2009, 103, 5–14. [Google Scholar] [CrossRef]
- Eggens, F.; Popp, M.; Nepokroeff, M.; Wagner, W.L.; Oxelman, B. The origin and number of introductions of the Hawaiian endemic Silene species (Caryophyllaceae). Am. J. Bot. 2007, 94, 210–218. [Google Scholar] [CrossRef]
- Erturk, O.; Kati, H.; Yayli, N.; Demirbag, Z. Antimicrobial properties of Silene multifida (Adams) Rohrb plant extract. Turk. J. Biol. 2006, 30, 17–21. [Google Scholar]
- Ahmad, V.; Ali, Z.; Ali, M.; Zahid, M. Chemical constituents of Silene conoidea. Fitoterapia 1998, 69, 406–408. [Google Scholar]
- Nasir, E.; Ali, S. Flora of Pakistan; Pakistan Agricultural Research Council: Pakistan, South Asia, 1986; pp. 1–187. [Google Scholar]
- Usher, G. A Dictionary of Plants Used by Man; Wiley-VCH Verlag GmbH: London, UK, 1975; p. 294. [Google Scholar]
- Uphof, J.C.Th. Dictionary of Economic Plants, 2nd edition; Verlag von J. Cramer: Lehre, Germany, 1968; p. 591. [Google Scholar]
- Lyndon, F.M.; Kinsey, A.C.; Rollins, R.C. Edible wild plants of Eastern North America; Courier Dover Publications: New York, NY, USA, 1958; p. 452. [Google Scholar]
- Hadjichambis, A.C.H.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; de Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- Alarcón, R.; Ortiz, L.-T.; García, P. Nutrient and fatty acid composition of wild edible bladder campion populations Silene vulgaris (Moench.) Garcke. Int. J. Food Sci. Technol. 2006, 41, 1239–1242. [Google Scholar]
- Ali, Z.; Ahmad, V.U.; Ali, M.S.; Iqbal, F.; Zahid, M.; Alam, N. Two new C-Glycosylflavones from Silene conoidea. Nat. Prod. Lett. 1999, 13, 121–129. [Google Scholar] [CrossRef]
- Krishnamurthi, S.; Chadha, Y. The Wealth of India; CSIR: New Delhi, India, 1972; pp. 177–183. [Google Scholar]
- Rongping, Z.; Cheng, Z.; Yineng, H.; Ninghua, T.; Jun, Z. Three new cyclopeptides from Silene szechuensis. Acta Bot. Yunnanica 1997, 304–310. [Google Scholar]
- Ballero, M.; Fresu, I. Le piante di uso officinale nella Barbagia di Seui (Sardegna Centrale). Fitoterapia 1993, 64, 141–150. [Google Scholar]
- Golovko, V.; Bushneva, O. Stabilizing effect of Silene pectin polysaccharide on electrical activity of the sinoatrial area in frog heart. Bull. Exp. Biol. Med. 2007, 143, 284–286. [Google Scholar] [CrossRef]
- Sobiecki, J. A review of plants used in divination in southern Africa and their psychoactive effects. South. Afr. Hum. 2008, 20, 333–351. [Google Scholar]
- Hirst, M. A river of metaphors: Interpreting the Xhosa diviner’s myth. Afr. Stud. 1997, 56, 217–250. [Google Scholar] [CrossRef]
- Hirst, M. Dreams and medicines: The perspective of Xhosa diviners and novices in the Eastern Cape, South Africa. Indo-Pac. J. Phenomenol. 2005, 5, 1–22. [Google Scholar]
- Wink, M. Annual Plant Reviews, Functions and Biotechnology of Plant Secondary Metabolites; Wiley-Blackwell: Chichester, UK, 2010; p. 424. [Google Scholar]
- Wink, M. Annual Plant Reviews, Biochemistry of Plant Secondary Metabolism; Wiley-Blackwell: Chichester, UK, 2011; p. 464. [Google Scholar]
- Mamadalieva, N.Z. Phytoecdysteroids from Silene plants: Distribution, diversity and biological (antitumour, antibacterial and antioxidant) activities. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2012, 11, 474–497. [Google Scholar]
- Gaidi, G.; Miyamoto, T.; Laurens, V.; Lacaille-Dubois, M.-A. New acylated triterpene saponins from Silene fortunei that modulate lymphocyte proliferation. J. Nat. Prod. 2002, 65, 1568–1572. [Google Scholar] [CrossRef]
- Darmograi, V. Flavonoids of plants of the genera Silene and Otites adans, family Caryophyllaceae. Chem. Nat. Compd. 1977, 13, 102–103. [Google Scholar]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef]
- Eshmirzaeva, N.E.; Khidyrova, N.K.; Khodzhaeva, M.; Mezhlumyan, L.G.; Shakhidoyatov, K.M. Chemical Composition of Silene viridiflora. Chem. Nat. Compd. 2005, 41, 451–453. [Google Scholar] [CrossRef]
- Arnetoli, M.; Montegrossi, G.; Buccianti, A.; Gonnelli, C. Determination of Organic Acids in Plants of Silene paradoxa L. by HPLC. J. Agric. Food Chem. 2008, 56, 789–795. [Google Scholar] [CrossRef]
- Tomczyk, M. Preliminary phytochemical investigation of Lychnis flos-cuculi herbs. J. Nat. Med. 2008, 62, 473–475. [Google Scholar]
- Báthori, M.; Lafont, R.; Girault, J.P.; Máthé, I. Structural diversity of ecdysteroids of Lychnis flos-cuculi. Acta Pharm. Hung. 2001, 71, 157–167. [Google Scholar]
- Mamadalieva, N.; Egamberdieva, D.; Lafont, R.; Girault, J. Phytoecdysteroids and antibacterial activity of the plant Coronaria flos-cuculi. Chem. Nat. Compd. 2008, 44, 404–406. [Google Scholar] [CrossRef]
- Kucukboyaci, N.; Ozcelik, B.; Adiguzel, N.; Goren, A. Fatty-acid compositions of Silene vulgaris and S. cserei subsp. aeoniopsis seeds and their antimicrobial activities. Chem. Nat. Compd. 2010, 46, 88–91. [Google Scholar] [CrossRef]
