Bariatric Surgery Improves HDL Function Examined by ApoA1 Exchange Rate and Cholesterol Efflux Capacity in Patients with Obesity and Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. ApoA1 Exchange Indicator
2.3. ApoA1 Exchange Rate Assay
2.4. Cellular Cholesterol Efflux Capacity
2.5. Statistical Analysis
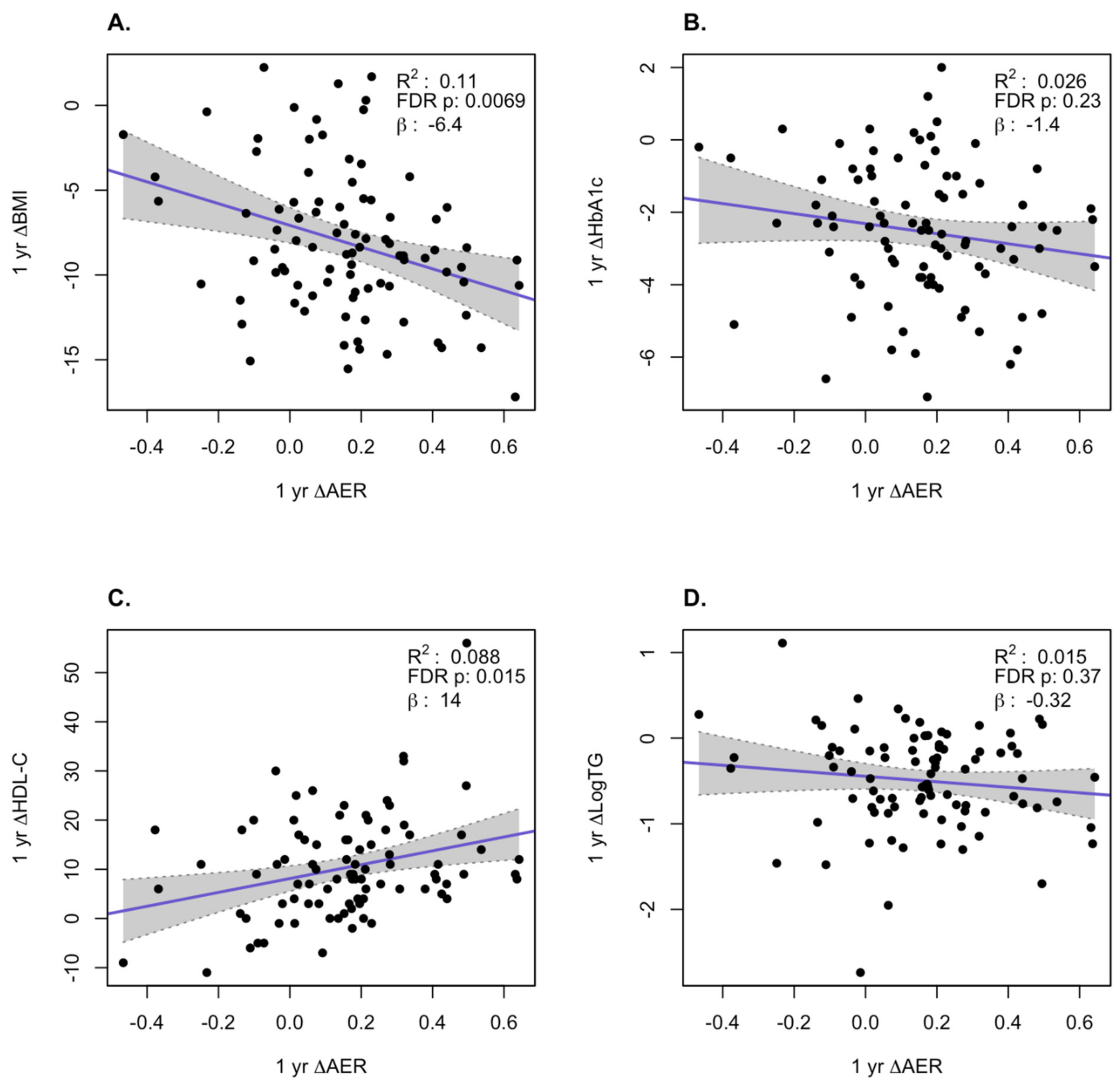
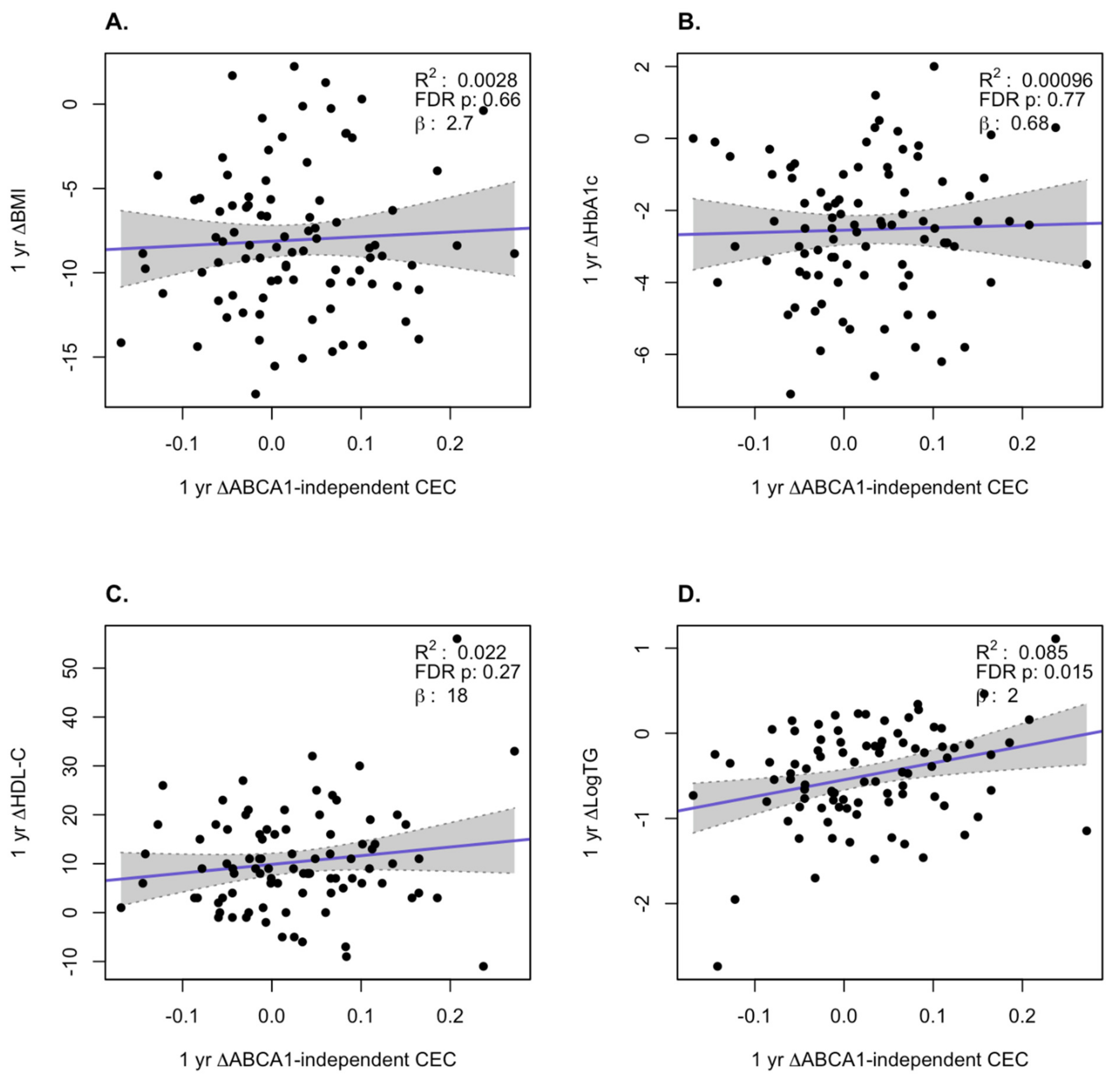
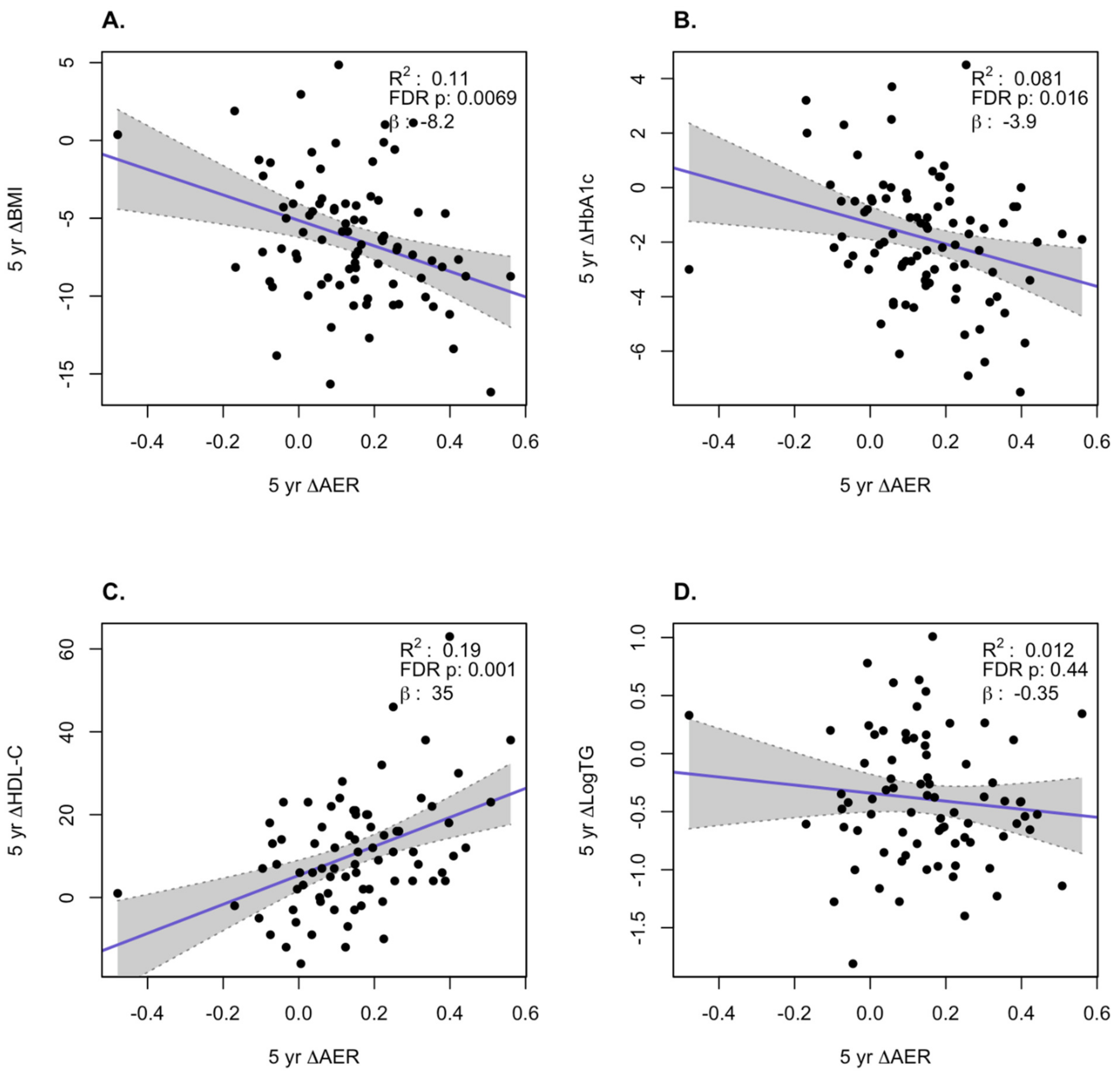
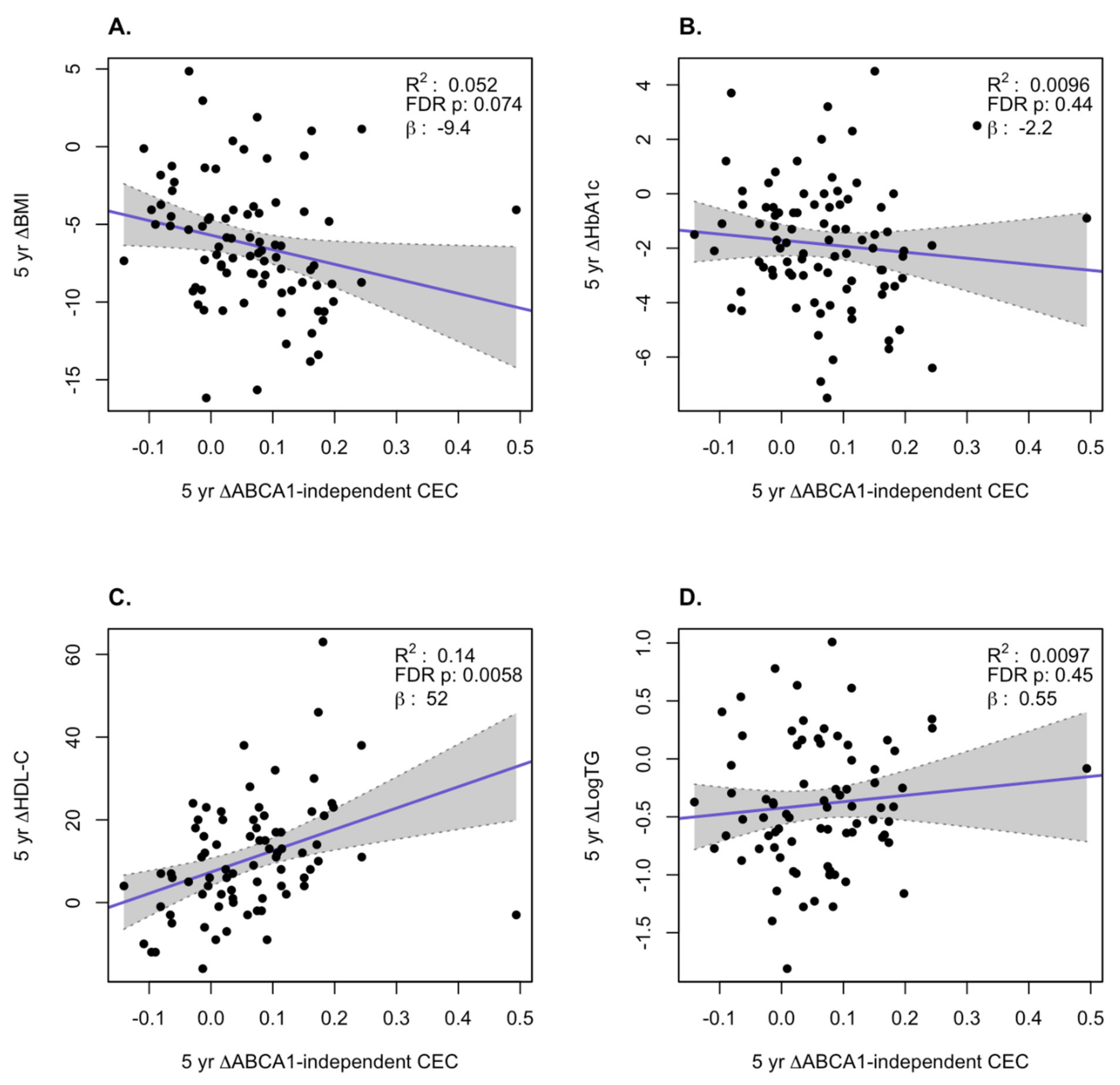
3. Results
HDL Function is Significantly Improved After Bariatric Surgery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Disclosures
References
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Thomas, A.J.; Connett, J.E.; Bantle, J.P.; Leslie, D.B.; Wang, Q.; Inabnet, W.B., 3rd; Jeffery, R.W.; et al. Lifestyle Intervention and Medical Management With vs Without Roux-en-Y Gastric Bypass and Control of Hemoglobin A1c, LDL Cholesterol, and Systolic Blood Pressure at 5 Years in the Diabetes Surgery Study. JAMA 2018, 319, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Aminian, A.; Zajichek, A.; Arterburn, D.E.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; Nissen, S.E. Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes and Obesity. JAMA 2019, 322, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Assmann, G.; Schulte, H.; von Eckardstein, A.; Huang, Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996, 124, S11–S20. [Google Scholar] [CrossRef]
