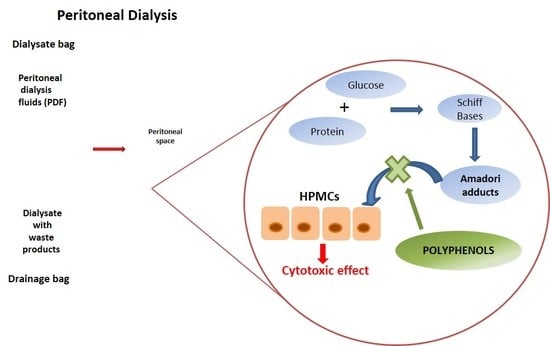
Polyphenols Attenuate Highly-Glycosylated Haemoglobin-Induced Damage in Human Peritoneal Mesothelial Cells
Abstract
1. Introduction
- -
- anti-oxidant actions (such as scavenging radicals and metal ion chelation ability) [21].
- -
- anti-inflammatory actions (through inhibition of the redox-sensitive transcription factors, such as NF-kB and/or inhibition of “pro-oxidant” enzymes like inducible Nitric Oxide synthetase (iNOS), COX, XO); induction of antioxidant enzymes, as glutathione S-transferase (GST and SOD) [22,23] or acting as scavengers of reactive oxygen (ROS) and nitrogen (RNS) species and as chelators of iron and copper, causal factors strictly correlated to inflammatory diseases [24,25,26,27,28,29,30].
- -
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Amadori Adducts
2.3. Polyphenols Administration
2.4. Intracellular Reactive Oxygen Species (ROS) Detection
2.5. Determination of Caspases-3/7
2.6. Quantitation of ATP-Levels
2.7. Annexin V Detection by Indirect Immunofluorescence
2.8. Reporter Plasmids
2.9. Transient Transfection
2.10. Determination of p53 Levels
2.11. Statistical Analysis
3. Results
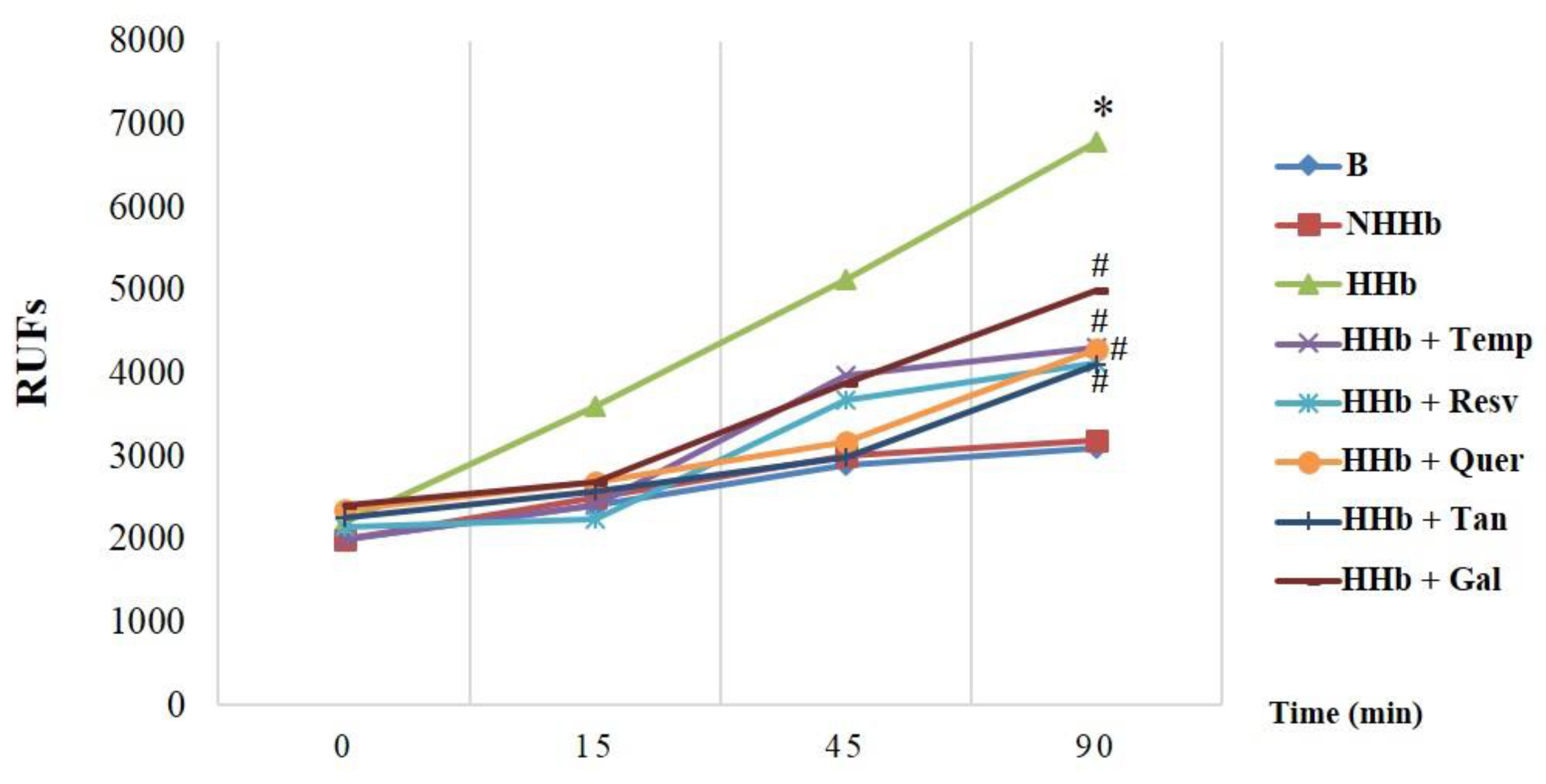
3.1. Polyphenols Decrease Amadori-Induced ROS
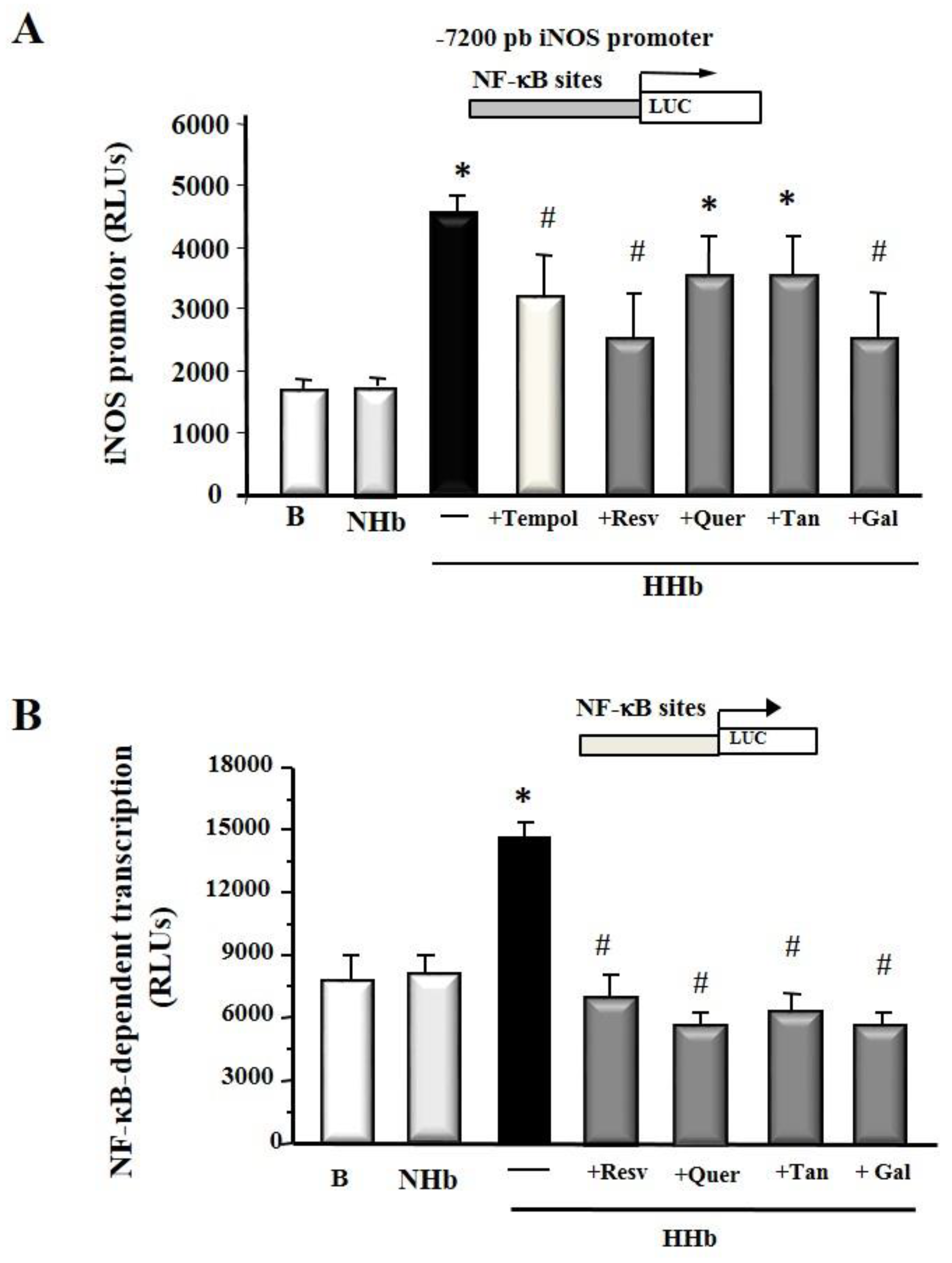
3.2. Polyphenols Decrease iNOS Gene Expression and NF-kB-Dependent Transcription Induced by Amadori Adducts in HPMCs
3.3. Polyphenols Block Amadori-Induced Apoptosis in HPMCs
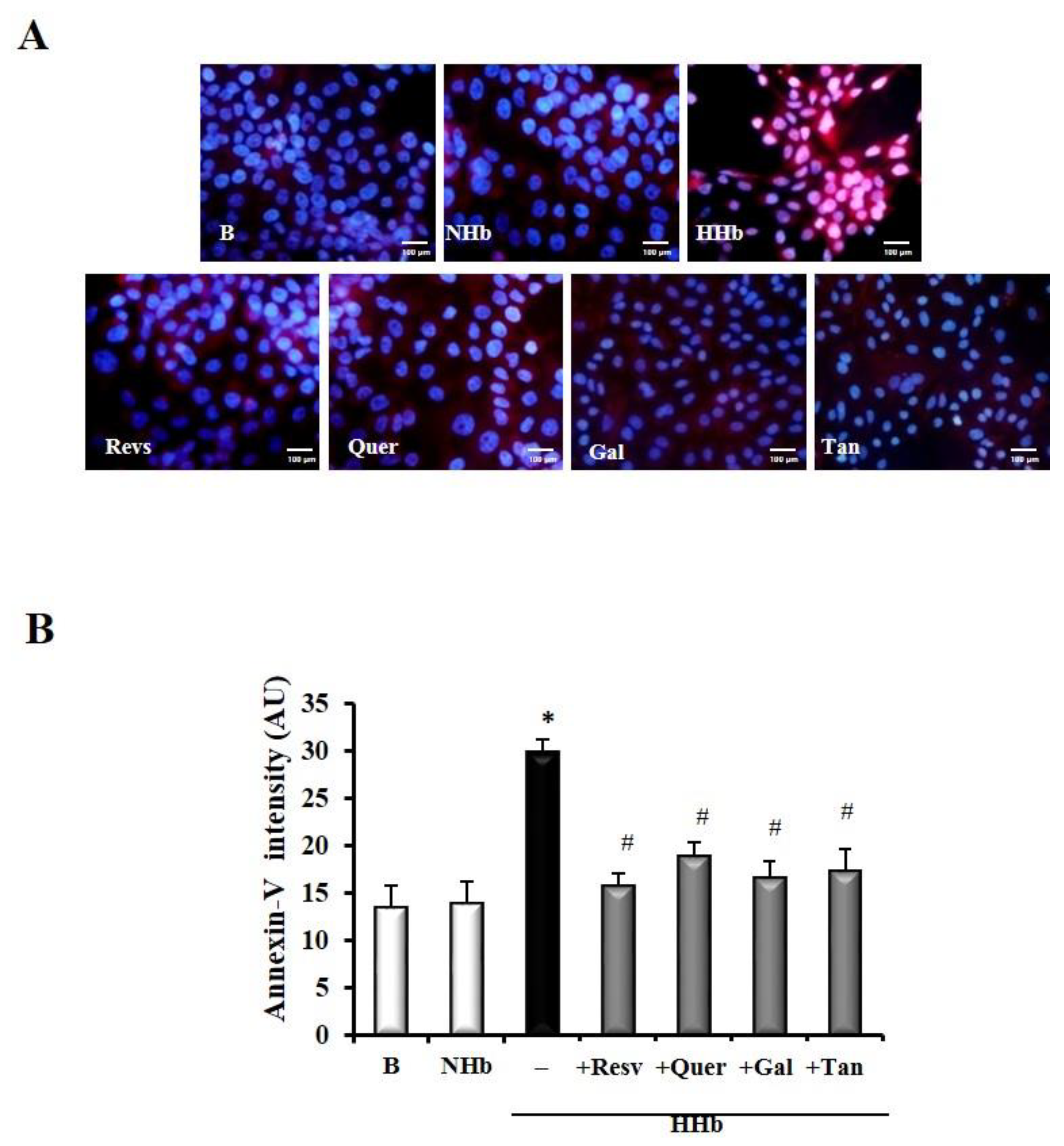
3.4. Polyphenols Decrease Levels of Amadori Adducts-Induced Annexin V in HPMCs
3.5. Polyphenols Decrease Tumour Suppressor Protein p53 Levels Induced by Amadori Adducts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuo, H.; Chen, H.; Hsiao, H.; Chen, H. Heat shock response protects human peritoneal mesothelial cells from dialysate-induced oxidative stress and mitochondrial injury. Nephrol. Dial. Transpl. 2009, 24, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Büchel, J.; Bartosova, M.; Eich, G.; Wittenberger, T.; Klein-Hitpass, L.; Steppan, S.; Hackert, T.; Schaefer, F.; Passlick-Deetjen, J.; Schmitt, C.P. Interference of peritoneal dialysis fluids with cell cycle mechanisms. Perit. Dial. Int. 2015, 35, 259–274. [Google Scholar] [CrossRef]