- Karamian, R.; Ghasemlou, F. Screening of total phenol and flavonoid content, antioxidant and antibacterial activities of the methanolic extracts of three Silene species from Iran. Int. J. Agric. Crop Sci. 2013, 5, 305–312. [Google Scholar]
- Bajpai, V.; Dung, N.; Kwon, O.; Kang, S. Analysis and the potential applications of essential oil and leaf extracts of Silene armeria L. to control food spoilage and food-borne pathogens. Eur. Food Res. Technol. 2008, 227, 1613–1620. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Egamberdieva, D.; Tiezzi, A. In vitro biological activities of the components from Silene wallichiana. Med. Aromat. Plant Sci. Biotechnol. 2013, 7, 1–6. [Google Scholar]
- Mamadalieva, N.Z.; Ul’chenko, N.T.; Yuldasheva, N.K.; Egamberdieva, D.R.; Zhanibekov, A.A.; Dzhukharova, M.K.; Glushenkova, A.I. Fatty-acid composition and antibacterial activity of CHCl3 extracts of three plants of the genus Silene. Chem. Nat. Compd. 2010, 46, 95–96. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Ul’chenko, N.T.; Yuldasheva, N.K.; Zhanibekov, A.A.; Egamberdieva, D.R.; Glushenkova, A.I. Neutral lipids and biological activity of the CHCl3 extract of the aerial part of Silene guntensis. Chem. Nat. Compd. 2010, 46, 621–622. [Google Scholar]
- Orhan, I.; Deliorman-Orhan, D.; Özcelik, B. Antiviral activity and cytotoxicity of the lipophilic extracts of various edible plants and their fatty acids. Food Chem. 2009, 115, 701–705. [Google Scholar] [CrossRef]
- Özcelik, B.; Aslan, M.; Orhan, I.; Karaoglu, T. Antibacterial, antifungal and antiviral activities of the lipophilic extracts of Pistacia vera. Microbiol. Res. 2005, 160, 159–164. [Google Scholar] [CrossRef]
- Conforti, F.; Marrelli, M.; Carmela, C.; Menichini, F.; Valentina, P.; Uzunov, D.; Statti, G.A.; Duez, P.; Menichini, F. Bioactive phytonutrients (omega fatty acids, tocopherols, polyphenols), in vitro inhibition of nitric oxide production and free radical scavenging activity of non-cultivated Mediterranean vegetables. Food Chem. 2011, 129, 1413–1419. [Google Scholar] [CrossRef]
- Taskin, T.; Bitis, L. Antioxidant activity of Silene alba subsp. divaricata and Stellaria media subsp. media from Caryophyllaceae. Spatula DD 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; El-Readi, M.Z.; Janibekov, A.A.; Tahrani, A.; Wink, M. Phytoecdysteroids of Silene guntensis and their in vitro cytotoxic and antioxidant activity. Z. Naturforschung C 2011, 66, 215–224. [Google Scholar] [CrossRef]
- Popov, S.V.; Popova, G.Y.; Ovodova, R.G.; Bushneva, O.A.; Ovodov, Y.S. Effects of polysaccharides from Silene vulgaris on phagocytes. Int. J. Immunopharmacol. 1999, 21, 617–624. [Google Scholar]
- Behzad, S.; Piranib, A.; Mosaddegh, M. Cytotoxic activity of some medicinal plants from Hamedan district of Iran. Iran. J. Pharm. Res. 2014, 13, 199–205. [Google Scholar]
- Gaidi, G.; Correia, M.; Chauffert, B.; Beltramo, J.-L.; Wagner, H.; Lacaille-Dubois, M.-A. Saponins -mediated potentiation of Cisplatin accumulation and cytotoxicity in human colon cancer cells. Planta Med. 2002, 68, 70–72. [Google Scholar] [CrossRef]
- Zibareva, L.; Eremina, V.; Ivanova, N.; Lazkov, G. Distribution of phytoecdysteriods in the tribe Sileneae Dumort. Fam. Caryophyllaaceae. Rastit. Resur. 2003, 39, 45–53. [Google Scholar]
- Burdette, W. Invertebrate Hormones and Tumors; Springer: Berlin, Germany, 1974; pp. 351–367. [Google Scholar]
- El-Mofty, M.; Sadek, I.; Soliman, A.; Mohamed, A.; Sakre, S. α-Ecdysone, a new bracken fern factor responsible for neoplasm induction in the Egyptian toad (Bufo regularis). Nutr. Cancer 1987, 9, 103–107. [Google Scholar] [CrossRef]
- El-Mofty, M.; Sakre, S.; Rizk, A.; Moussa, E. Induction of breast and lung neoplastic lesions in mice by alpha-ecdysone. Oncol. Rep. 1994, 1, 435–438. [Google Scholar]
- Takasaki, M.; Tokuda, H.; Nishino, H.; Konoshima, T. Cancer chemopreventive agents (antitumour promoters) from Ajuga decumbens. J. Nat. Prod. 1999, 62, 972–975. [Google Scholar] [CrossRef]
- Lagova, N.; Valueva, I. Effect of ecdysterone isolated from Rhaponticum carthamoides on the growth of experimental tumours. Eksp. Onkol. (Kiev.) 1981, 3, 69–71. [Google Scholar]
- Báthori, M.; Tóth, N.; Hunyadi, A.; Marki, A.; Zador, E. Phytoecdysteroids and anabolic-androgenic steroids-structure and effects on humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Christel, D.; Kapur, P.; Nguyen, B.; Jarry, H.; Wuttke, W. Beta-Ecdysone has bone protective but no estrogenic effects in ovariectomized rats. Phytomedicine 2010, 17, 884–899. [Google Scholar] [CrossRef]
- Shakhmurova, G.A.; Mamadalieva, N.Z.; Zhanibekov, A.A.; Khushbaktova, Z.A.; Syrov, V.N. Effect of total ecdysteroid preparation from Silene viridiflora on the immune state of experimental animals under normal and secondary immunodeficiency conditions. Pharm. Chem. J. 2012, 46, 222–224. [Google Scholar]