- Investigators, A.-H.; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Group, H.T.R.C.; Bowman, L.; Hopewell, J.C.; Chen, F.; Wallendszus, K.; Stevens, W.; Collins, R.; Wiviott, S.D.; Cannon, C.P.; Braunwald, E.; et al. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Holm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Cuchel, M.; Rohatgi, A.; Sacks, F.M.; Guyton, J.R. JCL roundtable: High-density lipoprotein function and reverse cholesterol transport. J. Clin. Lipidol. 2018, 12, 1086–1094. [Google Scholar] [CrossRef]
- Annema, W.; Dikkers, A.; de Boer, J.F.; van Greevenbroek, M.M.J.; van der Kallen, C.J.H.; Schalkwijk, C.G.; Stehouwer, C.D.A.; Dullaart, R.P.F.; Tietge, U.J.F. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: The CODAM study. Sci. Rep. 2016, 6, 27367. [Google Scholar] [CrossRef]
- Hoang, A.; Murphy, A.J.; Coughlan, M.T.; Thomas, M.C.; Forbes, J.M.; O’Brien, R.; Cooper, M.E.; Chin-Dusting, J.P.; Sviridov, D. Advanced glycation of apolipoprotein A-I impairs its anti-atherogenic properties. Diabetologia 2007, 50, 1770–1779. [Google Scholar] [CrossRef]
