The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Guided Irradiation of Liver Tissue of Sirt3+/+ and Sirt3−/− Male Mice
2.2. Immunohistochemistry and Histopathology Analysis
2.3. Real Time Quantitative Reverse Transcription PCR (qRT-PCR)
2.4. Antioxidant Enzyme Activity Measurements
2.5. Bilirubin Assay
2.6. Statistical Analysis
3. Results
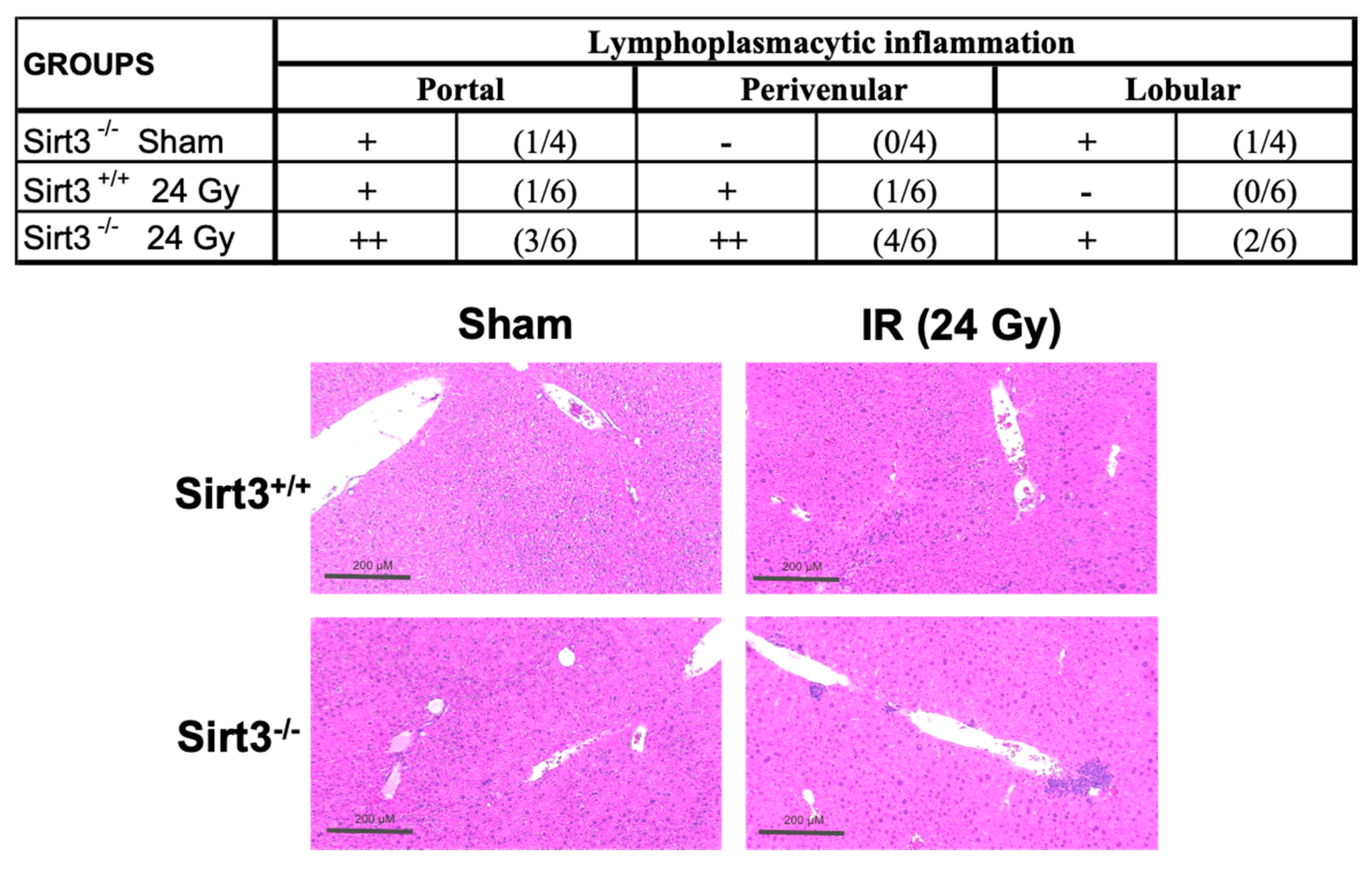
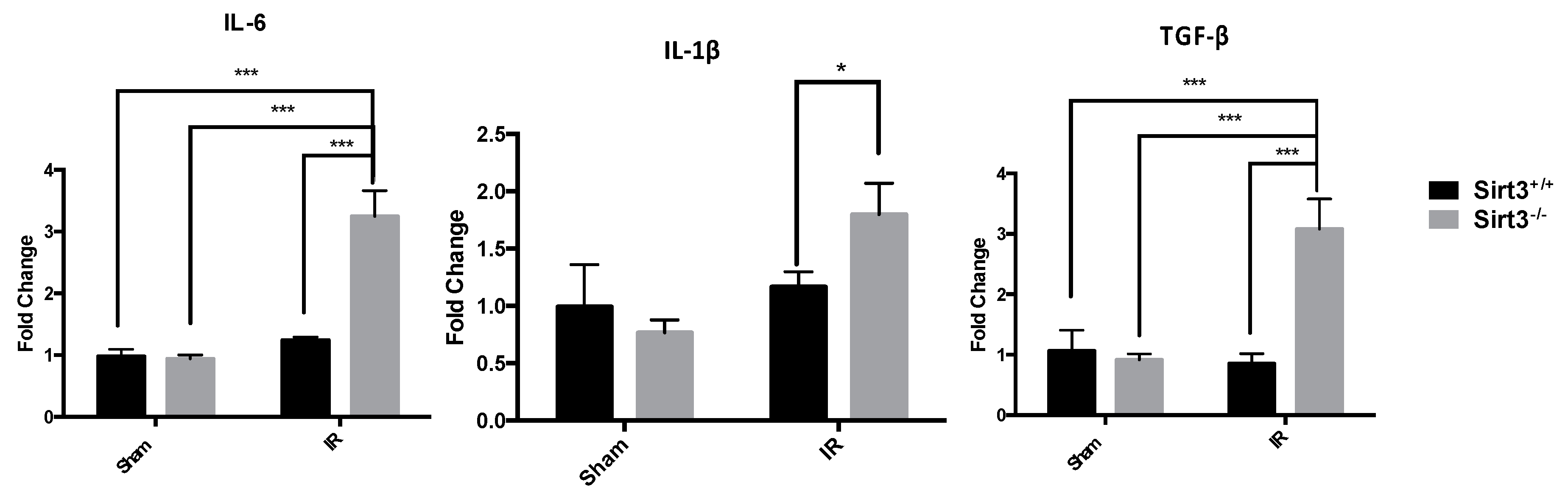
3.1. Exposure to 24 Gy IR Increased the Expression of Inflammatory Markers as Well as Lymphoplasmacytic Inflammation in the Livers of Sirt3−/− Mice
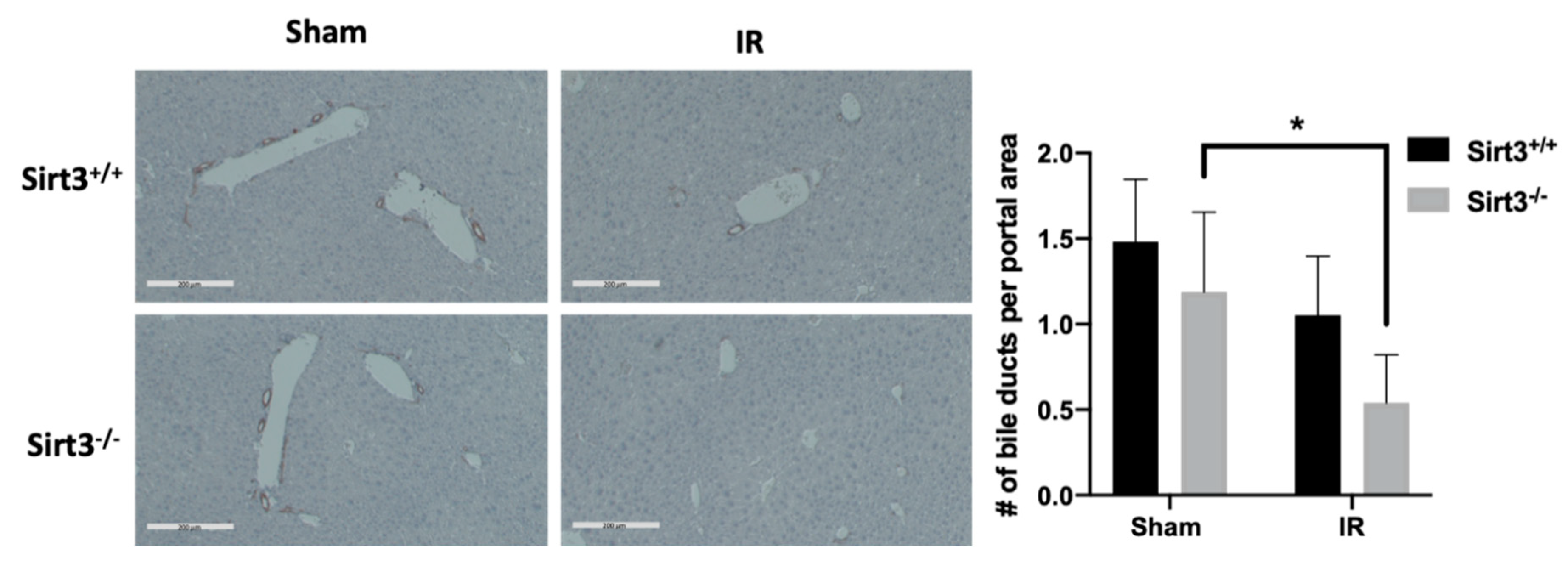
3.2. The Number of Bile Ducts Was Decreased in Irradiated Livers of Sirt3−/− Mice
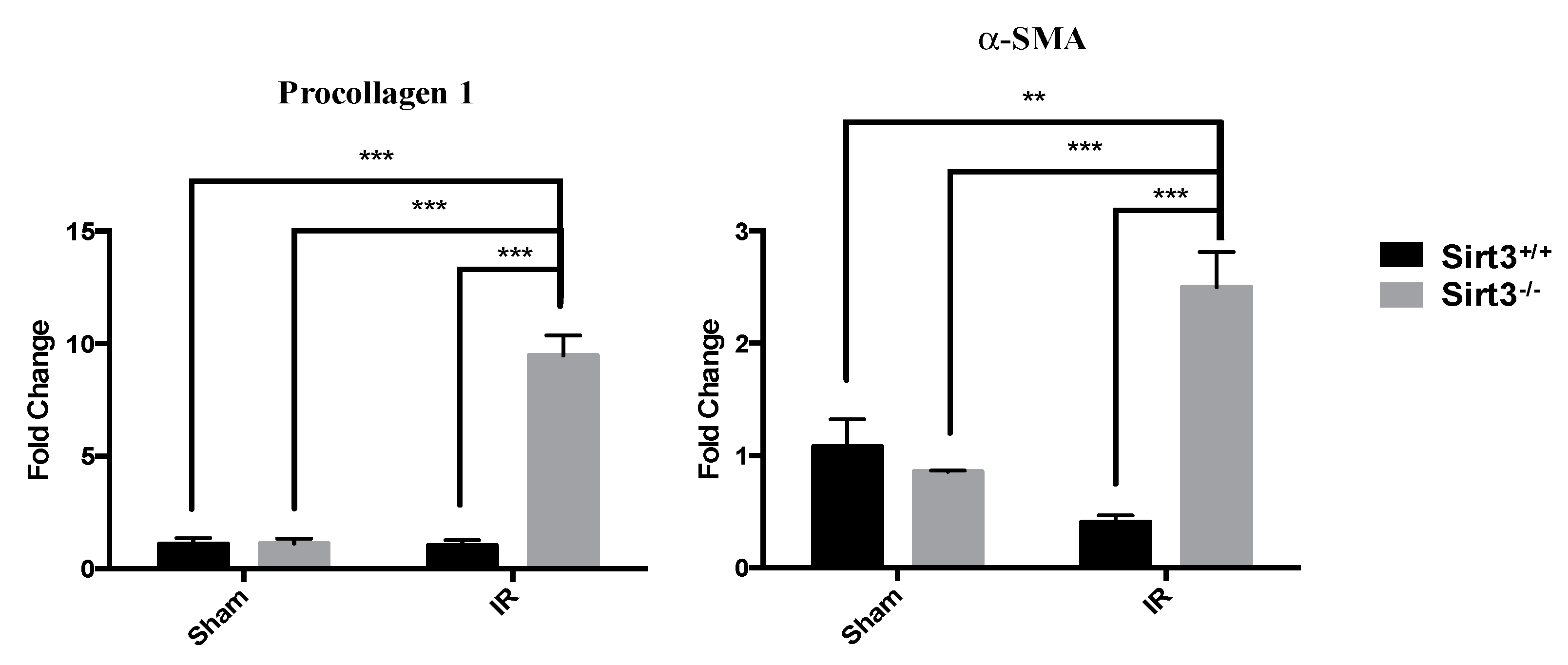
3.3. Exposure to 24 Gy IR Increased the Expression of Profibrotic Markers in the Livers of Sirt3−/− Mice but Did Not Cause Fibrosis at 6 Months after IR Exposure
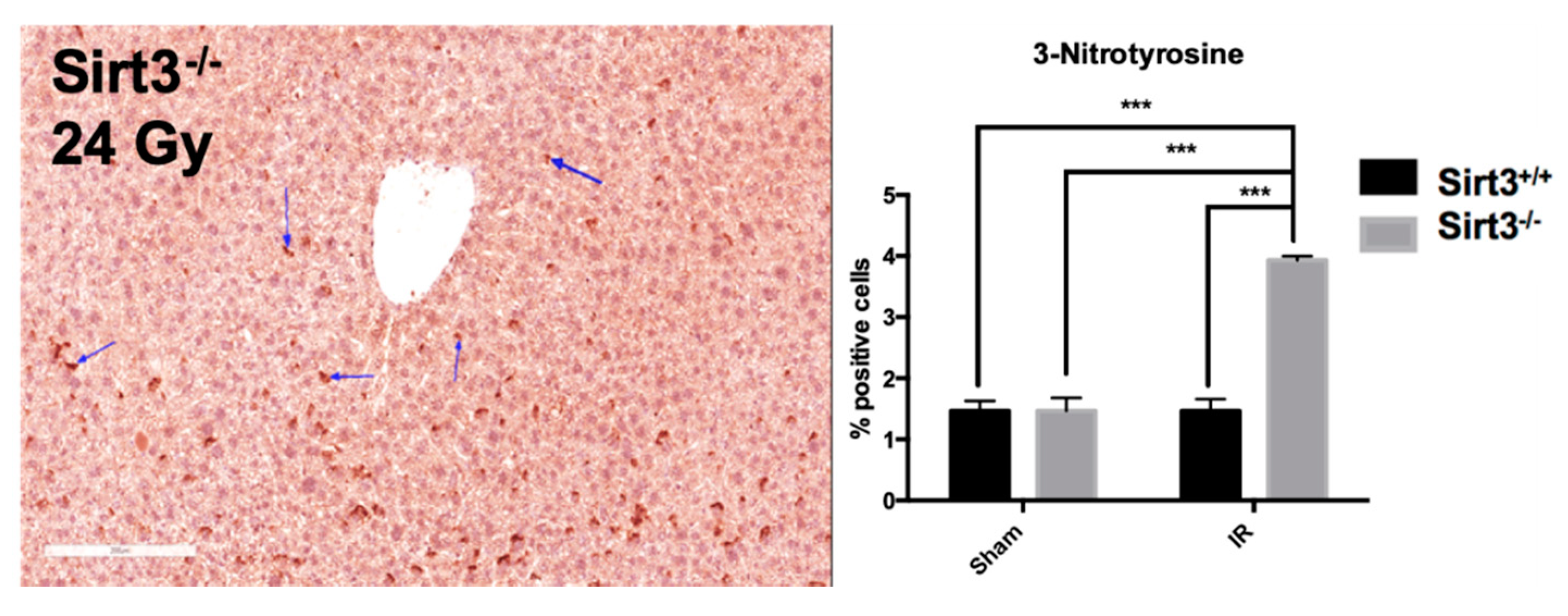
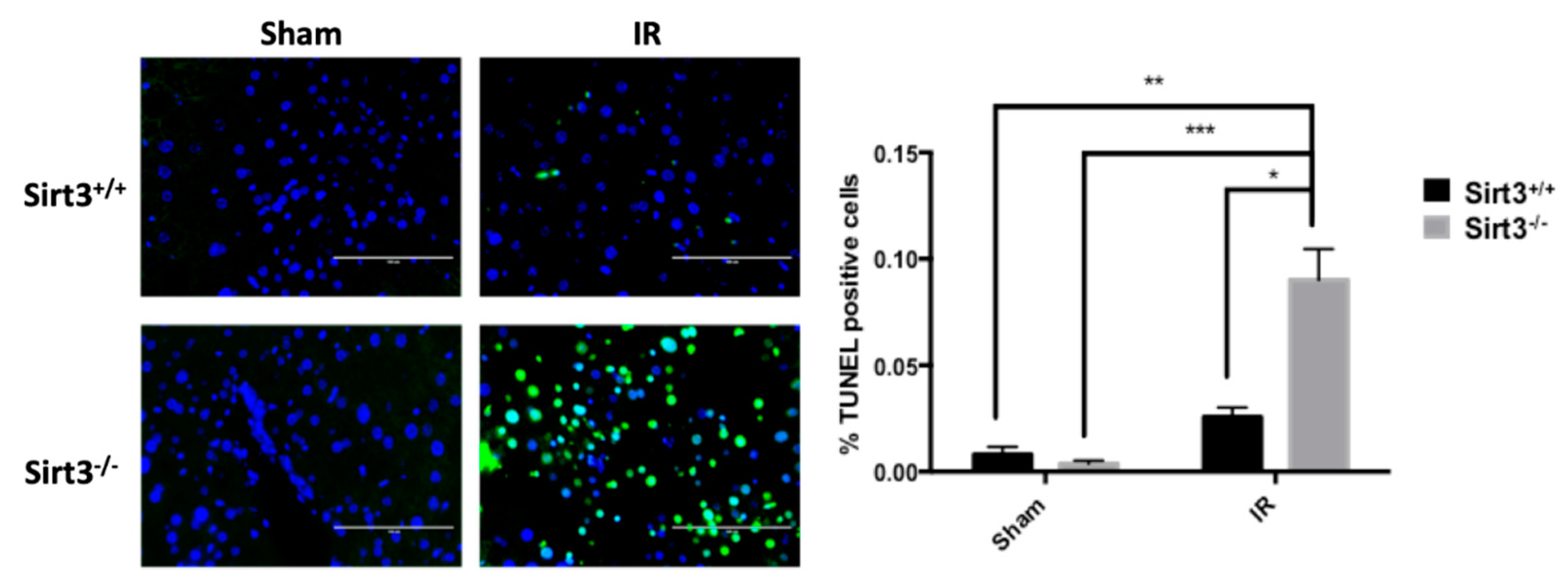
3.4. Persistent Significant Increases in DNA Damage and Oxidative Stress in Liver Tissue of Sirt3−/− Mice Following 24 Gy IR
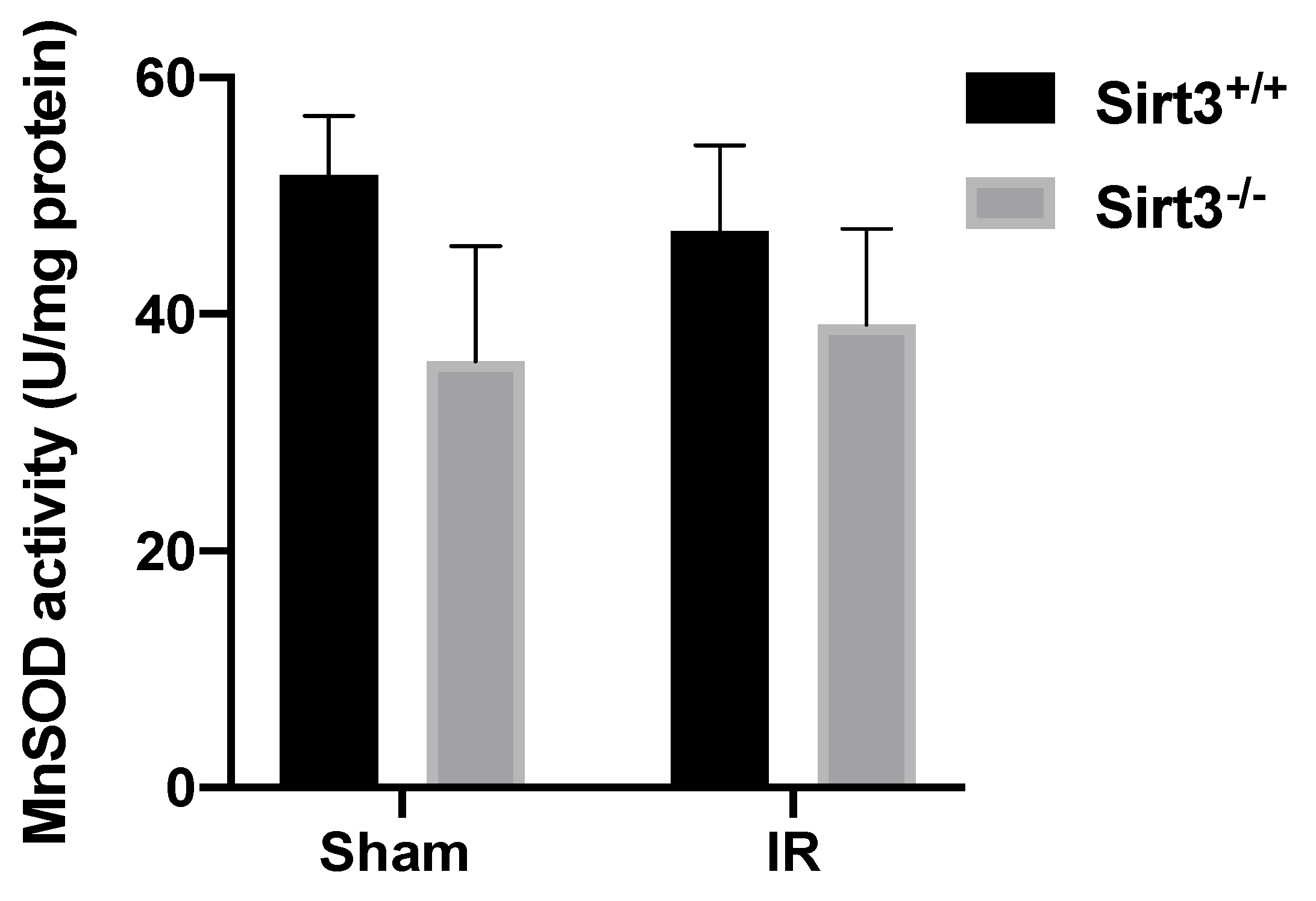
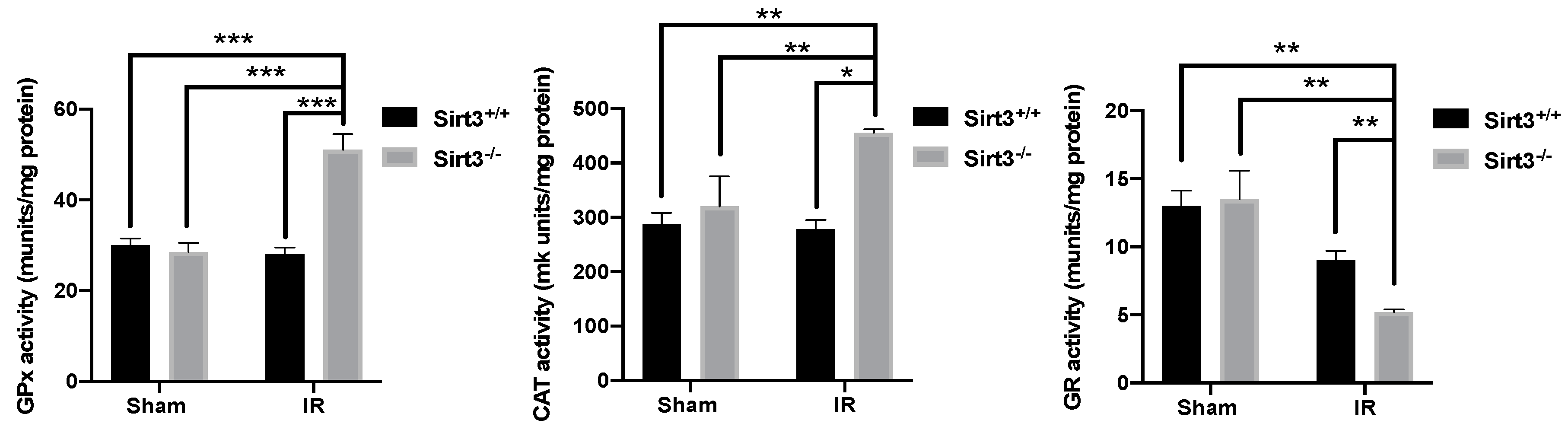
3.5. Mice Lacking SIRT3 Demonstrated Altered Activity of Antioxidant Enzymes, Which Are Responsible for Peroxide Removal in Liver Tissue Following 24 Gy IR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp. Mol. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.; Madan, R.; Kilambi, R.; Chander, S. Radiation induced liver disease: A clinical update. J. Egypt Natl. Canc. Inst. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Ten Haken, R.K. Radiation-Associated Liver Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Normolle, D.; Ensminger, W.D.; Walker, S.; Tatro, D.; Ten Haken, R.K.; Knol, J.; Dawson, L.A.; Pan, C.; Lawrence, T.S. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2005. [Google Scholar] [CrossRef]