- Boehm, M.; Bergmeister, H.; Kratochwill, K.; Vargha, R.; Lederhuber, H.; Aufricht, C. Cellular stress-response modulators in the acute rat model of peritoneal dialysis. Pediatr. Nephrol. 2010, 25, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Yu, M.R.; Song, J.S.; Ha, H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 2004, 65, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Cha, M.K.; Choi, H.N.; Lee, H.B. Effects of peritoneal dialysis solutions on the secretion of growth factors and extracellular matrix proteins by human peritoneal mesothelial cells. Perit. Dial. Int. 2002, 22, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Khangholi, S.; Majid, F.A.; Berwary, N.J.; Ahmad, F.; Aziz, R.B. The Mechanisms of Inhibition of Advanced Glycation End Products Formation through Polyphenols in Hyperglycemic Condition. Planta Med. 2016, 82, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W.; Thorpe, S.R. Glycoxidation and lipoxidation in atherogenesis. Free Radic. Biol. Med. 2000, 28, 1708–1716. [Google Scholar] [CrossRef]
- Honda, K.; Nitta, K.; Horita, S.; Yumura, W.; Nihei, H.; Nagai, R.; Ikeda, K.; Horiuchi, S. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol. Dial. Transplant 1999, 14, 1541–1549. [Google Scholar] [CrossRef]
- Nevado, J.; Peiró, C.; Vallejo, S.; El-Assar, M.; Lafuente, N.; Matesanz, N.; Azcutia, V.; Cercas, E.; Sánchez-Ferrer, C.F.; Rodríguez-Mañas, L. Amadori adducts activate NF-kB-related pro-inflammatory genes in human peritoneal mesothelial cells. Br. J. Pharmacol. 2005, 146, 268–279. [Google Scholar] [CrossRef]
- Peiró, C.; Lafuente, N.; Matesanz, N.; Cercas, E.; Llergo, J.L.; Vallejo, S.; Rodríguez-Mañas, L.; Sánchez-Ferrer, C.F. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br. J. Pharmacol. 2001, 133, 967–974. [Google Scholar]
- Peiro, C.; Matesanz, N.; Nevado, J.; Lafuente, N.; Cercas, E.; Azcutia, V.; Vallejo, S.; Rodriguez-Manas, L.; Sanchez-Ferrer, C.F. Glycosylated human oxyhaemoglobin activates nuclear factor-kappaB and activator protein-1 in cultured human aortic smooth muscle. Br. J. Pharmacol. 