- Patel, D.C.; Albrecht, C.; Pavitt, D.; Paul, V.; Pourreyron, C.; Newman, S.P.; Godsland, I.F.; Valabhji, J.; Johnston, D.G. Type 2 diabetes is associated with reduced ATP-binding cassette transporter A1 gene expression, protein and function. PLoS ONE 2011, 6, e22142. [Google Scholar] [CrossRef]
- Smith, J.D.; Le Goff, W.; Settle, M.; Brubaker, G.; Waelde, C.; Horwitz, A.; Oda, M.N. ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J. Lipid Res. 2004, 45, 635–644. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef]
- Ebtehaj, S.; Gruppen, E.G.; Bakker, S.J.L.; Dullaart, R.P.F.; Tietge, U.J.F. HDL (High-Density Lipoprotein) Cholesterol Efflux Capacity Is Associated With Incident Cardiovascular Disease in the General Population. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Stefater, M.A.; Wilson-Perez, H.E.; Chambers, A.P.; Sandoval, D.A.; Seeley, R.J. All bariatric surgeries are not created equal: Insights from mechanistic comparisons. Endocr. Rev. 2012, 33, 595–622. [Google Scholar] [CrossRef] [PubMed]
- Heffron, S.P.; Lin, B.X.; Parikh, M.; Scolaro, B.; Adelman, S.J.; Collins, H.L.; Berger, J.S.; Fisher, E.A. Changes in High-Density Lipoprotein Cholesterol Efflux Capacity After Bariatric Surgery Are Procedure Dependent. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kjellmo, C.A.; Karlsson, H.; Nestvold, T.K.; Ljunggren, S.; Cederbrant, K.; Marcusson-Stahl, M.; Mathisen, M.; Lappegard, K.T.; Hovland, A. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J. Clin. Lipidol. 2018, 12, 193–202. [Google Scholar] [CrossRef]
