Protein S for Portal Vein Thrombosis in Cirrhotic Patients Waiting for Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Examination and Data Collection
2.3. Statistical Analysis
3. Result
3.1. Patients
3.2. Clinical Difference between Patients with or without PVT
3.3. Risk Factors of PVT Development
3.4. Diagnostic Accuracy of PVT on Imaging Study
3.5. Liver Transplantation
3.6. Measurement of Portal Flow
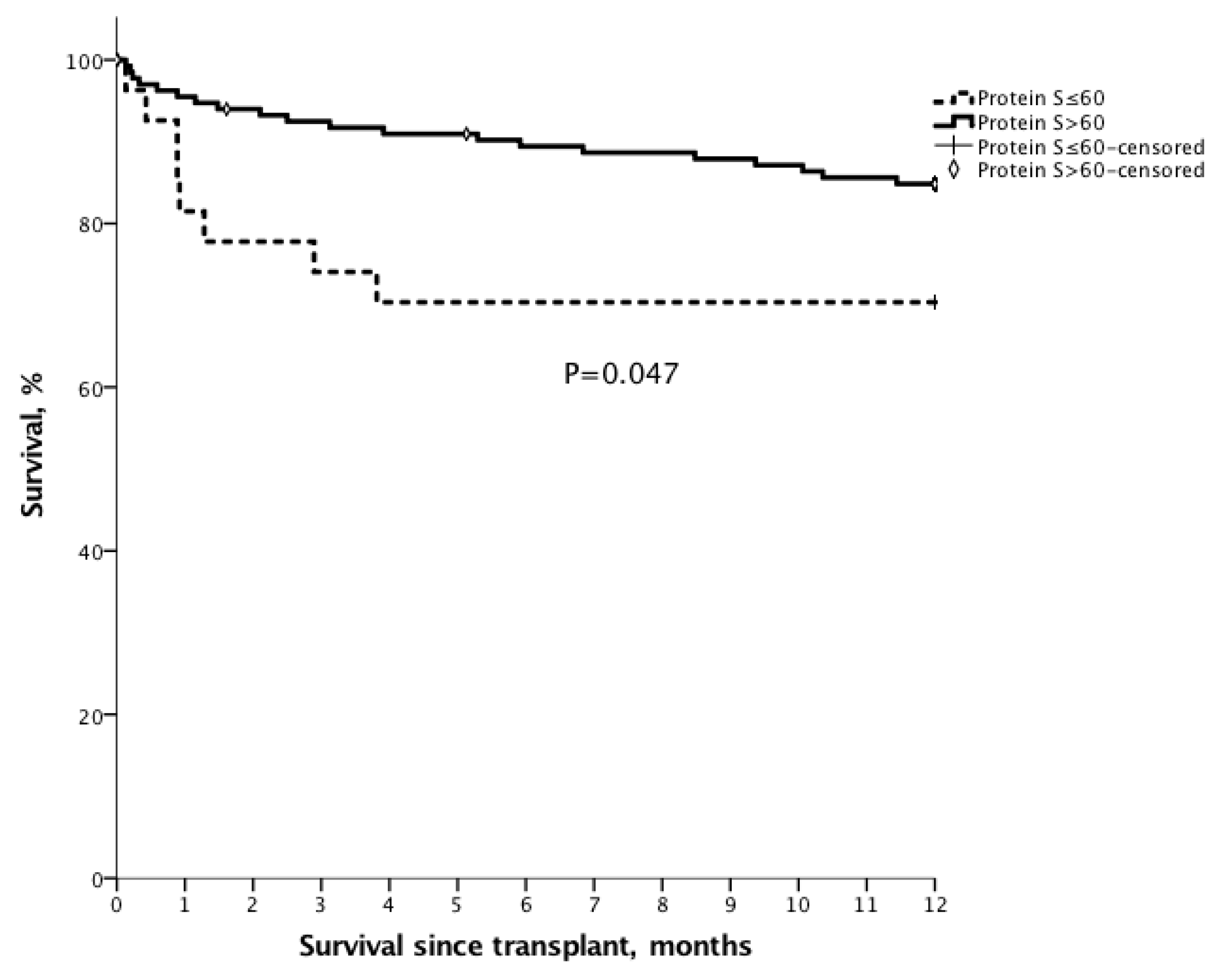
3.7. Outcomes after Liver Transplantation
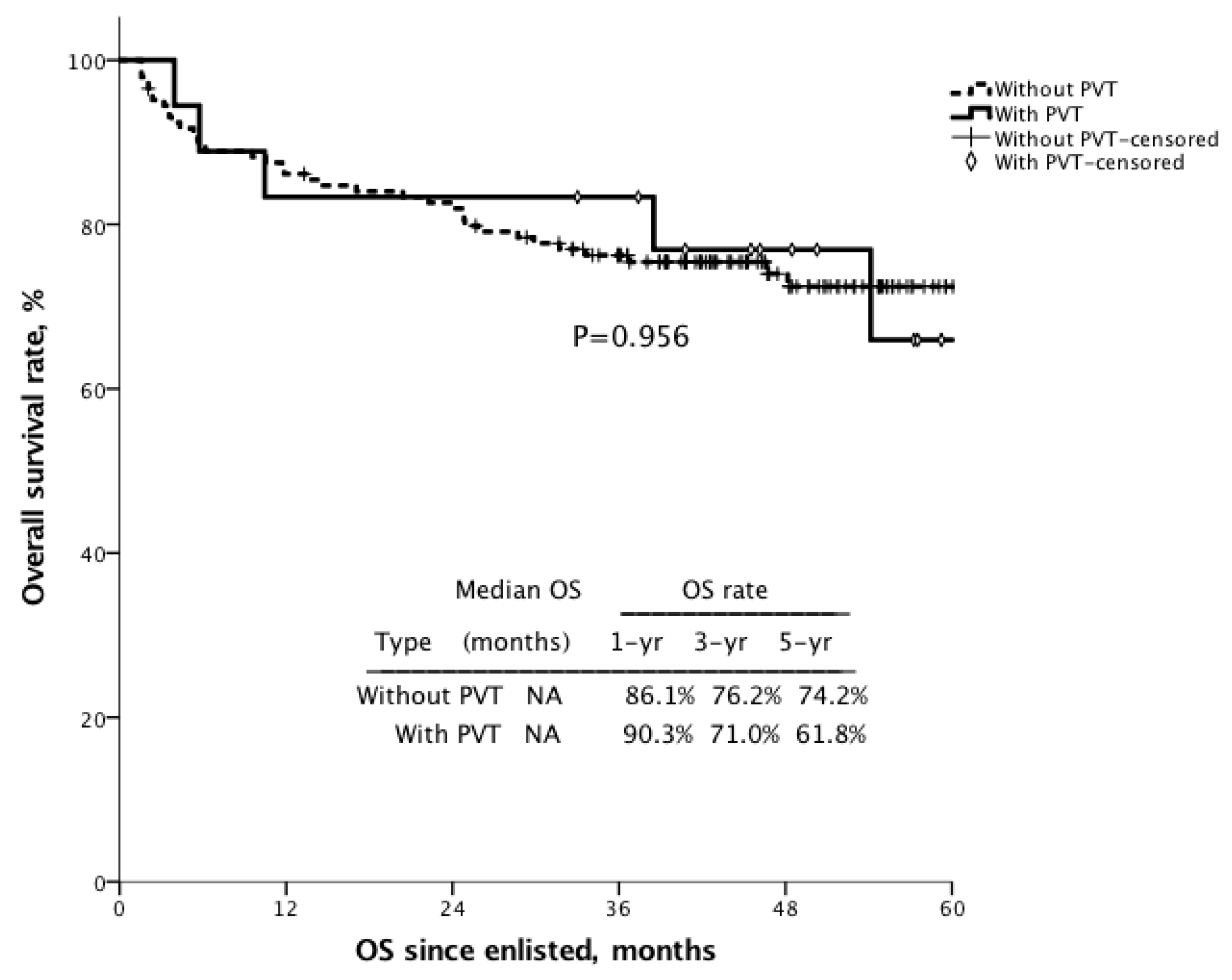
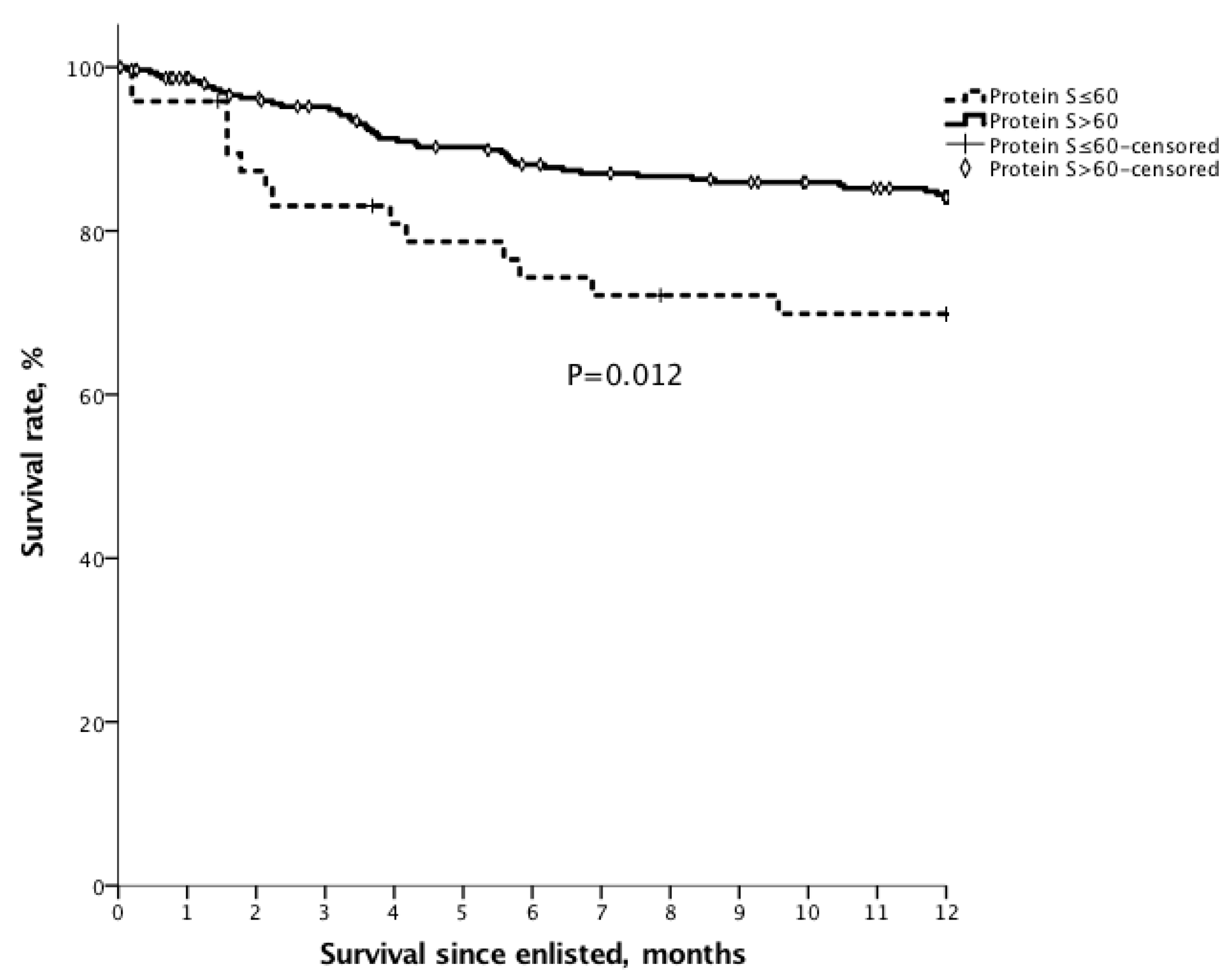
3.8. Survival
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PVT | portal vein thrombus |
| MELD | model for end-stage liver disease |
| HCC | hepatocellular carcinoma |
| UCSF | University of San Francisco |
| INR | international normalized ratio |
| AST | Aspartate aminotransferase |
| ALT | alanine aminotransferase |
| CT | computed tomography |
| POD | postoperative day |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| EV | esophageal varices |
| DDLT | deceased donor liver transplantation |
| LDLT | living donor liver transplantation |
References
- Garcia-Pagan, J.C.; Valla, D.C. Portal vein thrombosis: A predictable milestone in cirrhosis? J. Hepatol. 2009, 51, 632–634. [Google Scholar] [CrossRef]
- Kinjo, N.; Kawanaka, H.; Akahoshi, T.; Matsumoto, Y.; Kamori, M.; Nagao, Y.; Hashimoto, N.; Uehara, H.; Tomikawa, M.; Shirabe, K.; et al. Portal vein thrombosis in liver cirrhosis. World J. Hepatol. 2014, 6, 64–71. [Google Scholar] [CrossRef]
- Okuda, K.; Ohnishi, K.; Kimura, K.; Matsutani, S.; Sumida, M.; Goto, N.; Musha, H.; Takashi, M.; Suzuki, N.; Shinagawa, T. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology 1985, 89, 279–286. [Google Scholar] [CrossRef]
- Francoz, C.; Belghiti, J.; Vilgrain, V.; Sommacale, D.; Paradis, V.; Condat, B.; Denninger, M.H.; Sauvanet, A.; Valla, D.; Durand, F. Splanchnic vein thrombosis in candidates for liver transplantation: Usefulness of screening and anticoagulation. Gut 2005, 54, 691–697. [Google Scholar] [CrossRef]