- Bushneva, O.A.; Ovodova, R.G.; Shashkov, A.S.; Chizhov, A.O.; Ovodov, Y.S. Structure of Silenan, a Pectic Polysaccharide from Campion Silene vulgaris (Moench) Garcke. Biochemistry (Mosc.) 2003, 68, 1360–1368. [Google Scholar] [CrossRef]
- Ghonime, M.; Eldomany, R.; Abdelaziz, A.; Soliman, H. Evaluation of immunomodulatory effect of three herbal plants growing in Egypt. Immunopharmacol. Immunotoxicol. 2011, 33, 141–145. [Google Scholar] [CrossRef]
- Shakhmurova, G.; Djahangirova, M.; Batyrbekov, A.; Syrov, V.N. Evaluation of adaptogen and immunotrop effect of sum ecdysteroids from Silene viridiflora. Doklady Akademii Nauk RUz 2004, 55–59. (In Russian) [Google Scholar]
- Syrov, V.; Dzakhangirova, M.; Khushbaktova, Z. Effect of sum of ecdysteroids from Silene brachuica for working capacity on animals in experiments. Pharm. J. 2005, 56–59. (In Russian) [Google Scholar]
- Dzakhangirova, M.A.; Syrov, V.N. Experimental evaluation of effect of stimulation of sum of ecdysteroids from Silene brachuica and S. viridiflora for erythropoiesis in laboratory animals. Pathology 2005, 2, 7–9. [Google Scholar]
- Dzakhangirova, M. The Pharmacologic Investigation of the Sum Ecdysteroid Preparations Obtained from Silene brahuica, Silene viridiflora and Ajuga turkestanica Plants as Actoprotector Means. Ph.D. Thesis, Tashkent Medical Academy, Tashkent, Uzbekistan, 2007. [Google Scholar]
- Ko, Y.-J.; Hsieh, W.-T.; Wu, Y.-W.; Lin, A.W.-C. Ameliorative effect of Silene aprica on liver injuries induced by carbon tetrachloride and acetaminophen. Am. J. Chin. Med. 2002, 30, 235–243. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Choi, T.-S.; Jin, D.-H.; Cho, M.-K. Composition Comprising the Extract from Melandryum firmum for Improvement of Liver Function and Treatment of Liver Diseases. U.S. Patent WO2009117828A1, 19 October 2009. [Google Scholar]
- Dinan, L.; Harmatha, J.; Volodin, V.; Lafont, R. Phytoecdysteroids: Diversity, Biosynthesis and Distribution. In Ecdysone: Structures and Functions; Smagghe, G., Ed.; Springer-Verlag: Dordrecht, The Netherlands, 2009; pp. 3–46. [Google Scholar]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef]
- Chermenskaya, T.D.; Stepanycheva, E.A.; Shchenikova, A.V.; Chakaeva, A.S. Insectoacaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Ind. Crops Prod. 2010, 32, 157–163. [Google Scholar] [CrossRef]
- Dzhukharova, M.K.; Tashkhodzhaev, B.; Saatov, Z.; Abdullaev, N.D. Phytoecdysteroids of plants of the genus Silene XIV. Brahuisterone from Silene brahuica. Chem. Nat. Compd. 1993, 29, 484–489. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Zibareva, L.; Lafont, R.; Dinan, L.; Saatov, Z. Phytoecdysteroids from the Silene genus. Chem. Nat. Compd. 2004, 40, 574–578. [Google Scholar] [CrossRef]
- Meng, Y.; Whiting, P.; Zibareva, L.; Bertho, G.; Girault, J.P.; Lafont, R.; Dinan, L. Identification and quantitative analysis of the phytoecdysteroids in Silene species (Caryophyllaceae) by high-performance liquid chromatography. Novel ecdysteroids from S. pseudotites. J. Chromatogr. A 2001, 935, 309–319. [Google Scholar] [CrossRef]
- Bückmann, D.; Starnecker, G.; Tomaschko, K.-H.; Wilhelm, E.; Lafont, R.; Girault, J.-P. Isolation and identification of major ecdysteroids from the pycnogonid Pycnogonum litorale (Ström) (Arthropoda, Pantopoda). J. Comp. Physiol. B 1986, 156, 759–765. [Google Scholar]
- Girault, J.-P.; Báthori, M.; Varga, E.; Szendrei, K.; Lafont, R. Isolation and identification of new ecdysteroids from the Caryophyllaceae. J. Nat. Prod. 1990, 53, 279–293. [Google Scholar] [CrossRef]
- Báthori, M. HPLC analysis of ecdysteroids of Silene otites (L) Wib. In Chromatography ‘84; Kalász, H., Ettre, L.S., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1986; pp. 297–306. [Google Scholar]
- Louden, D.; Handley, A.; Lafont, R.; Taylor, S.; Sinclair, I.; Lenz, E.; Orton, T.; Wilson, I.D. HPLC analysis of ecdysteroids in plant extracts using superheated deuterium oxide with multiple on-line spectroscopic analysis (UV, IR, 1H NMR, and MS). Anal. Chem. 2001, 74, 288–294. [Google Scholar]
- Mamadalieva, N.; Ramazanov, N.S.; Dinan, L.; Saatov, Z. Phytoecdysteroids of Plants of the Silene Genus. 2-Dehydroxyecdysterone-3-O-benzoate from Silene wallichiana. Chem. Nat. Compd. 2000, 36, 513–515. [Google Scholar] [CrossRef]
- Munkhzhargal, N.; Zibareva, L.; Lafont, R.; Pribytkova, L.; Pisareva, S. Investigation of ecdysteroid content and composition of Silene repens indigenous in Mongolia and introduced into western Siberia. Russ. J. Bioorg. Chem. 2010, 36, 923–928. [Google Scholar] [CrossRef]
- Novozhilova, E.; Rybin, V.; Boltenkov, E. New phytoecdysteroids containing species from family of Caryophyllaceae. In Proceedings of the III Russian Conference “New Advances on Chemistry and Chemical Engineering of Plant Materials”, 23–27 April 2007; Altay University Publisher: Barnaul, Russia; pp. 204–208.