- Coimbra, S.; Reis, F.; Ferreira, C.; Nunes, S.; Viana, S.; Catarino, A.; Rocha-Pereira, P.; Belo, L.; Monteiro, L.; Catarino, C.; et al. Weight loss achieved by bariatric surgery modifies high-density lipoprotein subfractions and low-density lipoprotein oxidation towards atheroprotection. Clin. Biochem. 2019, 63, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Inge, T.H.; Sexmith, H.; Heink, A.; Elder, D.; Hui, D.Y.; Melchior, J.T.; Kelesidis, T.; Shah, A.S. Weight loss surgery in adolescents corrects high-density lipoprotein subspecies and their function. Int. J. Obes. (Lond) 2017, 41, 83–89. [Google Scholar] [CrossRef]
- Lorkowski, S.W.; Brubaker, G.; Li, L.; Li, X.S.; Hazen, S.L.; Smith, J.D. A Novel Cell-free Fluorescent Assay for HDL function: Low Apolipoprotein A1 Exchange Rate Associated with Increased Incident Cardiovascular Events. J. Appl. Lab. Med. 2020. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 30 December 2019).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Nambi, V.; Rohatgi, A.; Bhatt, D.L. Is RCT (Reverse Cholesterol Transport) Ready for an RCT (Randomized Controlled Trial)? J. Am. Coll. Cardiol. 2018, 72, 3270–3273. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Aminian, A.; Kashyap, S.R.