2003, 140, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañas, L.; Angulo, J.; Vallejo, S.; Sánchez-Ferrer, A.; Cercas, E.; López-Dóriga, P.; Sánchez-Ferrer, C.F. Early and intermediate Amadori glycosylation adducts, oxidative stress, and endothelial dysfunction in the streptozotocin-induced diabetic rats vasculature. Diabetologia 2003, 46, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, C.; Peiró, C.; Vallejo, S.; Matesanz, N.; El-Assar, M.; Azcutia, V.; Romacho, T.; Sánchez-Ferrer, C.F.; Rodríguez-Mañas, L.; Nevado, J. Pathways Responsible for Apoptosis Resulting from Amadori-Induced Oxidative and Nitrosative Stress in Human Mesothelial Cells. Am. J. Nephrol. 2011, 34, 104–114. [Google Scholar]
- Amore, A.; Cirina, P.; Conti, G.; Cerutti, F.; Bagheri, N.; Emancipator, S.N.; Coppo, R. Amadori-configurated albumin induces nitric oxide-dependent apoptosis of endothelial cells: A possible mechanism of diabetic vasculopathy. Nephrol. Dial. Transpl. 2004, 19, 53–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amore, A.; Cirina, P.; Conti, G.; Cerutti, F.; Bagheri, N.; Emancipator, S.N.; Coppo, R. Icodextrin avoids the enhancement of inducible nitric oxide (iNOS) activity in peritoneal mesothelial cells given by glucose solutions. J. Am. Soc. Nephrol. 1998, 9, 278. [Google Scholar]
- Drel, V.R.; Sybirna, N. Protective effects of polyphenolics in red wine on diabetes associated oxidative/nitrative stress in streptozotocindiabetic rats. Cell Biol. Int. 2010, 34, 1147–1153. [Google Scholar] [CrossRef]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, S67–S90. [Google Scholar] [CrossRef]
- Konic-Ristic, A.; Kroon, P.A.; Glibetic, M. Modulation of platelet function with dietary polyphenols: A promising strategy in the prevention of cardiovascular disease. Agro Food Ind. Hi Tech 2015, 26, 15–19. [Google Scholar]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateo, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals–just antioxidants or much more. Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Young Seo, A.; Carter, C.; Pal Yu, B.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Varga, Z.V.; Giricz, Z.; Liaudet, L.; Haskó, G.; Ferdinandy, P.; Pacher, P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 232–242. [Google Scholar] [CrossRef]