- Yerdel, M.A.; Gunson, B.; Mirza, D.; Karayalçin, K.; Olliff, S.; Buckels, J.; Mayer, D.; McMaster, P.; Pirenne, J. Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome. Transplantation 2000, 69, 1873–1881. [Google Scholar] [CrossRef]
- Fisher, N.C.; Wilde, J.T.; Roper, J.; Elias, E. Deficiency of natural anticoagulant proteins C, S, and antithrombin in portal vein thrombosis: A secondary phenomenon? Gut 2000, 46, 534–539. [Google Scholar] [CrossRef]
- Di Minno, M.N.; Ambrosino, P.; Ageno, W.; Rosendaal, F.; Di Minno, G.; Dentali, F. Natural anticoagulants deficiency and the risk of venous thromboembolism: A meta-analysis of observational studies. Thromb. Res. 2015, 135, 923–932. [Google Scholar] [CrossRef]
- Thalheimer, U.; Triantos, C.K.; Samonakis, D.N.; Patch, D.; Burroughs, A.K. Infection, coagulation, and variceal bleeding in cirrhosis. Gut 2005, 54, 556–563. [Google Scholar] [CrossRef]
- Amitrano, L.; Guardascione, M.A.; Brancaccio, V.; Margaglione, M.; Manguso, F.; Iannaccone, L.; Grandone, E.; Balzano, A. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J. Hepatol. 2004, 40, 736–741. [Google Scholar] [CrossRef]
- Mangia, A.; Villani, M.; Cappucci, G.; Santoro, R.; Ricciardi, R.; Facciorusso, D.; Leandro, G.; Caruso, N.; Andriulli, A. Causes of portal venous thrombosis in cirrhotic patients: The role of genetic and acquired factors. Eur. J. Gastroenterol. Hepatol. 2005, 17, 745–751. [Google Scholar] [CrossRef]
- Nonami, T.; Yokoyama, I.; Iwatsuki, S.; Starzl, T.E. The incidence of portal vein thrombosis at liver transplantation. Hepatology 1992, 16, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; O’Brien, M. Endogenous anticoagulants. Top Companion Anim. Med. 2012, 27, 81–87. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Ferrell, L.; Bass, N.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, C.; Han, G.; Zhang, W.; He, C.; Yin, Z.; Liu, Z.; Bai, W.; Li, R.; Bai, M.; et al. Prevalence of the JAK2V617F mutation in Chinese patients with Budd-Chiari syndrome and portal vein thrombosis: A prospective study. J. Gastroenterol. Hepatol. 2012, 27, 1036–1043. [Google Scholar] [CrossRef]