- Feng, M.; Smith, D.E.; Normolle, D.P.; Knol, J.A.; Pan, C.C.; Ben-Josef, E.; Lu, Z.; Feng, M.R.; Chen, J.; Ensminger, W.; et al. A phase i clinical and pharmacology study using amifostine as a radioprotector in dose-escalated whole liver radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012. [Google Scholar] [CrossRef]
- Munoz-Schuffenegger, P.; Ng, S.; Dawson, L.A. Radiation-Induced Liver Toxicity. Semin. Radiat. Oncol. 2017. [Google Scholar] [CrossRef]
- Tanguturi, S.K.; Wo, J.Y.; Zhu, A.X.; Dawson, L.A.; Hong, T.S. Radiation Therapy for Liver Tumors: Ready for Inclusion in Guidelines? Oncologist 2014. [Google Scholar] [CrossRef][Green Version]
- Toesca, D.A.S.; Ibragimov, B.; Koong, A.J.; Xing, L.; Koong, A.C.; Chang, D.T. Strategies for prediction and mitigation of radiation-induced liver toxicity. J. Radiat. Res. 2018, 59, i40–i49. [Google Scholar] [CrossRef]
- Tai, A.; Erickson, B.; Li, X.A. Extrapolation of Normal Tissue Complication Probability for Different Fractionations in Liver Irradiation. Int. J. Radiat. Onco.l Biol. Phys. 2009, 74, 283–289. [Google Scholar] [CrossRef]
- Lee, I.J.; Seong, J.; Shim, S.J.; Han, K.H. Radiotherapeutic Parameters Predictive of Liver Complications Induced by Liver Tumor Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, L.F.; Colby, T.V. Pathogenesis of veno-occlusive liver disease after radiation. Arch. Pathol. Lab. Med. 1980, 104, 584–588. [Google Scholar] [PubMed]
- Guha, C.; Kavanagh, B.D. Hepatic Radiation Toxicity: Avoidance and Amelioration. Semin. Radiat. Oncol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Coia, L.R.; Myerson, R.J.; Tepper, J.E. Late effects of radiation therapy on the gastrointestinal tract. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1213–1236. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Liu, C.Y.; Shueng, P.W.; Chong, N.S.; Chen, C.J.; Chen, M.J.; Lin, C.C.; Wang, T.E.; Lin, S.C.; Tai, H.C.; et al. Comparison of coplanar and noncoplanar intensity-modulated radiation therapy and helical tomotherapy for hepatocellular carcinoma. Radiat. Oncol. 2010. [Google Scholar] [CrossRef]