- Maurya, P.K.; Noto, C.; Rizzo, L.B.; Rios, A.C.; Nunes, S.O.; Barbosa, D.S.; Sethi, S.; Zeni, M.; Mansur, R.B.; Maes, M.; et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 2016, 65, 134–144. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J. Agric. Food Chem. 2014, 62, 1643–1648. [Google Scholar] [CrossRef]
- Guerra, P.V.; Yaylayan, V.A. Interaction of Flavanols with Amino Acids: Postoxidative Reactivity of the B-ring of Catechin with Glycine. J. Agric. Food Chem. 2014, 62, 3831–3836. [Google Scholar] [CrossRef]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Tea polyphenol (-)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
- Yin, J.; Hedegaard, R.V.; Skibsted, L.H.; Andersen, M.L. Epicatechin and Epigallocatechin Gallate Inhibit Formation of Intermediary Radicals during Heating of Lysine and Glucose. Food Chem. 2014, 146, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Srey, C.; Ames, J.M.; del Castillo, M.D. Glycation Is Regulated by Isoflavones. Food Funct. 2014, 5, 2036–2042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Tietz, N.W. Clinical Guide to Laboratory Tests; WB Saunders: Philadelphia, PA, USA, 1990; pp. 284–285. [Google Scholar]
- Ferruelo, A.; Romero, I.; Cabrera, P.M.; Arance, I.; Andrés, G.; Angulo, J.C. Effects of resveratrol and other wine polyphenols on the proliferation, apoptosis and androgen receptor expression in LNCaP cells. Actas Urol. Esp. 2014, 38, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ferruelo, A.; de Las Heras, M.M.; Redondo, C.; de Fata, F.R.; Romero, I.; Angulo, J.C. Wine polyphenols exert antineoplasic effect on androgen resistant PC-3 cell line through the inhibition of the transcriptional activity of COX-2 promoter mediated by NF-kβ. Actas Urol. Esp. 2014, 38, 429–437. [Google Scholar] [CrossRef]
- Mrvová, N.; Skandík, M.; Kuniaková, M.; Racková, L. Modulation of BV-2 microglia functions by novel quercetin pivaloyl ester. Neurochem. Int. 2015, 90, 246–254. [Google Scholar] [CrossRef]
- Ramyaa, P.; Krishnaswamy, R.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signaling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-𝜅B andCOX-2. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 681–692. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Dong, L.; Li, M.; Cai, W. Anti-inflammatory effect of resveratrol through the suppression of NF-κB and JAK/STAT signaling pathways. Acta Biochim. Biophys. Sin. 2015, 47, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol Content and Antioxidant Properties of Underutilized Fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Jardim, F.R.; de Rossi, F.T.; Nascimento, M.X.; Gabriele da Silva Barros, R.; Agrizzi Borges, P.; Cristina Prescilio, I.; Roberto de Oliveira, M. Resveratrol and Brain Mitochondria: A Review. Mol. Neurobiol. 2018, 55, 2085–2101. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Spencer, J.P.; Kuhnle, G.G.; Williams, R.J.; Rice-Evans, C. Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. Biochem. J. 2003, 372, 173–181. [Google Scholar] [CrossRef]
- Sengupta, B.; Uematsu, T.; Jacobsson, P.; Swenson, J. Exploring the antioxidant property of bioflavonoid quercetin in preventing DNA glycation: A calorimetric and spectroscopic study. Biochem. Biophys. Res. Commun. 2006, 339, 355–361. [Google Scholar] [CrossRef]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Peng, C.H.; Chyau, C.C.; Lin, Y.C.; Wang, H.E.; Peng, R.Y. Low-density lipoprotein, collagen, and thrombin models reveal that Rosemarinus officinalis L. exhibits potent antiglycative effects. J. Agric. Food Chem. 2007, 55, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Gerates, L.; Moonen, H.J.J.; Brauers, K.; Wouters, E.F.M.; Bast, A.; Hageman, G.J. Dietary flavones and flavonols are inhibitor of poly (ADP-ribose) polymerase-1 in pulmonary epithelial cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar] [CrossRef]
- Bureau, G.; Longpre, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J. Agric. Food Chem. 2010, 58, 815–5820. [Google Scholar] [CrossRef] [PubMed]
- Endale, M.; Park, S.C.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef]
- Yang, D.; Liu, X.; Liu, M.; Chi, H.; Liu, J.; Han, H. Protective effects of quercetin and taraxasterol against H2O2 -induced human umbilical vein endothelial cell injury in vitro. Exp. Ther. Med. 2015, 10, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Mainzen-Prince, P.S. Cardio protective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009, 179, 118–124. [Google Scholar] [CrossRef]