- Tripodi, A. Hemostasis in Acute and Chronic Liver Disease. Semin. Liver Dis. 2017, 37, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qi, X.; He, C.; Yin, Z.; Fan, D.; Han, G. Coagulation imbalance may not contribute to the development of portal vein thrombosis in patients with cirrhosis. Thromb. Res. 2013, 131, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Lankarani, K.; Homayon, K.; Motevalli, D.; Heidari, S.T.; Alavian, S.M.; Malek-Hosseini, S.A. Risk Factors for Portal Vein Thrombosis in Patients With Cirrhosis Awaiting Liver Transplantation in Shiraz, Iran. Hepat. Mon. 2015, 15, e26407. [Google Scholar] [CrossRef]
- Tripodi, A.; Primignani, M.; Chantarangkul, V.; Dell’Era, A.; Clerici, M.; De Franchis, R.; Colombo, M.; Mannucci, P.M. An Imbalance of Pro- vs Anti-Coagulation Factors in Plasma from Patients with Cirrhosis. Gastroenterology 2009, 137, 2105–2111. [Google Scholar] [CrossRef]
- Qi, X.; Chen, H.; Han, G. Effect of antithrombin, protein C and protein S on portal vein thrombosis in liver cirrhosis: A meta-analysis. Am. J. Med. Sci. 2013, 346, 38–44. [Google Scholar] [CrossRef]
- Zhang, D.L.; Hao, J.Y.; Yang, N. Value of D-dimer and protein S for diagnosis of portal vein thrombosis in patients with liver cirrhosis. J. Int. Med. Res. 2013, 41, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Molmenti, E.P.; Roodhouse, T.W.; Molmenti, H.; Jaiswal, K.; Jung, G.; Marubashi, S.; Sanchez, E.Q.; Gogel, B.; Levy, M.F.; Goldstein, R.M.; et al. Thrombendvenectomy for Organized Portal Vein Thrombosis at the Time of Liver Transplantation. Ann. Surg. 2002, 235, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.W., Jr.; Iwatsuki, S.; Bron, K.; Starzl, T.E. Portal vein grafts in hepatic transplantation. Surg. Gynecol. Obstet. 1985, 161, 66–68. [Google Scholar] [PubMed]