- Yamashita, H.; Onishi, H.; Murakami, N.; Matsumoto, Y.; Matsuo, Y.; Nomiya, T.; Nakagawa, K. Survival outcomes after stereotactic body radiotherapy for 79 Japanese patients with hepatocellular carcinoma. J. Radiat. Res. 2014. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.W.; Fan, C.C.; Ting, L.L.; Kuo, C.C.; Chiou, J.F. Clinical parameters for predicting radiation-induced liver disease after intrahepatic reirradiation for hepatocellular carcinoma. Radiat. Oncol. 2016. [Google Scholar] [CrossRef][Green Version]
- Jung, J.; Yoon, S.M.; Kim, S.Y.; Cho, B.; Park, J.H.; Kim, S.S.; Song, S.Y.; Lee, S.W.; Do Ahn, S.; Choi, E.K.; et al. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: Clinical and dose-volumetric parameters. Radiat. Oncol. 2013. [Google Scholar] [CrossRef]
- Fry, R.J.M.; Hall, E.J. Radiobiology for the Radiologist. Radiat Res. 1995. [Google Scholar] [CrossRef]
- Lautt, W.W. Hepatic Circulation Physiology and Pathophysiology; Morgan & Claypool Publishers: San Rafael, CA, USA, 2009. [Google Scholar] [CrossRef]
- Sharp, G.B. The Relationship between Internally Deposited Alpha-Particle Radiation and Subsite-Specific Liver Cancer and Liver Cirrhosis: An Analysis of Published Data. J. Radiat. Res. 2002, 43, 371–380. Available online: https://academic.oup.com/jrr/article-abstract/43/4/371/986795 (accessed on 30 March 2020). [CrossRef]
- Formenti, S.C.; Demaria, S. Local control by radiotherapy: Is that all there is? Breast Cancer Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.E.C.; Zhao, W. Chronic oxidative stress and radiation-induced late normal tissue injury: A review. Int. J. Radiat. Biol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011. [Google Scholar] [CrossRef] [PubMed]
