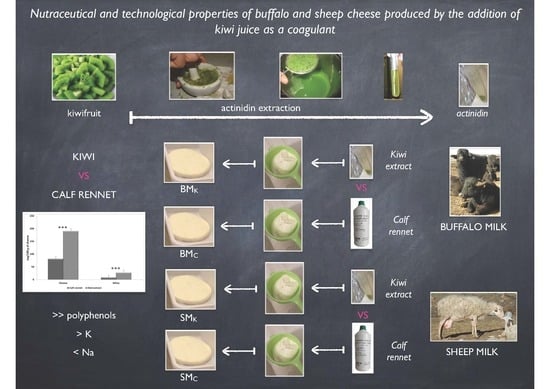
Nutraceutical and Technological Properties of Buffalo and Sheep Cheese Produced by the Addition of Kiwi Juice as a Coagulant
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analysis
2.2.1. Milk Clotting
2.2.2. Physical and Chemical Analysis
2.2.3. Lipid Composition of Kiwifruit and Cheeses
2.2.4. Phenol Extraction, Quantitation and Characterization
2.2.5. Volatile Organic Compounds Analysis
2.3. Statistical analysis
3. Results
3.1. Technological Parameters
3.2. Physico-Chemical Composition
3.3. Polyphenols
3.4. Sterols
3.5. Volatile Organic Compounds
4. Discussion
4.1. Tecnological Parameters
4.2. Physic-Chemical Composition
4.3. Polyphenols
4.4. Sterols
4.5. Volatile Organic Compounds
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopes, A.; Teixeira, G.; Liberato, M.C.; Pais, M.S.; Clemente, A. New vegetal sources for milk clotting enzymes. J. Mol. Catal. B Enzym. 1988, 5, 63–68. [Google Scholar] [CrossRef]
- Lo Piero, A.R.; Puglisi, I.; Petrone, G. Characterization of “Lettucine”, a serine-like protease from Lactuca sativa leaves, as a novel enzyme for milk clotting. J. Agric. Food Chem. 2002, 50, 2439–2443. [Google Scholar] [CrossRef]
- Egito, A.S.; Girardet, J.M.; Laguna, L.E.; Poirson, C.; Mollé, D.; Miclo, L.; Humbert, G.; Gaillard, J.L. Milk-clotting activity of enzyme extracts from sunflower and albizia seeds and specific hydrolysis of bovine-casein. Int. Dairy J. 2007, 17, 816–825. [Google Scholar] [CrossRef]
- Heimgartner, U.; Pietrzak, M.; Geertsen, R.; Brodelius, P.; Da Silva Figueiredo, A.C.; Pais, M.S. Purification and partial Characterization of milk clotting proteases from towers of Cynara cardunculus L. (Cardoon). Phytochemistry 1990, 29, 1405–1410. [Google Scholar] [CrossRef]
- Lo Piero, A.R.; Puglisi, I.; Petrone, G. Characterization of the purified actinidin as a plant coagulant of bovine milk. Eur. Food Res. Techol. 2011, 233, 517–524. [Google Scholar] [CrossRef]
- Saha, B.C.; Hayashi, K. Debittering of protein hydrolysates. Biotechol. Adv. 2001, 19, 355–370. [Google Scholar] [CrossRef]
- Jung, K.A.; Song, T.C.; Han, D.; Kim, I.H.; Kim, Y.E.; Lee, C.H. Cardiovascular protective properties of kiwifruit extract in vitro. Biol. Pharm. Bull. 2005, 28, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Patel, M.; Plank, L.D.; Ferguson, L.R. Kiwifruit promotes laxation in the elderly. Asia Pac. J. Clin. Nutr. 2002, 11, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Namiesnik, J.; Vearasilp, K.; Leontowicz, H.; Leontowicz, M.; Barasch, D.; Nemirovski, A.; Trakhtenberg, S.; Gorinstein, S. Bioactive compounds and the antioxidant capacity in new kiwi fruit cultivar. Food Chem. 2014, 165, 354–361. [Google Scholar] [CrossRef]