- Harding, D.J.; Perera, M.T.; Chen, F.; Olliff, S.; Tripathi, D. Portal vein thrombosis in cirrhosis: Controversies and latest developments. World J. Gastroenterol. 2015, 21, 6769–6784. [Google Scholar] [CrossRef] [PubMed]
- Lendoire, J.C.; Raffin, G.; Cejas, N.; Duek, F.; Schelotto, P.B.; Trigo, P.; Quarin, C.; Garay, V.; Imventarza, O. Liver transplantation in adult patients with portal vein thrombosis: risk factors, management and outcome. HPB 2007, 9, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Zanetto, A.; Rodriguez-Kastro, K.I.; Germani, G.; Ferrarese, A.; Cillo, U.; Burra, P.; Senzolo, M. Mortality in liver transplant recipients with portal vein thrombosis—An updated meta-analysis. Transpl. Int. 2018, 31, 1318–1329. [Google Scholar] [CrossRef]
- Troisi, R.; Cammu, G.; Militerno, G.; De Baerdemaeker, L.; Decruyenaere, J.; Hoste, E.; Smeets, P.; Colle, I.; Van Vlierberghe, H.; Petrovic, M.; et al. Modulation of Portal Graft Inflow: A Necessity in Adult Living-Donor Liver Transplantation? Ann. Surg. 2003, 237, 429–436. [Google Scholar] [CrossRef]
- Shimamura, T.; Taniguchi, M.; Jin, M.B.; Suzuki, T.; Matsushita, M.; Furukawa, H.; Todo, S. Excessive portal venous inflow as a cause of allograft dysfunction in small-for-size living donor liver transplantation. Transplant. Proc. 2001, 33, 1331. [Google Scholar] [CrossRef]
- Dubuisson, C.; Boyer-Neumann, C.; Wolf, M.; Meyer, D.; Bernard, O. Protein C, protein S and antithrombin III in children with portal vein obstruction. J. Hepatol. 1997, 27, 132–135. [Google Scholar] [CrossRef]
- Humar, A.; Kosari, K.; Sielaff, T.D.; Glessing, B.; Gomes, M.; Dietz, C.; Rosen, G.; Lake, J.; Payne, W.D. Liver regeneration after adult living donor and deceased donor split-liver transplants. Liver Transplant. 2004, 10, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Schoffski, P.; Trautwein, C.; Manns, M.P.; Ganser, A.; von Depka, M. Tissue factor and thrombomodulin levels are correlated with stage of cirrhosis in patients with liver disease. Blood Coagul. Fibrinolysis 2001, 12, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.H. Hypercoagulability syndromes. Arch. Intern. Med. 2001, 161, 2433–2439. [Google Scholar] [CrossRef]