- Castilla, A.; Prieto, J.; Fausto, N. Transforming Growth Factors β1 and α in Chronic Liver Disease Effects of Interferon Alfa Therapy. N. Engl. J. Med. 1991. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hao, J.; Zhang, X.; Yu, B.; Ren, J.; Luo, C.; Li, Q.; Huang, Q.; Shi, X.; Li, W.; et al. The polyhydroxylated fullerene derivative C60(OH)24 protects mice from ionizing-radiation-induced immune and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018. [Google Scholar] [CrossRef]
- Richardson, R.B.; Harper, M.E. Mitochondrial stress controls the radiosensitivity of the oxygen effect: Implications for radiotherapy. Oncotarget 2016. [Google Scholar] [CrossRef]
- Coleman, M.C.; Olivier, A.K.; Jacobus, J.A.; Mapuskar, K.A.; Mao, G.; Martin, S.M.; Riley, D.P.; Gius, D.; Spitz, D.R. Superoxide mediates acute liver injury in irradiated mice lacking Sirtuin 3. Antioxid. Redox Signal. 2014. [Google Scholar] [CrossRef]
- Tao, R.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.H.; Jiang, H.; Kim, H.S.; Flynn, C.R.; Hill, S.; McDonald, W.H.; et al. Sirt3-Mediated Deacetylation of Evolutionarily Conserved Lysine 122 Regulates MnSOD Activity in Response to Stress. Mol. Cell. 2010. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, Y.; Park, S.H.; Liu, G.; O’Brien, J.; Jiang, H.; Gius, D. SIRT3-mediated dimerization of IDH2 directs cancer cell metabolism and tumor growth. Cancer Res. 2017. [Google Scholar] [CrossRef]
- Yu, W.; Dittenhafer-Reed, K.E.; Denu, J.M. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 2012. [Google Scholar] [CrossRef] [PubMed]