- Katsaros, G.I.; Tavantzis, G.; Taoukis, P.S. Production of novel dairy products using actinidin and high pressure as enzyme activity regulator. Innov. Food Sci. Emerg. Technol. 2010, 11, 47–51. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Perea-Gutiérrez, T.C.; Lugo-Sánchez, M.E.; Ramirez-Suarez, J.C.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Cordoba, B. Comparison of the milk-clotting properties of three plant extracts. Food Chem. 2013, 141, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, I.; Petrone, G.; Lo Piero, A.R. A kiwi juice aqueous solution as coagulant of bovine milk and its potential in Mozzarella cheese manufacture. Food Bioprod. Process. 2014, 92, 67–72. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Uchikoba, T.; Kaneda, M. Milk-clotting activity of cucumisin, a plant serine protease from melon fruit. Appl. Biochem. Biotechnol. 1996, 56, 325–330. [Google Scholar] [CrossRef]
- Bittante, G.; Penasa, M.; Cecchinato, A. Genetics and modeling of milk coagulation properties. J. Dairy Sci. 2012, 95, 6843–6870. [Google Scholar] [CrossRef]
- MacDougall, D.B. The chemistry of colour and appearance. Food Chem. 1986, 21, 283–299. [Google Scholar] [CrossRef]
- Wyszeck, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Sanz, T.; Salvador, A.; Baixauli, R.; Fiszman, S.M. Evaluation of four types of resistant starch in muffins. II. Effects in texture, colour and consumer response. Eur. Food Res. Technol. 2009, 229, 197–204. [Google Scholar] [CrossRef]
- Rodriguez-Estrada, M.T.; Penazzi, G.; Caboni, M.F.; Bertacco, G.; Lercker, G. Effect of different cooking methods on some lipid and protein components of hamburgers. Meat Sci. 1997, 45, 365–375. [Google Scholar] [CrossRef]
- Sander, B.D.; Addis, P.B.; Park, S.W.; Smith, D.E. Quantification of cholesterol oxidation products in a variety of foods. J. Food Prot. 1989, 52, 109–114. [Google Scholar] [CrossRef]
- Suárez, M.; Macià, A.; Romero, M.P.; Motilva, M.J. Improved liquid chromatography tandem mass spectrometry method for the determination of phenolic compounds in virgin olive oil. J. Chromatogr. A 2008, 1214, 90–99. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phy-tochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of polyphenolic and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Buccioni, A.; Rodriguez-Estrada, M.T.; Conte, G.; Cappucci, A.; Mele, M. Fatty acid composition oxidation status and volatile organic com-pounds in “Colonnata” lard from Large White or Cinta Senese pigs as affected by curing time. Meat Sci. 2014, 97, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.N.; Sun, B.G.; Tian, D.T.; Qu, W.Y. Analysis of volatile compounds in traditional smoke-cured bacon (CSCB) with different fiber coatings using SPME. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties (2005). Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Perez-Cacho, P.R.; Davenport, T.; Rouseff, R. Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. J. Agric. Food Chem. 2007, 55, 1939–1944. [Google Scholar] [CrossRef]
- Povolo, M.; Contarini, G.; Mele, M.; Secchiari, P. Study of the influence of pasture on volatile fraction of sheeps dairy products by solid-phase microextraction and gas chromatography-mass spectrometry. J. Dairy Sci. 2007, 90, 556–559. [Google Scholar] [CrossRef]
- Hilario, M.C.; Delgadillo Puga, C.; Ocaña, A.N.; Pèrez-Gil Romo, F. Antioxidant activity, bioactive polyphenols in Mexican goats’ milk cheeses on summer grazing. J. Dairy Res. 2010, 77, 20–26. [Google Scholar] [CrossRef]
- Piironen, V.; Toivo, J.; Puupponen-Pimiä, R.; Lampi, A.M. Plant sterols in vegetables, fruits and berries. J. Sci. Food Agric. 2003, 83, 330–337. [Google Scholar] [CrossRef]
- Garcia, C.V.; Quek, S.Y.; Stevenson, R.J.; Winz, R.A. Kiwifruit flavour: A review. Trends Food Sci. Technol. 2012, 24, 82–91. [Google Scholar] [CrossRef]
- Uzun, P.; Masucci, F.; Serrapica, F.; Napolitano, F.; Braghieri, A.; Romano, R.; Manzo, N.; Esposito, G.; Di Francia, A. The inclusion of fresh forage in the lactating buffalo diet affects fatty acid and sensory profile of mozzarella cheese. J. Dairy Sci. 2018, 101, 6752–6761. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Conte, G.; Ciucci, F.; Bulleri, E.; Corrales-Retana, L.; Cappucci, A.; Buccioni, A.; Mele, M. Dietary linseed supplementation affects the fatty acid composition of the sn-2 position of triglycerides in sheep milk. J. Dairy Sci. 2018, 101, 6742–6751. [Google Scholar] [CrossRef] [PubMed]
- Cichoscki, A.J.; Valduga, E.; Valduga, A.T.; Tornadijo, M.E.; Fresno, J.M. Characterization of Prato cheese, a Brazilian semi-hard cow variety: Evolution of physico-chemical parameters and mineral composition during ripening. Food Control 2002, 13, 329–336. [Google Scholar] [CrossRef]
- Mukherjee, K.K.; Hutkins, R.W. Isolation of galactose fermenting thermophilic cultures and their use in the manufacture of low browning Mozzarella cheese. J. Dairy Sci. 1994, 77, 2839–2849. [Google Scholar] [CrossRef]
- Cieslik, E.; Greda, A.; Adamus, W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Cuchillo, H.M.; Puga, D.C.; Wrage-Mönning, N.; Espinosa, M.J.G.; Montaño, B.S.; Navarro-Ocaña, A.; Ledesma, J.A.; Díaz, M.M.; Pérez-Gil, R.F. Chemical composition, antioxidant activity and bioactive compounds of vegetation species ingested by goats on semiarid rangelands. J. Anim. Feed Sci. 2013, 22, 106–115. [Google Scholar] [CrossRef]