- Ramazanov, N.; Maksimov, E.; Saatov, Z.; Abdullaev, N. Phytoecdysteroms of plants of the genus Silene. XVII. Tomentesterone from Silene tomentella. Chem. Nat. Compd. 1995, 31, 600–603. [Google Scholar]
- Revina, T.; Revushkina, A.; Rakitin, A. Ecdysteroid-containing species in flora of the Altai Mountains. Rastit. Resur. 1988, 34, 565–570. [Google Scholar]
- Saatov, Z.; Usmanov, B.; Abubakirov, N. Phytoecdysones of Silene praemixta. I. Silenosterone. Chem. Nat. Compd. 1979, 15, 700–703. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.B.; Melibaev, S.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Silene. IX. Ecdysterone 22-O-benzoate from Silene scabrifolia. Chem. Nat. Compd. 1986, 22, 71–73. [Google Scholar] [CrossRef]
- Saatov, Z.; Abdullaev, N.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. X. Sileneoside E: 2-deoxy-alpha-ecdysone 3-O-beta-d-glucopyranoside from Silene brahuica. Chem. Nat. Compd. 1986, 22, 297–300. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. XV. 2-Deoxy-α-ecdysone 22-O-benzoate from Silene wallichiana. Chem. Nat. Compd. 1987, 23, 708–711. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. Chem. Nat. Compd. 1993, 29, 551–557. [Google Scholar] [CrossRef]
- Sadikov, Z.; Saatov, Z.; Garcia, M.; Girault, J.P.; Lafont, R. Ecdysteroids from Silene claviformis. Chem. Nat. Compd. 2001, 37, 262–265. [Google Scholar] [CrossRef]
- Sviridova, T.; Zibareva, L.N.; Kritskaja, S. Biological and Chemical Specialities of Species of the Genus Silene L. Grown in the South of the Tomsk Region. In Proceedings of the International Conference “Specialities of Acclimitization of Perennial Plants Accumulating Biologically Active Substances”, Krasnodar, Russia, 25–27 September 1995.
- Zibareva, L. The chromatographic detection of ecdysteroids in plants. In Proceedings of the Advances and Applications of Chromatography in Industry, Bratislava, Slovakia, 30 June–4 July 1996.
- Zibareva, L. The prognosis of the presence of ecdysteroids among the species Silene L. and Chenopodium L. to their contents in the seeds. Rastit. Resur. 1997, 33, 89–92. [Google Scholar]
- Zibareva, L. Occurrence of phytoecdysteroids in Silene L. genus and dynamics of their contents. Rastit. Resur. 1999, 35, 79–87. [Google Scholar]
- Zibareva, L. Distribution and levels of phytoecdysteroids in plants of the genus Silene during development. Arch. Insect Biochem. Physiol. 2000, 43, 1–8. [Google Scholar]
- Zibareva, L.; Yeryomina, V.; Ivanova, N. New ecdysteroidiferous species of the genus Silene L. and the dynamics of ecdysteroids contents in them. Rastit. Resur. 1997, 33, 73–75. [Google Scholar]
- Zibareva, L.; Volodin, V.; Saatov, Z.; Savchenko, T.; Whiting, P.; Lafont, R.; Dinan, L. Distribution of phytoecdysteroids in the Caryophyllaceae. Phytochemistry 2003, 64, 499–517. [Google Scholar] [CrossRef]
- Zibareva, L.; Yeriomina, V.I.; Munkhjargal, N.; Girault, J.-P.; Dinan, L.; Lafont, R. The phytoecdysteroid profiles of 7 species of Silene (Caryophyllaceae). Arch. Insect Biochem. Physiol. 2009, 72, 234–248. [Google Scholar]
- Zibareva, L.; Yeryomina, V.I. Dynamics of the contents ecdysteroids in species of the genus Silene grown in the Siberian botanical garden. Rastit. Resur. 1996, 106–110. [Google Scholar]
- Zibareva, L.; Ebel, A.; Gashkova, L. Physiology-biochemical aspects of study of herbal plants. In Proceedings of the International Meeting, Novosibirsk, Russia, 24–31 August 1998; p. 28.