- Jing, E.; O’Neill, B.T.; Rardin, M.J.; Kleinridders, A.; Ilkeyeva, O.R.; Ussar, S.; Bain, J.R.; Lee, K.Y.; Verdin, E.M.; Newgard, C.B.; et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 2013. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, A.A.; Choudhury, M.; Rahman, S.M.; McCURDY, C.E.; Friederich, M.; Van Hove, J.L.; Watson, P.A.; Birdsey, N.; Bao, J.; Gius, D.; et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem. J. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, B.; Bossy-Wetzel, E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging. Neurosci. 2013. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010. [Google Scholar] [CrossRef]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 Reverses Aging-Associated Degeneration. Cell Rep. 2013. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Zhang, T.; Tong, Q.; Ye, R.D.; Lin, L. SIRT3 protects hepatocytes from oxidative injury by enhancing ROS scavenging and mitochondrial integrity. Cell Death Dis. 2017. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Samant, S.A.; Pillai, V.B.; Rajamohan, S.B.; Gupta, M.P. SIRT3 Is a Stress-Responsive Deacetylase in Cardiomyocytes That Protects Cells from Stress-Mediated Cell Death by Deacetylation of Ku70. Mol. Cell Biol. 2008. [Google Scholar] [CrossRef]
- Ansari, A.; Rahman, M.S.; Saha, S.K.; Saikot, F.K.; Deep, A.; Kim, K.H. unction of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell. 2017. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011. [Google Scholar] [CrossRef]
- Kim, H.S.; Patel, K.; Muldoon-Jacobs, K.; Bisht, K.S.; Aykin-Burns, N.; Pennington, J.D.; van der Meer, R.; Nguyen, P.; Savage, J.; Owens, K.M.; et al. SIRT3 Is a Mitochondria-Localized Tumor Suppressor Required for Maintenance of Mitochondrial Integrity and Metabolism during Stress. Cancer Cell. 2010. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ozden, O.; Jiang, H.; Cha, Y.I.; Pennington, J.D.; Aykin-Burns, N.; Spitz, D.R.; Gius, D.; Kim, H.S. Sirt3, mitochondrial ROS, ageing, and carcinogenesis. Int. J. Mol. Sci. 2011, 12, 6226–6239. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under Caloric Restriction. Cell 2010. [Google Scholar] [CrossRef]