- Pinelli, P.; Romani, A.; Fierini, E.; Remorini, D.; Agati, G. Characterisation of the Polyphenol Content in the Kiwifruit (Actinidia deliciosa) Exocarp for the Calibration of a Fruit-sorting Optical Sensor. Phytochem. Anal. 2013, 24, 460–466. [Google Scholar] [CrossRef]
- Pandev, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Lehtonen, P.J.; Hopia, A.I. Antioxidant activity of berry and fruit wines and liquors. J. Agric. Food Chem. 1998, 46, 25–31. [Google Scholar] [CrossRef]
- Hagerman, A.E. Phenolic Compounds in Food and Their Effects on Health. I—Analysis, Occurrence and Chemistry; ACS symposium series 506 237–247; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Han, J.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Salmieri, S.; Lacroix, M. Polyphenolic compounds as functional ingredients in cheese. Food Chem. 2011, 124, 1589–1594. [Google Scholar] [CrossRef]
- Bartolomé, B.; Estrella, I.; Hernández, M.T. Interaction of low molecular weight phenolics with proteins (BSA). J. Food Sci. 2000, 65, 617–621. [Google Scholar] [CrossRef]
- Naczk, M.; Oickle, D.; Pink, D.; Shahidi, F. Protein precipitating ca-pacity of crude canola tannins: Effect of pH, tannin and protein concentrations. J. Agric. Food Chem. 1996, 44, 2144–2148. [Google Scholar] [CrossRef]
- Ricardo-da-Silva, J.M.; Cheynier, V.; Souquet, J.M.; Moutounet, M. Interaction of grape seed procyanidins with various proteins in relation to wine fining. J. Sci. Food Agric. 1991, 57, 111–125. [Google Scholar] [CrossRef]
- Serafini, M.; Maiani, G.; Ferro-Luzzi, A. Effect of ethanol on red wine tannin-protein (BSA) interactions. J. Agric. Food Chem. 1997, 45, 3148–3151. [Google Scholar] [CrossRef]
- Spencer, C.M.; Cai, Y.; Martin, R.; Gaffney, S.H.; Goulding, P.N.; Magnolato, D.; Lilley, T.H.; Haslam, E. Polyphenol complexation—Some thoughts and observations. Phytochemistry 1988, 27, 2397–2409. [Google Scholar] [CrossRef]
- Pollack, O.J. Reduction of blood cholesterol in man. Circulation 1953, 7, 702–706. [Google Scholar] [CrossRef]
- Grundy, S.M.; Mok, H.Y. Determination of cholesterol absorption in man by intestinal perfusion. J. Lipid Res. 1977, 18, 263–271. [Google Scholar]
- Mattson, F.H.; Volpenhein, R.A.; Erickson, B.A. Effect of plant sterol esters on the absorption of dietary cholesterol. J. Nutr. 1977, 107, 1139–1146. [Google Scholar] [CrossRef]