- Agzamova, M.; Isaev, I.; Mamathanov, A.; Isaev, M.; Ibragimov, T. Phytoecdysteroids from Silene praemixta. Adv. Biol. Chem. 2014, 4, 1–4. [Google Scholar]
- Saatov, Z.; Gorovich, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. XI. 2-Deoxy-α-ecdysone 3-acetate from Silene scabrifolia. Chem. Nat. Compd. 1986, 22, 409–411. [Google Scholar]
- Dzhukharova, M.K.; Saatov, Z.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Silene. XVIII. 2-Deoxyecdysterone 20,22-monoacetonide from Silene brahuica. Chem. Nat. Compd. 1991, 27, 207–209. [Google Scholar] [CrossRef]
- Báthori, M.; Lafont, R.; Girault, J.-P.; Mathe, I. Occurrence of phytoecdysteroids in Silene species. J. Toxicol. Toxin Rev. 1995, 14, 223. [Google Scholar]
- Báthori, M.; Girault, J.P.; Kalasz, H.; Mathé, I.; Dinan, L.N.; Lafont, R. Complex phytoecdysteroid cocktail of Silene otites (Caryophyllaceae). Arch. Insect Biochem. Physiol. 1999, 41, 1–8. [Google Scholar]
- Báthori, M.; Girault, J.P.; Mathé, I.; Lafont, R. Isolation of 5 alpha- and 5 beta-dihydrorubrosterone from Silene otites L. (Wib). Biomed. Chromatogr. 2000, 14, 464–467. [Google Scholar] [CrossRef]
- Báthori, M.; Pongracz, Z.; Tóth, G.; Simon, A.; Kandra, L.; Kele, Z.; Ohmacht, R. Isolation of a new member of the ecdysteroid glycoside family: 2-deoxy-20-hydroxyecdysone 22-O-beta-d-glucopyranoside. J. Chromatogr. Sci. 2002, 40, 409–415. [Google Scholar]
- Báthori, M.; Kalasz, H.; Pongracz, Z.; Mathé, I.; Kalman, A.; Argay, G. 5-Alpha- and 5-beta-2-deoxyintegristerone A, a 5-alpha and 5-beta isomer pair of ecdysteroids isolated from the Silene genus. Biomed. Chromatogr. 2002, 16, 373–378. [Google Scholar] [CrossRef]
- Lafont, R.; Horn, D. Phytoecdysteroids: Structures and Occurrence. In Ecdysone: From Chemistry to Mode of Action; Koolman, J., Ed.; Georg Thieme-Verlag: Stuttgart, Germany, 1989; pp. 39–64. [Google Scholar]
- Mamadalieva, N.; Zibareva, L.; Saatov, Z. Phytoecdysteroids of Silene linicola. Chem. Nat. Compd. 2002, 38, 268–271. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Zibareva, L.; Saatov, Z.; Lafont, R. Phytoecdysteroids of Silene viridiflora. Chem. Nat. Compd. 2003, 39, 199–203. [Google Scholar] [CrossRef]
- Pongrácz, Z.; Báthori, M.; Tóth, G.; Simon, A.; Mák, M.; Máthé, I. 9α, 20-Dihydroxyecdysone, a new natural ecdysteroid from Silene italica ssp. nemoralis. J. Nat. Prod. 2003, 66, 450–451. [Google Scholar] [CrossRef]
- Simon, A.; Pongrácz, Z.; Tóth, G.; Mák, M.; Máthé, I.; Báthori, M. A new ecdysteroid with unique 9β-OH and four other ecdysteroids from Silene italica ssp. nemoralis. Steroids 2004, 69, 389–394. [Google Scholar]
- Zibareva, L. Study of peculiarities to accumulate ecdysteroids in the plants of the Silene genus. In Proceedings of the Fundamental and Applied Problems of Environmental Protection, Tomsk, Russia, 12–16 September 1995.
- Zibareva, L. Phytoecdysteroids of Caryophyllaceae Juss. Contemp. Probl. Ecol. 2009, 2, 476–488. [Google Scholar] [CrossRef]
- Zibareva, L.; Yeryomina, V.; Zibarev, P. The Method of Detection and Quantity Definition of Phytoecdysteroids in Plant Objects; Pat. Russ. Fed: Moskow, Russia, 1997. [Google Scholar]
- Bathori, M.; Girault, J.P.; Kalasz, H.; Mathe, I.; Lafont, R. New minor ecdysteroids from Silene otites (L.) Wib. J. Pharm. Biomed. Anal. 1997, 16, 327–336. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abdullaev, N.; Usmanov, B.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. VIII. 2-Deoxyecdysterone 3-acetate from Silene praemixta. Chem. Nat. Compd. 1985, 21, 56–58. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Saatov, Z.; Kachala, V.; Shashkov, A. Phytoecdysteroids of plants of the Silene genus. 2-Deoxyecdysterone-25-acetate from Silene wallichiana. Chem. Nat. Compd. 2002, 38, 179–181. [Google Scholar] [CrossRef]
- Bathori, M. Purification and characterization of plant ecdysteroids of Silene species. Trends Anal. Chem. 1998, 17, 372–383. [Google Scholar] [CrossRef]
- Bathori, M.; Szendrei, K.; Herke, I. The ecdysteriods of Silene otites L Wib. Herba Hung. 1986, 25, 105–117. [Google Scholar]
- Báthori, M.; Szendrei, K.; Miklós, P.; Pelczer, I.; Solymosi, P. Ecdysteroids from Silene nutans L. In Chromatography‘85; Kalász, H., Ettre, L.S., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1986; pp. 241–250. [Google Scholar]
- Bathori, M.; Mathe, I.; Solymosi, P.; Szendrei, K. Phytoecdysteroids in some species of Caryophyllaceae and Chenopodiaceae. Acta Bot. Hung. 1987, 33, 1938–1945. [Google Scholar]
- Ramazanov, N.; Sultanov, S.; Saatov, Z.; Nigmatullaev, A. Phytoecdysteroids of plants of the Silene genus and the dynamics of their accumulation. Chem. Nat. Compd. 1997, 33, 558–562. [Google Scholar] [CrossRef]
- Toth, N.; Simon, A.; Toth, G.; Kele, Z.; Hunyadi, A.; Bathori, M. 26-Hydroxylated ecdysteroids from Silene viridiflora. J. Nat. Prod. 2008, 71, 1461–1463. [Google Scholar]
- Mamadalieva, N.Z.; Janibekov, A.A.; Girault, J.P.; Lafont, R. Two minor phytoecdysteroids of the plant Silene viridiflora. Nat. Prod. Commun. 2010, 5, 1579–1582. [Google Scholar]
- Mamadalieva, N.Z. Study of the phytoecdysteroids of some Caryophyllaceae plants. In Proceedings of the 7th International Symposium on the Chemistry of Natural Compounds, Tashkent, Uzbekistan, 16–18 October 2007.