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Hazen, S.L.; Nissen, S.E.; Schauer, P.R. Cardiovascular Biomarkers After Metabolic Surgery Versus Medical Therapy for Diabetes. J. Am. Coll. Cardiol. 2019, 74, 261–263. [Google Scholar] [CrossRef]
- Sasahara, T.; Nestel, P.; Fidge, N.; Sviridov, D. Cholesterol transport between cells and high density lipoprotein subfractions from obese and lean subjects. J. Lipid. Res. 1998, 39, 544–554. [Google Scholar] [PubMed]
- De la Llera-Moya, M.; Drazul-Schrader, D.; Asztalos, B.F.; Cuchel, M.; Rader, D.J.; Rothblat, G.H. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; de la Llera-Moya, M.; Dallal, G.E.; Horvath, K.V.; Schaefer, E.J.; Rothblat, G.H. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J. Lipid. Res. 2005, 46, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef] [PubMed]
- Cavigiolio, G.; Geier, E.G.; Shao, B.; Heinecke, J.W.; Oda, M.N. Exchange of apolipoprotein A-I between lipid-associated and lipid-free states: A potential target for oxidative generation of dysfunctional high density lipoproteins. J. Biol. Chem. 2010, 285, 18847–18857. [Google Scholar] [CrossRef]
- Osto, E.; Doytcheva, P.; Corteville, C.; Bueter, M.; Dorig, C.; Stivala, S.; Buhmann, H.; Colin, S.; Rohrer, L.; Hasballa, R.; et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: Role of glucagon-like peptide-1. Circulation 2015, 131, 871–881. [Google Scholar] [CrossRef]

