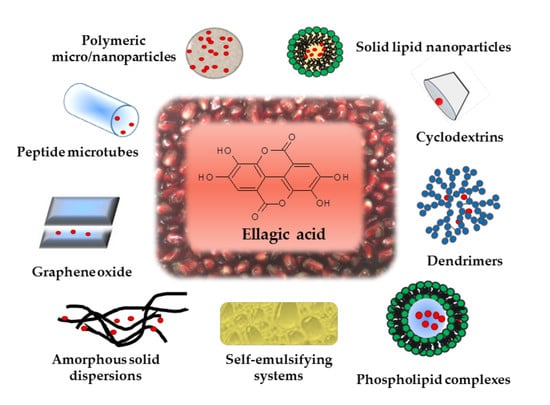
Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid
Abstract
Share and Cite
Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Appl. Sci. 2020, 10, 3353. https://doi.org/10.3390/app10103353
Zuccari G, Baldassari S, Ailuno G, Turrini F, Alfei S, Caviglioli G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Applied Sciences. 2020; 10(10):3353. https://doi.org/10.3390/app10103353
Chicago/Turabian StyleZuccari, Guendalina, Sara Baldassari, Giorgia Ailuno, Federica Turrini, Silvana Alfei, and Gabriele Caviglioli. 2020. "Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid" Applied Sciences 10, no. 10: 3353. https://doi.org/10.3390/app10103353
APA StyleZuccari, G., Baldassari, S., Ailuno, G., Turrini, F., Alfei, S., & Caviglioli, G. (2020). Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Applied Sciences, 10(10), 3353. https://doi.org/10.3390/app10103353