- Mamadalieva, N.; Zibareva, L.; Evrard-Todeschi, N.; Girault, J.-P.; Maria, A.; Ramazanov, N.; Saatov, Z.; Lafont, R. New minor ecdysteroids from Silene viridiflora. Collect. Czech. Chem. Commun. 2004, 69, 1675–1680. [Google Scholar] [CrossRef]
- Saatov, Z.; Abdullaev, N.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. VI. α-Ecdysone 22-sulfate—A new ecdysteroid from Silene brahuica. Chem. Nat. Compd. 1984, 20, 441–444. [Google Scholar]
- Baltayev, U. Ecdysteroside, a phytoecdysteroid from Silene tatarica. Phytochemistry 1998, 47, 1233–1235. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abdullaev, N.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene XII. 5α-Ecdysterone 22-O-benzoate from Silene scabrifolia. Chem. Nat. Compd. 1987, 23, 563–565. [Google Scholar] [CrossRef]
- Abubakirov, N.K. Ecdysteroids of flowering plants (Angiospermae). Proc. Indian Natl. Sci. Acad. Phys. Sci. 1982, 48A, 122–138. [Google Scholar]
- Abubakirov, N.K. Insect moulting hormones in plants from Central Asia. Uzb. Acad. Sci. Ser.: Chem. 1984, 4, 49–53. [Google Scholar]
- Baltaev, U.; Belov, Y.; Chumachenko, M.; Abubakirov, N. High-performance liquid chromatography of the phytoecdysteroids of Melandrium nutans. Chem. Nat. Compd. 1984, 20, 300–301. [Google Scholar] [CrossRef]
- Bergamasco, R.; Horn, D.H.S. Distribution and Role of Insect Hormones in Plants. In Endocrinology of Insects; Downer, R.G.H., Laufer, H., Eds.; Alan R. Liss: New York, NY, USA, 1983; pp. 627–654. [Google Scholar]
- Girault, J.P.; Báthori, M.; Kalász, H.; Mathé, I.; Lafont, R. Sidisterone, a C24 Ecdysteroid from Silene dioica and Silene otites. J. Nat. Prod. 1996, 59, 522–524. [Google Scholar]
- Saatov, Z.; Usmanov, B.Z.; Abubakirov, N.K. Phytoecdysones of Silene praemixta. II. Premixisterone. Chem. Nat. Compd. 1979, 15, 703–705. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abdullaev, N.; Usmanov, B.; Abubakirov, N. Phytoecdysteroids of Silene plants. III. Silenoside A—A new ecdysteroid glycoside from Silene brahuica. Chem. Nat. Compd. 1981, 17, 534–539. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abdullaev, N.; Usmanov, B.; Abubakirov, N. Phytoecdysteroids of Silene plants. V. Silenoside B-digalactoside of ecdysterone from Silene brahuica. Chem. Nat. Compd. 1982, 18, 578–582. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abdullaev, N.; Usmanov, B.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. IV. Sileneoside C—A galactoside of intergristerone A from Silene brahuica. Chem. Nat. Compd. 1982, 18, 193–196. [Google Scholar]
- Volodin, V.; Chadin, I.; Whiting, P.; Dinan, L. Screening plants of European North-East Russia for ecdysteroids. Biochem. Syst. Ecol. 2002, 30, 525–578. [Google Scholar]
- Zibareva, L.; Yeryomina, V. 20-Hydroxyecdysone distribution of different parts of Silene bellidifolia Juss. ex Jacq. и S. squamigera Boiss., cultivating in Siberian Botanical garden (Tomsk). Rastit. Resur. 2002, 38, 81–85. [Google Scholar]
- Cui, Z.-H.; Qiao, L.; Gao, C.-Y.; Lou, Z.-C. Chemical constituents of Silene jenisseensis. J. Chin. Pharm. Sci. 1995, 4, 30–31. [Google Scholar]
- Baltaev, U.; Darmograi, V.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. XIV. Ecdysterone 20-O-benzoate from Silene tatarica. Chem. Nat. Compd. 1987, 23, 706–708. [Google Scholar] [CrossRef]
- Saatov, Z.; Abdullaev, N.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. XVII. Ecdysterone 22, 25-di-O-benzoate from Silene scabrifolia. Chem. Nat. Compd. 1990, 26, 301–303. [Google Scholar]
- Ramazonov, N.; Mamadalieva, N.; Bobaev, I. Phytoecdysteroids from five species of the genus Silene. Chem. Nat. Compd. 2007, 43, 117–118. [Google Scholar] [CrossRef]
- Sadikov, Z.T.; Saatov, Z. Phytoecdysteroids of plants of the genus Silene XX. Integristerone A 25-acetate from Silene brahuica. Chem. Nat. Compd. 1999, 35, 440–441. [Google Scholar] [CrossRef]
- Saatov, Z.; Abdullaev, N.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. VII. Sileneoside D—ecdysterone 3-O-α-d-galactopyranoside from Silene brahuica. Chem. Nat. Compd. 1984, 20, 700–703. [Google Scholar] [CrossRef]
- Dzhukarova, M.K.; Saatov, Z.; Abdullaev, N.D. Phytoecdysteroids of plants of the genus Silene XVI. 5α-sileneoside E from Silene brahuica. Chem. Nat. Compd. 1995, 31, 207–210. [Google Scholar] [CrossRef]