| Characteristic | Medical Therapy (N = 20) | Roux-en-Y Gastric Bypass (N = 37) | Sleeve Gastrectomy (N = 33) | p-Value |
|---|---|---|---|---|
| Duration of diabetes (yr) | 10.15 ± 5.75 | 7.60 ± 5.22 | 8.61 ± 4.38 | 0.20 |
| Glycated hemoglobin (%) | 8.81 ± 0.98 | 9.18 ± 1.37 | 9.69 ± 1.89 | 0.11 |
| Use of insulin (%) | 50.0 | 45.9 | 54.5 | 0.77 |
| Age (yr) | 52.58 ± 5.46 | 48.52 ± 8.43 | 48.54 ± 7.43 | 0.11 |
| Female (%) | 70.0 | 54.1 | 81.8 | 0.045 |
| Body Mass Index (kg/m2) | 35.84 ± 2.92 | 37.01 ± 3.32 | 35.87 ± 4.32 | 0.34 |
| Body weight (kg) | 102.1 ± 10.94 | 107.3 ± 15.91 | 99.28 ± 17.56 | 0.10 |
| Waist (cm) | 112.7 ± 6.93 | 117.0 ± 9.51 | 113.5 ± 10.98 | 0.18 |
| Waist-to-hip ratio | 0.95 ± 0.07 | 0.97 ± 0.07 | 0.95 ± 0.08 | 0.39 |
| White race (%) | 70.0 | 75.7 | 72.7 | 0.89 |
| Smoking (%) | 20.0 | 35.1 | 15.2 | 0.13 |
| Metabolic Syndrome (%) | 85.0 | 97.3 | 93.9 | 0.20 |
| Hypertension (%) | 75.0 | 75.7 | 60.6 | 0.33 |
| Dyslipidemia (%) | 85.0 | 89.2 | 84.8 | 0.84 |
| HDL-C (mg/dl) | 51.50 ± 12.45 | 44.16 ± 11.64 | 41.91 ± 9.10 | 0.0094 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorkowski, S.W.; Brubaker, G.; Rotroff, D.M.; Kashyap, S.R.; Bhatt, D.L.; Nissen, S.E.; Schauer, P.R.; Aminian, A.; Smith, J.D. Bariatric