- Thompson, G.R. Additive effects of plant sterol and stanol esters to statin therapy. Am. J. Cardiol. 2005, 96, 37–39. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Chen, S.C. Phytosterols—Health benefits and potential concerns: A review. Nutr. Res. 2005, 25, 413–428. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [PubMed]
| Buffalo | Sheep | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMC | BMK | SEM | S | SMC | SMK | SEM | S | ||
| Yield (%) | 51.25 | 33.18 | 2.82 | ** | 24.90 | 21.27 | 1.10 | * | |
| Whey volume (mL) | 84.67 | 120.67 | 7.12 | ** | 130.83 | 143.83 | 1.77 | *** | |
| Yield after 24 h (%) | 38.95 | 27.85 | 1.71 | ** | 23.15 | 20.52 | 0.87 | * | |
| Weight reduction (%) | 23.78 | 15.18 | 1.83 | ** | 6.73 | 3.52 | 0.87 | * | |
| pH whey 24 h | 5.23 | 5.16 | 0.14 | ns | 4.62 | 4.92 | 0.02 | ns | |
| Clotting time (min) | 13.50 | 21.00 | 0.23 | *** | 11.50 | 16.50 | 0.22 | *** | |
| r (min) | 10.96 | n.r. | 3.7 | ne | 7.38 | n.r. | 6.03 | ne | |
| k20 (min) | 1.95 | n.r. | 0.41 | ne | 1.33 | n.r. | 1.66 | ne | |
| a30 (mm) | 42.47 | n.r. | 6.20 | ne | 41.67 | n.r. | 2.86 | ne | |
| Buffalo | Sheep | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMC | BMK | SEM | S | SMC | SMK | SEM | S | ||
| Total solid (g/100 g) | 35.89 | 44.44 | 1.83 | +++ | 43.68 | 50.69 | 0.46 | +++ | |
| Proteins (g/100 g) | 10.76 | 11.74 | 0.26 | + | 15.83 | 15.74 | 0.23 | ns | |
| Lipids (g/100 g) | 20.60 | 27.40 | 1.24 | ++ | 21.72 | 27.55 | 0.36 | +++ | |
| Ashes (g/100 g) | 0.99 | 1.27 | 0.03 | +++ | 1.33 | 1.69 | 0.05 | +++ | |
| Carbohydrates | 4.05 | 4.53 | 0.75 | ns | 5.16 | 5.52 | 0.47 | ns | |
| Fe (μg/g) | 0.71 | 0.81 | 0.09 | ns | 1.57 | 1.93 | 0.09 | ns | |
| Mg (μg/g) | 266.01 | 254.73 | 14.05 | ns | 268.27 | 271.63 | 4.58 | ns | |
| K (μg/g) | 1721.61 | 2136.11 | 132.05 | ns | 1727.81 | 1696.26 | 56.39 | ns | |
| Ca (μg/g) | 7985.18 | 7938.71 | 259.73 | ns | 8131.28 | 8689.25 | 285.27 | ns | |
| Na (μg/g) | 522.83 | 346.34 | 42.47 | + | 1173.25 | 1004.39 | 23.37 | + | |
| Colour | |||||||||
| L* | 93.73 | 92.15 | 0.37 | +++ | 93.12 | 92.09 | 0.24 | + | |
| a* | −1.75 | −1.83 | 0.04 | ns | −1.88 | −1.73 | 0.05 | ns | |
| b* | 8.68 | 10.62 | 0.45 | + | 12.17 | 12.33 | 0.17 | ns | |
| E* | 1.84 | 0.68 | 1.05 | 0.36 | |||||
| Buffalo | Sheep | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMC | BMK | SEM | S | SMC | SMK | SEM | S | ||
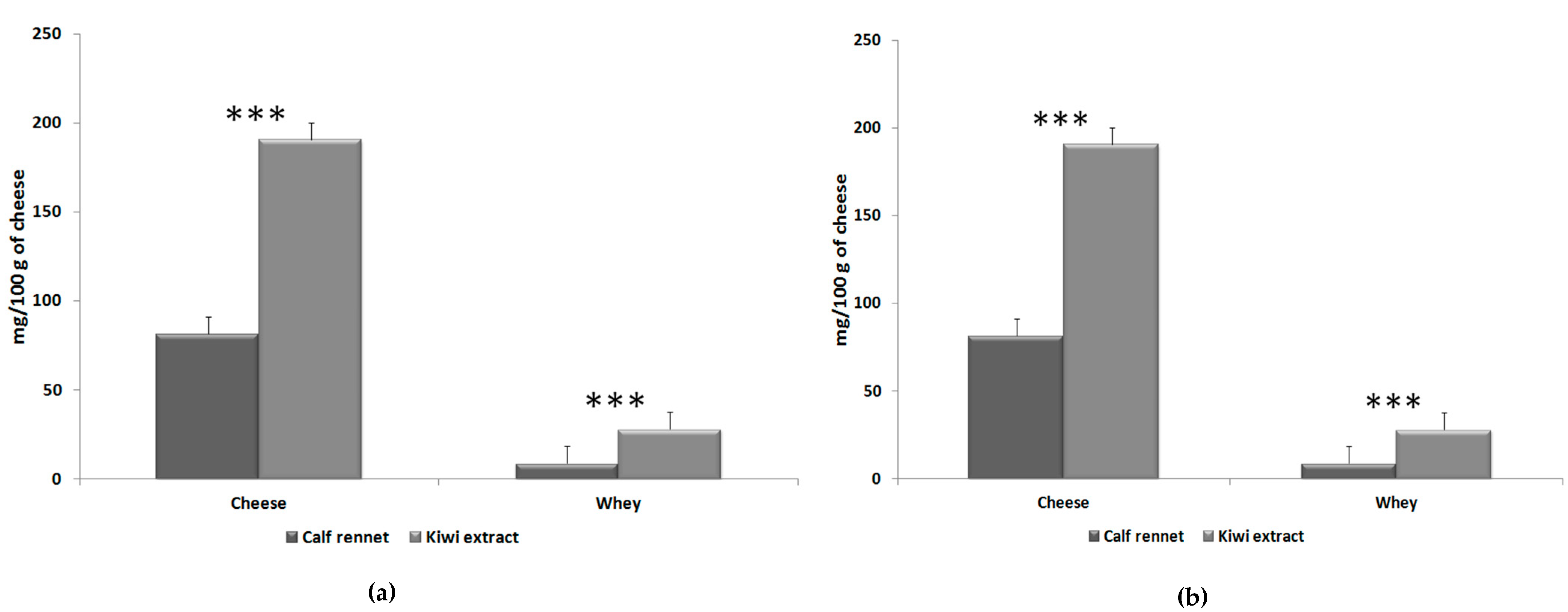
| Total polyphenols | 32.13 | 149.94 | 7.65 | *** | 68.02 | 189.28 | 13.40 | *** | |
| Gallic acid | 15.39 | 15.76 | 1.99 | ns | 34.24 | 15.18 | 4.97 | * | |
| Caffeic acid | 4.16 | 35.97 | 2.72 | * | 6.32 | 11.52 | 1.50 | * | |
| Coumaric acid | - | 21.60 | 1.93 | ne | - | 33.35 | 6.32 | ne | |
| Cinnamic acid | - | 10.78 | 1.31 | ne | - | 27.33 | 6.08 | ne | |