- Goodwin, A.J.; Rosendaal, F.R.; Kottke-Marchant, K.; Bovill, E.G. A review of the technical, diagnostic, and epidemiologic considerations for protein S assays. Arch. Pathol. Lab Med. 2002, 126, 1349–1366. [Google Scholar]
- Imai, H.; Egawa, H.; Kajiwara, M.; Nakajima, A.; Ogura, Y.; Hatano, E.; Ueda, M.; Kawaguchi, Y.; Kaido, T.; Takada, Y.; et al. Resolution of Preoperative Portal Vein Thrombosis After Administration of Antithrombin III in Living Donor Liver Transplantation: Case Report. Transplant. Proc. 2009, 41, 3931–3933. [Google Scholar] [CrossRef]
- Basili, S.; Pastori, D.; Raparelli, V.; Violi, F. Anticoagulant therapy in patients with liver cirrhosis and portal vein thrombosis: Insights for the clinician. Ther. Adv. Gastroenterol. 2018, 11, 1756284818793561. [Google Scholar] [CrossRef]
- Makris, M.; Van Veen, J.J.; Tait, C.R.; Mumford, A.D.; Laffan, M. British Committee for Standards in H. Guideline on the management of bleeding in patients on antithrombotic agents. Br. J. Haematol. 2013, 160, 35–46. [Google Scholar] [CrossRef]
| Total, n = 349 | Non-PVT, n = 301 | PVT, n = 48 | p-Value | |
|---|---|---|---|---|
| Age, years | 53.5 ± 9.0 | 53.7 ± 8.8 | 52.6 ± 10.4 | 0.429 |
| Gender, male | 265 (75.9%) | 233 (77.4%) | 32 (66.7%) | 0.106 |
| Hepatitis | 0.718 | |||
| HBV | 151 (43.2%) | 132 (43.8%) | 19 (39.5%) | |
| HCV | 69 (19.7%) | 57 (18.9%) | 12 (25.0%) | |
| Both | 13 (3.7%) | 12 (3.9%) | 1 (2.0%) | |
| Alcohol use | 130 (37.2%) | 115 (38.2%) | 15 (31.3%) | 0.355 |
| HCC, presence | 148 (42.8%) | 131 (44.0%) | 17 (35.4%) | 0.267 |
| EV, presence | 229 (65.6%) | 191 (63.5%) | 38 (79.2%) | 0.033 |
| EV bleeding history, presence | 101 (28.9%) | 78 (25.9%) | 23 (47.9%) | 0.001 |