- Kim, S.H.; Jun, C.D.; Suk, K. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.-J.; Choi, C.-H. Inhibitory effect of methyl gallate and Gallic Acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef]
- Kratz, J.M.; Andrighetti-Fröhner, C.R.; Leal, P.C.; Nunes, R.J.; Yunes, R.A.; Trybala, E.; Bergström, T.; Barardi, C.R.; Simões, C.M. Evaluation of anti-HSV-2 activity of Gallic Acid and pentylgallate. Biol. Pharm. Bull. 2008, 31, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sameermahmood, Z.; Raji, L.; Saravanan, T.; Vaidya, A.; Mohan, V.; Balasubramanyam, M. Gallic acid protects RINm5F beta-cells from glucolipotoxicity by its antiapoptotic and insulin-secretagogue actions. Phytother. Res. 2010, 24, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, S.; Gopi, V.; Elangovan, V. Regulatory Mechanism of Gallic Acid Against Advanced Glycation End Products Induced Cardiac Remodeling in Experimental Rats. Chem. Biol. Interact. 2014, 208, 28–36. [Google Scholar] [CrossRef]
- Ahmad, S.T.; Sultana, S. Tannic acid mitigates cisplatin-induced nephrotoxicity in mice. Hum. Exp. Toxicol. 2012, 31, 145–156. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Tannic acid modulates NFκB signaling pathway and skin inflammation in NC/Nga mice through PPARγ expression. Cytokine 2015, 76, 206–213. [Google Scholar] [CrossRef]
- Huang, Q.; Chai, W.M.; Ma, Z.Y.; Ou-Yang, C.; Wei, Q.-M.; Song, S.; Zou, Z.-R.; Peng, Y.-Y. Inhibition of α-glucosidase activity and non-enzymatic glycation by tannic acid: Inhibitory activity and molecular mechanism. Int. J. Biol. Macromol. 2019, 141, 358–368. [Google Scholar] [CrossRef]
- Nevado, J.; Sanz, R.; Casqueiro, J.C.; Ayala, A.; García-Berrocal, J.R.; Ramírez-Camacho, R. Ageing evokes an intrinsic pro-apoptotic signalling pathway in rat cochlea. Acta Otolaryngol. 2006, 126, 1134–1139. [Google Scholar] [CrossRef]
- García-Berrocal, J.R.; Nevado, J.; Ramírez-Camacho, R.; Sanz, R.; González-García, J.A.; Sánchez-Rodríguez, C.; Cantos, B.; España, P.; Verdaguer, J.M.; Trinidad Cabezas, A. The anticancer drug cisplatin induces an intrinsic apoptotic pathway inside the inner ear. Br. J. Pharmacol. 2007, 152, 1012–1020. [Google Scholar] [CrossRef]