| Quercetin | 2.93 | 9.19 | 0.91 | * | 10.83 | 10.03 | 2.25 | ns | |
| Catechin | 5.49 | 24.03 | 4.44 | * | 5.17 | 43.49 | 3.28 | * | |
| Rutin | 4.15 | 43.85 | 2.91 | *** | - | 58.61 | 9.85 | ne | |
| Sterols | |||||||||
| Cholesterol | 5.71 | 5.51 | 0.14 | ns | 5.96 | 5.76 | 0.18 | ns | |
| Stigmasterol | - | 0.17 | 0.00 | ne | - | 0.83 | 0.04 | ne | |
| Campesterol | - | 0.20 | 0.00 | ne | - | 0.69 | 0.02 | ne | |
| β-sitosterol | - | 0.63 | 0.02 | ne | - | 2.29 | 0.15 | ne | |
| Kiwi | Buffalo | Sheep | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMC | BMK | SEM | S | SMC | SMK | SEM | S | ||||
| Acids | |||||||||||
| Acetic acid | 4.00 | 9.06 | 9.06 | 2.58 | ns | 2.58 | 3.03 | 0.43 | ns | ||
| Butyric acid | 1.56 | 2.26 | 1.58 | 0.48 | ns | 2.72 | 3.27 | 0.74 | ns | ||
| Caproic acid | 3.22 | 6.28 | 3.63 | 1.34 | ns | 7.65 | 9.65 | 2.38 | ns | ||
| heptanoic acid | 1.31 | 0.95 | 1.11 | 0.16 | ns | 0.79 | 0.57 | 0.07 | * | ||
| caprylic acid | 2.31 | 6.17 | 7.23 | 2.88 | ns | 10.53 | 13.95 | 2.83 | ns | ||
| n-nonanoic acid | 3.55 | 4.97 | 3.34 | 1.04 | ns | 3.83 | 4.83 | 0.83 | ns | ||
| n-decanoic acid | 1.69 | 1.78 | 2.61 | 0.76 | ns | 5.36 | 8.21 | 1.60 | ns | ||
| Total | 17.64 | 31.46 | 28.56 | 6.77 | ns | 33.46 | 43.50 | 8.21 | ns | ||
| Alcohols | |||||||||||
| 2-methyl-3-pentanol | - | 1.00 | 2.03 | 0.28 | * | 2.15 | 0.58 | 0.14 | *** | ||
| 2-methyl-1-undecanol | - | 1.39 | 1.98 | 0.53 | ns | 1.83 | 0.90 | 0.13 | *** | ||
| 2-buthyl-1-octanol | - | 0.77 | 0.81 | 0.19 | ns | 1.65 | 0.88 | 0.35 | ns | ||
| 1-pentanol | 2.92 | 0.51 | 0.49 | 0.06 | ns | 0.87 | 1.29 | 0.16 | * | ||
| 1-hexanol | 11.12 | 1.31 | 0.91 | 0.16 | ns | 0.67 | 0.91 | - | ns | ||
| 3,4-hexane diol | - | 3.31 | 6.11 | 1.26 | ns | 3.67 | 9.52 | 0.98 | ** | ||
| 3-hexen-1-ol | 1.67 | - | - | - | - | - | - | - | - | ||
| 2-hexen 1-ol | 12.96 | - | 0.34 | 0.05 | ne | - | 0.46 | 0.07 | ne | ||
| 1-octen-3-ol | 0.99 | 0.30 | 0.49 | 0.03 | ** | 0.11 | 0.15 | 0.01 | * | ||
| 2,4,7,9-tetramethyl-5-decyne-4,7-diol | 1.49 | 2.84 | 0.82 | 0.23 | *** | 1.61 | 2.73 | 0.77 | ns | ||
| Total | 31.11 | 11.42 | 13.97 | 1.64 | ns | 12.56 | 17.41 | 1.79 | ns | ||
| Aldehydes | |||||||||||
| Acetaldehyde | 1.65 | - | - | - | - | 0.16 | 0.21 | 0.09 | ns | ||
| Hexanal | 24.48 | 3.82 | 3.22 | 1.01 | ns | 3.21 | 2.67 | 0.70 | ns | ||
| (E)-2-hexenal | 710.37 | - | 1.06 | 0.09 | ne | 0.09 | 1.82 | 0.26 | *** | ||
| 2-heptenal | 0.52 | - | - | - | - | - | - | - | - | ||
| Nonanal | 6.66 | 0.49 | 0.55 | 0.13 | ns | 0.57 | 0.63 | 0.07 | ns | ||
| 2-octenal | 1.79 | - | - | - | - | - | - | - | - | ||
| Decanal | 0.89 | - | - | - | - | - | - | - | - | ||