- Dzhukharova, M.K.; Saatov, Z.; Abdullaev, N.D.; Abubakirov, N.K. Phytoecdysteroids of the plants of the genus Silene XV. Silenoside F—Brahuisterone 3-O-β-d-glucopyranoside from Silene brahuica. Chem. Nat. Compd. 1994, 30, 680–683. [Google Scholar] [CrossRef]
- Sadykov, Z.T.; Saatov, Z. Phytoecdysteroids of plants of the Silene genus. XIX. The structure of sileneoside G. Chem. Nat. Compd. 1998, 34, 602–604. [Google Scholar] [CrossRef]
- Sadikov, Z.T.; Saatov, Z.; Girault, J.P.; Lafont, R. Sileneoside H, a new phytoecdysteroid from Silene brahuica. J. Nat. Prod. 2000, 63, 987–988. [Google Scholar]
- Baltaev, U.; Rashkes, Y.; Darmograi, V.; Belov, Y.; Abubakirov, N. Phytoecdysteroids of Silene nutans. II. 22-Deoxyecdysterone and features of its mass spectrum. Chem. Nat. Compd. 1985, 21, 59–62. [Google Scholar]
- Mamadalieva, N.; Egamberdieva, D.; Zhanibekov, A.; Triggiani, D.; Tiezzi, A. Chemical components of Silene viridiflora and their biological properties. Chem. Nat. Compd. 2009, 45, 589–591. [Google Scholar]
- Ramazanov, N.; Maksimov, E.; Saatov, Z.; Abdullaev, N. Phytoecdysteroids of plants of the Silene genus. XVIII. Tomentesterone from Silene tomentella. Chem. Nat. Compd. 1996, 32, 47–49. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene. XVI. Viticosterone E 22-O-benzoate from Silene wallichiana. Chem. Nat. Compd. 1988, 24, 463–465. [Google Scholar] [CrossRef]
- Baltaev, U.; Rashkes, Y.; Abubakirov, N. Phytoecdysteroids of Silene nutans. III. Nusilsterone. Chem. Nat. Compd. 1985, 21, 489–491. [Google Scholar] [CrossRef]
- Simon, A.; Tóth, N.; Tóth, G.; Kele, Z.; Groska, J.; Báthori, M. Ecdysteroids from Silene viridiflora. Helv. Chim. Acta 2009, 92, 753–761. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.; Abubakirov, N. Phytoecdysteroids of plants of the genus Silene XIII. Ecdysterone 20, 22-monoacetonide from Silene scabrifolia. Chem. Nat. Compd. 1987, 23, 643. [Google Scholar] [CrossRef]
- Glensk, M.; Wray, V.; Nimtz, M.; Schöpke, T. Silenosides A−C, triterpenoid saponins from Silene vulgaris. J. Nat. Prod. 1999, 62, 717–721. [Google Scholar] [CrossRef]
- Fu, H.; Koike, K.; Li, W.; Nikaido, T.; Lin, W.; Guo, D. Silenorubicosides A−D, Triterpenoid Saponins from Silene rubicunda. J. Nat. Prod. 2005, 68, 754–758. [Google Scholar]
- Larhsini, M.; Marston, A.; Hostettmann, K. Triterpenoid saponins from the roots of Silene cucubalus. Fitoterapia 2003, 74, 237–241. [Google Scholar] [CrossRef]
- Bukharov, V.G.; Karneeva, L.N. Nutanoside—A triterpene glycoside from Silene nutans. Chem. Nat. Compd. 1971, 7, 200–200. [Google Scholar] [CrossRef]
- Bouguet Bonnet, S.; Rochd, M.; Mutzenhardt, P.; Henry, M. Total assignment of 1H and 13C NMR spectra of three triterpene saponins from roots of Silene vulgaris (Moench) Garcke. Magn. Reson. Chem. 2002, 40, 618–621. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.-A.; Hanquet, B.; Cui, Z.-H.; Lou, Z.-C.; Wagner, H. Acylated triterpene saponins from Silene jenisseensis. Phytochemistry 1995, 40, 509–514. [Google Scholar]
- Lacaille-Dubois, M.-A.; Hanquet, B.; Cui, Z.-H.; Lou, Z.-C.; Wagner, H. Jenisseensosides C and D, biologically active acylated triterpene saponins from Silene jenisseensis. Phytochemistry 1997, 45, 985–990. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.-A.; Hanquet, B.; Cui, Z.-H.; Lou, Z.-C.; Wagner, H. A new biologically active acylated triterpene saponin from Silene fortunei. J. Nat. Prod. 1999, 62, 133–136. [Google Scholar] [CrossRef]
- Zou, C.; Jiang, J.; Chen, C.; Zhou, J.; Zhao, Q. Diacylated triterpenoid saponin from Silene szechuensis. Chin. Chem. Lett. 1999, 10, 33–36. [Google Scholar]
- Xu, W.; Wu, J.; Zhu, Z.; Sha, Y.; Fang, J.; Li, Y. Pentacyclic triterpenoid saponins from Silene viscidula. Helv. Chim. Acta 2010, 93, 2007–2014. [Google Scholar] [CrossRef]
- Tan, N.; Zhou, J.; Zhao, S.; Cheng, C. Rubicunosides B, C, D-three new triterpenoids saponins with acetylated saccharides from Silene rubicunda. Acta Chim. Sin. 1996, 54, 722–728. [Google Scholar]
- Wu, Q.; Tu, G.; Fu, H. A new triterpenoid saponin from Silene rubicunda Franch. J. Chin. Pharm. Sci. 2014, 23, 246–250. [Google Scholar]
- Elgamal, M.H.A.; Soliman, H.S.M.; Karawya, M.S.; Duddeck, H. Villosagenin I and II Two Novel Pentacyclic 28-Nortriterpenes from Silene villosa (Forssk). Nat. Prod. Lett. 1994, 4, 297–301. [Google Scholar] [CrossRef]
- Karawya, M.; Elgamal, M.; Shalaby, N.; Soliman, H. Saponins of Silene succulenta forssk, growing locally. Egypt. J. Pharm. Sci. 1991, 32, 879. [Google Scholar]