| Ascites, presence | 192 (55.0%) | 167 (55.5%) | 25 (52.1%) | 0.66 |
| INR, >1.3 | 203 (58.2%) | 172 (57.1%) | 34 (64.6%) | 0.332 |
| PLT, ≤100 (103/μL) | 225 (64.5%) | 187 (62.1%) | 38 (79.2%) | 0.022 |
| Protein C, ≤70 (%) | 262 (75.1%) | 219 (72.8%) | 43 (89.6%) | 0.012 |
| Protein S, ≤60 (%) | 48 (13.8%) | 35 (11.6%) | 13 (27.1%) | 0.004 |
| Albumin, ≤2.8 (g/dL) | 105 (30.1%) | 94 (31.2%) | 11 (22.9%) | 0.244 |
| Total bilirubin, >2 (mg/dL) | 185 (53.0%) | 159 (52.8%) | 26 (54.2%) | 0.863 |
| ALK, >122 (U/L) | 172 (49.3%) | 151 (50.2%) | 21 (43.9%) | 0.408 |
| Creatinine, >1.2 (mg/dL) | 71 (20.3%) | 62 (20.6%) | 9 (18.8%) | 0.768 |
| e-GFR, ≤100 (mL/min/1.73m2) | 169 (48.4%) | 146 (48.5%) | 23 (47.9%) | 0.94 |
| MELD score | 16.4 ± 7.5 | 16.5 ± 7.8 | 15.9 ± 6.1 | 0.59 |
| Spontaneous shunt | 211 (60.5%) | 181 (60.1%) | 30 (62.5%) | 0.755 |
| Engorged CV | 173 (49.6%) | 146 (48.5%) | 27 (56.3%) | 0.319 |
| Splenorenal shunt | 107 (30.7%) | 91 (30.2%) | 16 (33.3%) | 0.665 |
| PVT grade, CT | NA | |||
| Grade 1–2 | 29 (60.4%) | |||
| Grade 3–4 | 14 (29.2%) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| PLT, PLT, 103/μL | ||||||
| ≤100 | 1.66 | 0.99–3.94 | 0.051 | |||
| >100 | 1 | |||||
| Protein C, % | ||||||
| ≤70 | 3.22 | 1.23–8.41 | 0.017 | |||
| >70 | 1 | |||||
| Protein S, % | ||||||
| ≤60 | 2.82 | 1.36–5.84 | 0.005 | 2.46 | 1.17–5.16 | 0.017 |
| >60 | 1 | 1 | ||||
| Esophageal varices | ||||||
| Absence | 1 | |||||
| Presence | 2.19 | 1.05–4.56 | 0.037 | |||
| Liver Transplantation | Non-PVT, n = 145 | PVT, n = 18 | p |
|---|---|---|---|
| Op method | 0.068 | ||
| LDLT | 116 (80.0%) | 11 (61.1%) | |
| DDLT | 29 (20.0%) | 7 (38.9%) | |
| OP duration, hours | 10.1 ± 2.0 | 10.2 ± 1.7 | 0.899 |
| Blood loss, mL | 2321 ± 2805 | 1825 ± 1100 | 0.459 |
| Spontaneous shunt ligation | 4 (2.8%) | 2 (11.1%) | 0.076 |
| PV anastomosis | NA | ||
| PV–PV classic style | 143 (98.6%) | 16 (88.8%) | |
| CV–PV interposition style | 2 (1.4%) | 1 (5.6%) | |