- Zeng, H.; Li, L.; Chen, J.X. Loss of Sirt3 limits bone marrow cell-mediated angiogenesis and cardiac repair in post-myocardial infarction. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Alam, S.; Carter, G.S.; Krager, K.J.; Li, X.; Lehmler, H.-J.; Aykin-Burns, N. PCB11 Metabolite, 3,3′-Dichlorobiphenyl-4-ol, Exposure Alters the Expression of Genes Governing Fatty Acid Metabolism in the Absence of Functional Sirtuin 3: Examining the Contribution of MnSOD. Antioxidants 2018, 7, 121. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976. [Google Scholar] [CrossRef]
- Pinto, R.E.; Bartley, W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem. J. 1969. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989. [Google Scholar] [CrossRef]
- Barshishat-Kupper, M.; Tipton, A.J.; McCart, E.A.; McCue, J.; Mueller, G.P.; Day, R.M. Effect of ionizing radiation on liver protein oxidation and metabolic function in C57BL/6J mice. Int. J. Radiat. Biol. 2014. [Google Scholar] [CrossRef]
- Maeda, M.; Ishikawa, H.; Yoshida, Y.; Takahashi, T.; Ohkubo, Y.; Musha, A.; Komachi, M.; Nakazato, Y.; Nakano, T. Long-term pathological and immunohistochemical features in the liver after intraoperative whole-liver irradiation in rats. J. Radiat. Res. 2014. [Google Scholar] [CrossRef]
- Osmundson, E.C.; Wu, Y.; Luxton, G.; Bazan, J.G.; Koong, A.C.; Chang, D.T. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int. J. Radiat. Oncol. Biol. Phys. 2015. [Google Scholar] [CrossRef] [PubMed]