- Tolibaev, I.; Mukhamedova, K.S.; Glushenkova, A.I. Lipids of Silene brahuica. Chem. Nat. Compd. 1993, 29, 444–446. [Google Scholar] [CrossRef]
- Salt, T.; Adler, J. Dominance of Δ7-sterols in the family caryophyllaceae in the family Caryophyllaceae. Lipids 1986, 21, 754–758. [Google Scholar] [CrossRef]
- Zemtsova, G.; Glyzin, V.Y.; Dzhumyrko, S. Flavones and their C-glycosides from Silene saxatilis. Chem. Nat. Compd. 1975, 11, 538–538. [Google Scholar] [CrossRef]
- Chopin, M.J.; Bouillant, M.L.; Wagner, H.; Galle, K. Endgültige Struktur von Schaftosid aus Silene schafta. Phytochemistry 1974, 13, 2583–2586. [Google Scholar]
- Heinsbroek, R.; van Brederode, J.; van Nigtevecht, G.; Maas, J.; Kamsteeg, J.; Besson, E.; Chopin, J. The 2″-O-glucosylation of vitexin and isovitexin in petals of Silene alba is catalysed by two different enzymes. Phytochemistry 1980, 19, 1935–1937. [Google Scholar]
- Richardson, M. Flavonols and C-Glycosylflavonoids of the Caryophyllales. Biochem. Syst. Ecol. 1978, 6, 283–286. [Google Scholar] [CrossRef]
- Iwashina, T.; Ootani, S. Characterization of C-glycosylflavones and anthocyanins in several species of Caryophyllaceae. Ann. Tsukuba Bot. Gard. 1987, 20, 19–30. [Google Scholar]
- Kamsteeg, J.; van Brederode, J.; van Nigtevecht, G. Pleiotropic effect of a pelargonidin-hydroxylation gene in Silene dioica. Phytochemistry 1976, 15, 1917–1918. [Google Scholar]
- Bajpai, V.; Shukla, S.; Kang, S. Chemical composition and antifungal activity of essential oil and various extract of Silene armeria L. Bioresour. Technol. 2008, 99, 8903–8908. [Google Scholar] [CrossRef]
- Dötterl, S.; Jahreiß, K.; Jhumur, U.S.; Jürgens, A. Temporal variation of flower scent in Silene otites (Caryophyllaceae): A species with a mixed pollination system. Bot. J. Linn. Soc. 2012, 169, 447–460. [Google Scholar] [CrossRef]
- Jhumur, U.; Dötterl, S.; Jürgens, A. Floral odors of Silene otites: Their variability and attractiveness to mosquitoes. J. Chem. Ecol. 2008, 34, 14–25. [Google Scholar]
- Andersson, S.; Nilsson, L.A.A.; Groth, I.; Bergstrom, G. Floral scents in butterfly-pollinated plants: Possible convergence in chemical composition. Bot. J. Linn. Soc. 2002, 140, 129–153. [Google Scholar] [CrossRef]
- Jürgens, A.; Witt, T.; Gottsberger, G. Flower scent composition in night-flowering Silene species (Caryophyllaceae). Biochem. Syst. Ecol. 2002, 30, 383–397. [Google Scholar]
- Jürgens, A. Flower scent composition in diurnal Silene species (Caryophyllaceae): Phylogenetic constraints or adaption to flower visitors? Biochem. Syst. Ecol. 2004, 32, 841–859. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L. Trends in floral scent chemistry in pollination syndromes: Floral scent composition in moth pollinated taxa. Bot. J. Linn. Soc. 1993, 113, 263–284. [Google Scholar] [CrossRef]
- Dötterl, S.; Burkhardt, D.; Weißbecker, B.; Jürgens, A.; Schütz, S.; Mosandl, A. Linalool and lilac aldehyde/alcohol in flower scents: Electrophysiological detection of lilac aldehyde stereoisomers by a moth. J. Chromatogr. A 2006, 1113, 231–238. [Google Scholar]
- Vardavas, C.I.; Majchrzak, D.; Wagner, K.H.; Elmadfa, I.; Kafatos, A. Lipid concentrations of wild edible greens in Crete. Food Chem. 2006, 99, 822–834. [Google Scholar] [CrossRef]
- Asai, T.; Fujimoto, Y. Cyclic fatty acyl glycosides in the glandular trichome exudate of Silene gallica. Phytochemistry 2010, 71, 1410–1417. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Majchrzak, D.; Wagner, K.H.; Elmadfa, I.; Kafatos, A. The antioxidant and phylloquinone content of wildly grown greens in Crete. Food Chem. 2006, 99, 813–821. [Google Scholar] [CrossRef]
- Morales, P.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Molina, M.; Ferreira, I.C. Tocopherol composition and antioxidant activity of Spanish wild vegetables. Genet. Resour. Crop Evol. 2012, 59, 851–863. [Google Scholar] [CrossRef]
- Günter, Y.; Ovodov, Y. Pectin substances of the callus culture of Silene vulgaris (M.). Appl. Biochem. Microbiol. 2011, 47, 82–86. [Google Scholar] [CrossRef]
- Borris, R.P.; Hesse, M.; Seifert, K.; Johne, S. 2-[6′-(O-trans-Cinnamoyl)-β-d-glucopyranosyloxy]-3-methyl 4H-pyran-4-one, a New Acylated Pyrone Glucoside from Silene vulgaris (Caryophyllaceae) Part 186. Papers on Organic Natural Products. Helv. Chim. Acta 1982, 65, 2481–2485. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).