- Benevolensky, D.; Belikova, Y.; Mohammadzadeh, R.; Trouvé, P.; Marotte, F.; Russo-Marie, F.; Samuel, J.L.; Charlemagne, D. Expression and localization of the annexins II, V, and VI in myocardium from patients with end-stage heart failure. Lab. Investig. 2000, 80, 123–133. [Google Scholar] [CrossRef]
- Neelofar, K.; Ahmad, J. Amadori albumin in diabetic nephropathy. Indian J. Endocrinol. Metab. 2015, 19, 39–46. [Google Scholar]
- Bodega, F.; Zocchi, L.; Agostoni, E. Albumin transcytosis in mesothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L3–L11. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Albertoni, G.; Schor, N. Resveratrol plays important role in protective mechanisms in renal disease--mini-review. J. Bras. Nefrol. 2015, 37, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs. metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef]
- Wei, J.; Jin, F.; Wu, Q.; Jiang, Y.; Gao, D.; Liu, H. Molecular interaction study of flavonoid derivative 3d with human serum albumin using multi spectroscopic and molecular modeling approach. Talanta 2014, 126, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Richard, T.; Lefeuvre, D.; Descendit, A.; Quideau, S.; Monti, J.P. Recognition characters in peptide-polyphenol complex formation. Biochim. Biophys. Acta 2006, 1760, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef] [PubMed]
- Švajger, U.; Jeras, M. Anti-inflammatory Effects of Resveratrol and Its Potential Use in Therapy of Immune-Mediated Diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.J.; Vellaichamy, E. Protective Activity of Gallic Acid Against Glyoxal -Induced Renal Fibrosis in Experimental Rats. Toxicol. Rep. 2015, 2, 1246–1254. [Google Scholar] [CrossRef]
- Lupinacci, S.; Perri, A.; Toteda, G.; Vizza, D.; Puoci, F.; Parisi, O.I.; Giordano, F.; Lofaro, D.; La Russa, A.; Bonofiglio, M.; et al. Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFβ1 signaling. Cell Biol. Toxicol. 2018, 35, 95–109. [Google Scholar] [CrossRef]
- Lupinacci, S.; Toteda, G.; Vizza, D.; Perri, A.; Benincasa, C.; Mollica, A.; La Russa, A.; Gigliotti, P.; Leone, F.; Lofaro, D.; et al. Active Compounds Extracted From Extra Virgin Olive Oil Counteract Mesothelial-To-Mesenchymal Transition of Peritoneal Mesothelium Cells Exposed to Conventional Peritoneal Dialysate: In Vitro and in Vivo Evidences. J. Nephrol. 2017, 30, 841–850. [Google Scholar] [CrossRef]