- Seidensticker, M.; Burak, M.; Kalinski, T.; Garlipp, B.; Koelble, K.; Wust, P.; Antweiler, K.; Seidensticker, R.; Mohnike, K.; Pech, M.; et al. Radiation-Induced Liver Damage: Correlation of Histopathology with Hepatobiliary Magnetic Resonance Imaging, a Feasibility Study. Cardiovasc. Interven. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Hauer-Jensen, M. Ionizing radiation-induced responses: Where free radical chemistry meets redox biology and medicine. Antioxidants Redox Signal. 2014. [Google Scholar] [CrossRef]
- Tamminga, J.; Kovalchuk, O. Role of DNA Damage and Epigenetic DNA Methylation Changes in Radiation-Induced Genomic Instability and Bystander Effects in Germline In Vivo. Curr. Mol. Pharmacol. 2012. [Google Scholar] [CrossRef]
- Barjaktarovic, Z.; Shyla, A.; Azimzadeh, O.; Schulz, S.; Haagen, J.; Dörr, W.; Sarioglu, H.; Atkinson, M.J.; Zischka, H.; Tapio, S. Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiother. Oncol. 2013. [Google Scholar] [CrossRef]
- Morgan, W.F.; Day, J.P.; Kaplan, M.I.; McGhee, E.M.; Limoli, C.L. Genomic Instability Induced by Ionizing Radiation. Radiat. Res. 1996. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010. [Google Scholar] [CrossRef]
- Fausto, N.; Mead, J.E.; Gruppuso, P.A.; Castilla, A.; Jakowlew, S.B. Effects of TGF-beta s in the liver: Cell proliferation and fibrogenesis. Ciba Found. Symp. 1991. [Google Scholar] [CrossRef]
- Jin, X.; Zimmers, T.A.; Perez, E.A.; Pierce, R.H.; Zhang, Z.; Koniaris, L.G. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology 2006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective Effects of Sirtuin 3 in a Murine Model of Sepsis-Induced Acute Kidney Injury. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Salunga, T.L.; Cui, Z.G.; Shimoda, S.; Zheng, H.C.; Nomoto, K.; Kondo, T.; Takano, Y.; Selmi, C.; Alpini, G.; Gershwin, M.E.; et al. Oxidative Stress-Induced Apoptosis of Bile Duct Cells in Primary Biliary Cirrhosis. J. Autoimmun. 2007. [Google Scholar] [CrossRef] [PubMed]