| trans-2-decenal | 2.11 | - | - | - | ns | - | - | - | - | ||
| Undec-2-enal | 1.50 | - | - | - | - | - | - | - | |||
| Dibutyl formaldehyde | 7.12 | - | 0.48 | - | ne | - | 0.21 | 0.02 | ne | ||
| Total | 757.09 | 4.31 | 5.31 | 1.17 | ns | 4.03 | 5.54 | 0.88 | ns | ||
| Alkanes and Alkenes | |||||||||||
| 2,2 dimethyl decane | - | 1.07 | 1.21 | 0.37 | ns | 1.53 | 1.01 | 0.34 | ns | ||
| 2,5,6-trimethyldecane | - | 2.90 | 3.79 | 0.61 | ns | 4.86 | 2.79 | 0.66 | * | ||
| 2,5-dimethylnonane | - | 1.52 | - | 0.01 | ne | 1.77 | - | 0.16 | ne | ||
| 2,6,11-trimethyldodecane | - | 1.45 | - | 0.04 | ne | 0.85 | - | 0.12 | ne | ||
| 2,5-dimethylundecane | - | 1.40 | 1.14 | 0.37 | ns | 1.29 | 1.07 | 0.33 | ns | ||
| 2,3 dimethyl nonane | - | 1.13 | 1.32 | 0.28 | ns | 1.53 | 1.30 | 0.26 | ns | ||
| 3 methyl decane | 1.16 | 1.46 | 0.67 | 0.18 | * | 1.27 | 0.98 | 0.24 | ns | ||
| 3-methyl eicosane | 5.19 | - | 0.80 | 0.01 | ne | - | 0.75 | 0.03 | ne | ||
| 5-methyl-undecane | 1.65 | 0.60 | 0.54 | 0.08 | ns | 0.38 | 0.82 | 0.07 | ** | ||
| 4,5 dipropyloctane | - | 1.19 | 2.11 | 0.31 | * | 1.71 | 1.06 | 0.30 | ns | ||
| 5-ethyl decane | - | 2.30 | 1.99 | 0.47 | ns | 2.27 | 2.24 | 0.39 | ns | ||
| 3,5-dimethyl undecane | 1.55 | 0.46 | 0.47 | 0.10 | ns | 0.27 | 0.34 | 0.04 | ns | ||
| 2,4-dimethyl-1-heptene | - | 1.11 | 1.64 | 0.35 | ns | 3.11 | 1.68 | 0.80 | ns | ||
| 5-methyl-1-undecene | - | 1.69 | - | 0.53 | ne | 1.73 | - | 0.11 | ne | ||
| Total | 9.55 | 15.76 | 13.20 | 1.47 | ns | 19.74 | 11.66 | 2.26 | * | ||
| Aromatic hydrocarbons | |||||||||||
| p-xylene | - | 1.28 | 2.16 | 0.97 | ns | 2.34 | 1.40 | 0.57 | ns | ||
| o-xylene | - | 0.29 | 1.79 | 0.61 | ns | 1.65 | 1.09 | 0.47 | ns | ||
| Total | - | 1.57 | 3.95 | 1.54 | ns | 3.99 | 2.48 | 1.03 | ns | ||
| Esters | |||||||||||
| Ethyl acetate | 7.86 | 0.50 | 0.54 | 0.06 | ns | 0.88 | 0.55 | 0.14 | ns | ||
| Ethyl butyrate | 11.98 | - | - | - | - | - | - | - | - | ||
| Ethyl caproate | 11.43 | - | 0.42 | 0.01 | ne | - | 0.15 | - | - | ||
| Ethyl caprilate | 1.39 | - | - | - | - | - | - | - | - | ||
| n-heptyl formate | 1.84 | 0.42 | 0.37 | 0.06 | ns | - | - | - | - | ||
| Total | 34.50 | 0.92 | 1.33 | 0.07 | ** | 0.88 | 0.70 | 0.13 | ns | ||
| Ketones | |||||||||||
| 2,3-butenedione | - | 7.66 | 6.53 | 1.11 | ns | 4.94 | 5.17 | 0.72 | ns | ||
| 1-penten-3-one | 2.05 | - | - | - | - | - | - | - | - | ||
| 2,3 pentanedione | - | 4.70 | 5.20 | 0.77 | ns | 2.82 | 2.23 | 0.41 | ns | ||
| 2-butanone | - | 30.77 | 38.30 | 4.69 | ns | 38.93 | 40.90 | 5.36 | ns | ||
| 3-pentanone 2-hydroxy | - | 2.14 | 3.90 | 0.64 | * | 2.32 | 5.70 | 0.54 | ** | ||