| MCV–PV interposition style | 0 (0.0%) | 1 (5.6%) | |
| PVT grade, intraoperative | NA | ||
| Grade 1–2 | 13 (72.2%) | ||
| Grade 3–4 | 5 (27.8%) | ||
| PV flow assessment | |||
| Pre-LT inflow, mL/min | 561.4 ± 281.1 | 408.2 ± 155.8 | 0.095 |
| Intraoperative inflow, mL/min | 1517.3 ± 662.8 | 1360 ± 729.3 | 0.35 |
| Post-LT day 1 inflow, mL/min | 932.7 ± 258.8 | 1008.9 ± 218.0 | 0.233 |
| Use of anticoagulant agent | 3 (2.1%) | 6 (33.3%) | <0.001 |
| Postoperative complications | 0.898 | ||
| None | 76 (52.4%) | 10 (55.6%) | |
| Grade 1–2 | 26 (17.9%) | 2 (11.1%) | |
| Grade 3–4 | 27 (18.6%) | 4 (22.2%) | |
| Grade 5 | 16 (11.0%) | 2 (11.1%) | |
| Acute rejection | 11 (7.6%) | 1 (5.6%) | 0.756 |
| Graft failure | 8 (5.5%) | 1 (5.6%) | 0.995 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-C.; Lee, J.-C.; Cheng, C.-H.; Wang, Y.-C.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Protein S for Portal Vein Thrombosis in Cirrhotic Patients Waiting for Liver Transplantation. J. Clin. Med. 2020, 9, 1181. https://doi.org/10.3390/jcm9041181
Hung H-C, Lee J-C, Cheng C-H, Wang Y-C, Wu T-H, Lee C-F, Wu T-J, Chou H-S, Chan K-M, Lee W-C. Protein S for Portal Vein Thrombosis in Cirrhotic Patients Waiting for Liver Transplantation. Journal of Clinical Medicine. 2020; 9(4):1181. https://doi.org/10.3390/jcm9041181
Chicago/Turabian StyleHung, Hao-Chien, Jin-Chiao Lee, Chih-Hsien Cheng, Yu-Chao Wang, Tsung-Han Wu, Chen-Fang Lee, Ting-Jung Wu, Hong-Shiue Chou, Kun-Ming Chan, and Wei-Chen Lee. 2020. "Protein S for Portal Vein Thrombosis in Cirrhotic Patients Waiting for Liver Transplantation" Journal of Clinical Medicine 9, no. 4: 1181. https://doi.org/10.3390/jcm9041181
APA StyleHung, H.-C., Lee, J.-C., Cheng, C.-H., Wang, Y.-C., Wu, T.-H., Lee, C.-F., Wu, T.-J., Chou, H.-S., Chan, K.-M., & Lee, W.-C. (2020). Protein S for Portal Vein Thrombosis in Cirrhotic Patients Waiting for Liver Transplantation. Journal of Clinical Medicine, 9(4), 1181. https://doi.org/10.3390/jcm9041181