Surgery Improves HDL Function Examined by ApoA1 Exchange Rate and Cholesterol Efflux Capacity in Patients with Obesity and Type 2 Diabetes. Biomolecules 2020, 10, 551. https://doi.org/10.3390/biom10040551
Lorkowski SW, Brubaker G, Rotroff DM, Kashyap SR, Bhatt DL, Nissen SE, Schauer PR, Aminian A, Smith JD. Bariatric Surgery Improves HDL Function Examined by ApoA1 Exchange Rate and Cholesterol Efflux Capacity in Patients with Obesity and Type 2 Diabetes. Biomolecules. 2020; 10(4):551. https://doi.org/10.3390/biom10040551
Chicago/Turabian StyleLorkowski, Shuhui Wang, Gregory Brubaker, Daniel M. Rotroff, Sangeeta R. Kashyap, Deepak L. Bhatt, Steven E. Nissen, Philip R. Schauer, Ali Aminian, and Jonathan D. Smith. 2020. "Bariatric Surgery Improves HDL Function Examined by ApoA1 Exchange Rate and Cholesterol Efflux Capacity in Patients with Obesity and Type 2 Diabetes" Biomolecules 10, no. 4: 551. https://doi.org/10.3390/biom10040551
APA StyleLorkowski, S. W., Brubaker, G., Rotroff, D. M., Kashyap, S. R., Bhatt, D. L., Nissen, S. E., Schauer, P. R., Aminian, A., & Smith, J. D. (2020). Bariatric Surgery Improves HDL Function Examined by ApoA1 Exchange Rate and Cholesterol Efflux Capacity in Patients with Obesity and Type 2 Diabetes. Biomolecules, 10(4), 551. https://doi.org/10.3390/biom10040551