| 2-nonanone | - | 0.66 | 0.43 | 0.07 | ns | 0.66 | 0.77 | 0.12 | ns | ||
| Total | 2.05 | 46.42 | 52.66 | 6.62 | ns | 49.54 | 55.72 | 6.29 | ns | ||
| Terpenes | |||||||||||
| β-phellandrene | 2.95 | 0.12 | 0.25 | 0.01 | *** | 0.45 | 0.66 | 0.04 | ** | ||
| m-cymene | 1.79 | - | - | - | - | - | - | - | - | ||
| p-mentha-1,4(8)-diene | 2.26 | - | - | - | - | - | - | - | - | ||
| Pinocanphone | 2.61 | - | - | - | - | - | - | - | - | ||
| 3-pinanone | 6.21 | - | - | - | - | - | - | - | - | ||
| 2-pinen-4-one | 4.64 | - | - | - | - | - | - | - | - | ||
| Total | 20.46 | 0.12 | 0.25 | 0.01 | *** | 0.45 | 0.66 | 0.04 | ** | ||
| Others | |||||||||||
| 2-ethyl-hexyl tert-butyl ether | - | 1.44 | 1.92 | 0.28 | ns | 1.28 | 0.96 | 0.21 | ns | ||
| Dimethyl disulfide | - | 0.57 | 0.95 | 0.24 | ns | - | - | - | - | ||
| 2 ethyl hexyl chloroformate | - | 2.95 | 6.15 | 2.80 | ns | 2.81 | 3.61 | 0.83 | ns | ||
| a-ethyl-furan | 8.12 | - | - | - | - | 0.42 | 0.33 | 0.09 | ns | ||
| m-d-tert-butyl-benzene | - | 0.26 | 0.45 | 0.09 | ns | 0.71 | 0.60 | 0.15 | ns | ||
| Ethylhexanol | 4.83 | - | - | - | - | - | - | - | - | ||
| 1-cyclopropyl pentane | 2.33 | 0.23 | 0.22 | 0.04 | ns | 0.39 | 0.33 | 0.05 | ns | ||
| 1-hexyl-2-methylcyclopropane | 1.78 | 0.42 | 0.44 | 0.10 | ns | 0.40 | 0.23 | 0.03 | ns | ||
| Ethyl benzene carboxylate | 3.88 | - | - | - | - | - | - | - | |||
| Total | 20.94 | 5.85 | 10.11 | 2.48 | ns | 6.01 | 6.06 | 1.13 | ns | ||
| Total VOCs | 893.34 | 117.85 | 129.34 | 15.61 | ns | 130.88 | 143.55 | 11.91 | ns | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, A.; Conte, G.; Corrales-Retana, L.; Casarosa, L.; Ciucci, F.; Mele, M. Nutraceutical and Technological Properties of Buffalo and Sheep Cheese Produced by the Addition of Kiwi Juice as a Coagulant. Foods 2020, 9, 637. https://doi.org/10.3390/foods9050637
Serra A, Conte G, Corrales-Retana L, Casarosa L, Ciucci F, Mele M. Nutraceutical and Technological Properties of Buffalo and Sheep Cheese Produced by the Addition of Kiwi Juice as a Coagulant. Foods. 2020; 9(5):637. https://doi.org/10.3390/foods9050637
Chicago/Turabian StyleSerra, Andrea, Giuseppe Conte, Leonor Corrales-Retana, Laura Casarosa, Francesca Ciucci, and Marcello Mele. 2020. "Nutraceutical and Technological Properties of Buffalo and Sheep Cheese Produced by the Addition of Kiwi Juice as a Coagulant" Foods 9, no. 5: 637. https://doi.org/10.3390/foods9050637
APA StyleSerra, A., Conte, G., Corrales-Retana, L., Casarosa, L., Ciucci, F., & Mele, M. (2020). Nutraceutical and Technological Properties of Buffalo and Sheep Cheese Produced by the Addition of Kiwi Juice as a Coagulant. Foods, 9(5), 637. https://doi.org/10.3390/foods9050637