- Riesenhuber, A.; Kasper, D.C.; Vargha, R.; Endemann, M.; Aufricht, C. Quercetin protects human mesothelial cells against exposure to peritoneal dialysis fluid. Pediatr. Nephrol. 2007, 22, 1205–1208. [Google Scholar] [CrossRef]
- Lin, C.; Sun, X.; Lin, A. Supplementation with high-dose trans-resveratrol improves ultrafiltration in peritoneal dialysis patients: A prospective, randomized, double-blind study. Renal Fail. 2016, 38, 214–221. [Google Scholar] [CrossRef]
- Firuzi, O.; Khajehrezaei, S.; Ezzatzadegan, S.; Nejati, M.; Jahanshahi, K.-A.; Roozbeh, J. Effects of silymarin on biochemical and oxidative stress markers in end-stage renal disease patients undergoing peritoneal dialysis. Hemodial. Int. 2016, 20, 558–563. [Google Scholar] [CrossRef]
- Singh, R.G.; Negi, P.S.; Radha, C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringaoleifera seed flour. J. Funct. Foods 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal-glucosidase and pancreatic-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Alhamdani, M.S.; Al-Azzawie, H.F.; Abbas, F.K. Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit. Dial. Int. 2007, 27, 86–89. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Rodríguez, C.; Peiró, C.; Rodríguez-Mañas, L.; Nevado, J. Polyphenols Attenuate Highly-Glycosylated Haemoglobin-Induced Damage in Human Peritoneal Mesothelial Cells. Antioxidants 2020, 9, 572. https://doi.org/10.3390/antiox9070572
Sánchez-Rodríguez C, Peiró C, Rodríguez-Mañas L, Nevado J. Polyphenols Attenuate Highly-Glycosylated Haemoglobin-Induced Damage in Human Peritoneal Mesothelial Cells. Antioxidants. 2020; 9(7):572. https://doi.org/10.3390/antiox9070572
Chicago/Turabian StyleSánchez-Rodríguez, Carolina, Concepción Peiró, Leocadio Rodríguez-Mañas, and Julián Nevado. 2020. "Polyphenols Attenuate Highly-Glycosylated Haemoglobin-Induced Damage in Human Peritoneal Mesothelial Cells" Antioxidants 9, no. 7: 572. https://doi.org/10.3390/antiox9070572
APA StyleSánchez-Rodríguez, C., Peiró, C., Rodríguez-Mañas, L., & Nevado, J. (2020). Polyphenols Attenuate Highly-Glycosylated Haemoglobin-Induced Damage in Human Peritoneal Mesothelial Cells. Antioxidants, 9(7), 572. https://doi.org/10.3390/antiox9070572