- Erice, O.; Munoz-Garrido, P.; Vaquero, J.; Perugorria, M.J.; Fernandez-Barrena, M.G.; Saez, E.; Santos-Laso, A.; Arbelaiz, A.; Jimenez-Agüero, R.; Fernandez-Irigoyen, J.; et al. MicroRNA-506 Promotes Primary Biliary Cholangitis–like Features in Cholangiocytes and Immune Activation. Hepatology 2018. [Google Scholar] [CrossRef]
- Degott, C.; Feldmann, G.; Larrey, D.; Durand-Schneider, A.M.; Grange, D.; Machayekhi, J.P.; Moreau, A.; Potet, F.; Benhamou, J.P. Drug-induced prolonged cholestasis in adults: A histological semiquantitative study demonstrating progressive ductopenia. Hepatology 1992. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, R.; Wang, G.; Chen, Z.; Li, Y.; Zhao, Y.; Liu, D.; Zhao, H.; Zhang, F.; Yao, J.; et al. SIRT3-Mediated Deacetylation of PRDX3 Alleviates Mitochondrial Oxidative Damage and Apoptosis Induced by Intestinal Ischemia/Reperfusion Injury. Redox Biol. 2019. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
LoBianco, F.V.; Krager, K.J.; Carter, G.S.; Alam, S.; Yuan, Y.; Lavoie, E.G.; Dranoff, J.A.; Aykin-Burns, N. The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury. Antioxidants 2020, 9, 409. https://doi.org/10.3390/antiox9050409
LoBianco FV, Krager KJ, Carter GS, Alam S, Yuan Y, Lavoie EG, Dranoff JA, Aykin-Burns N. The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury. Antioxidants. 2020; 9(5):409. https://doi.org/10.3390/antiox9050409
Chicago/Turabian StyleLoBianco, Francesca V., Kimberly J. Krager, Gwendolyn S. Carter, Sinthia Alam, Youzhong Yuan, Elise G. Lavoie, Jonathan A. Dranoff, and Nukhet Aykin-Burns. 2020. "The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury" Antioxidants 9, no. 5: 409. https://doi.org/10.3390/antiox9050409
APA StyleLoBianco, F. V., Krager, K. J., Carter, G. S., Alam, S., Yuan, Y., Lavoie, E. G., Dranoff, J. A., & Aykin-Burns, N. (2020). The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury. Antioxidants, 9(5), 409. https://doi.org/10.3390/antiox9050409





