Aquatic Plants, Landoltia punctata, and Azolla filiculoides as Bio-Converters of Wastewater to Biofuel
Abstract
1. Introduction
1.1. Duckweed and Azolla as Phytoremediation Species
1.2. Duckweed and Azolla as Universal Feedstock for Biofuel Production
2. Results and Discussion
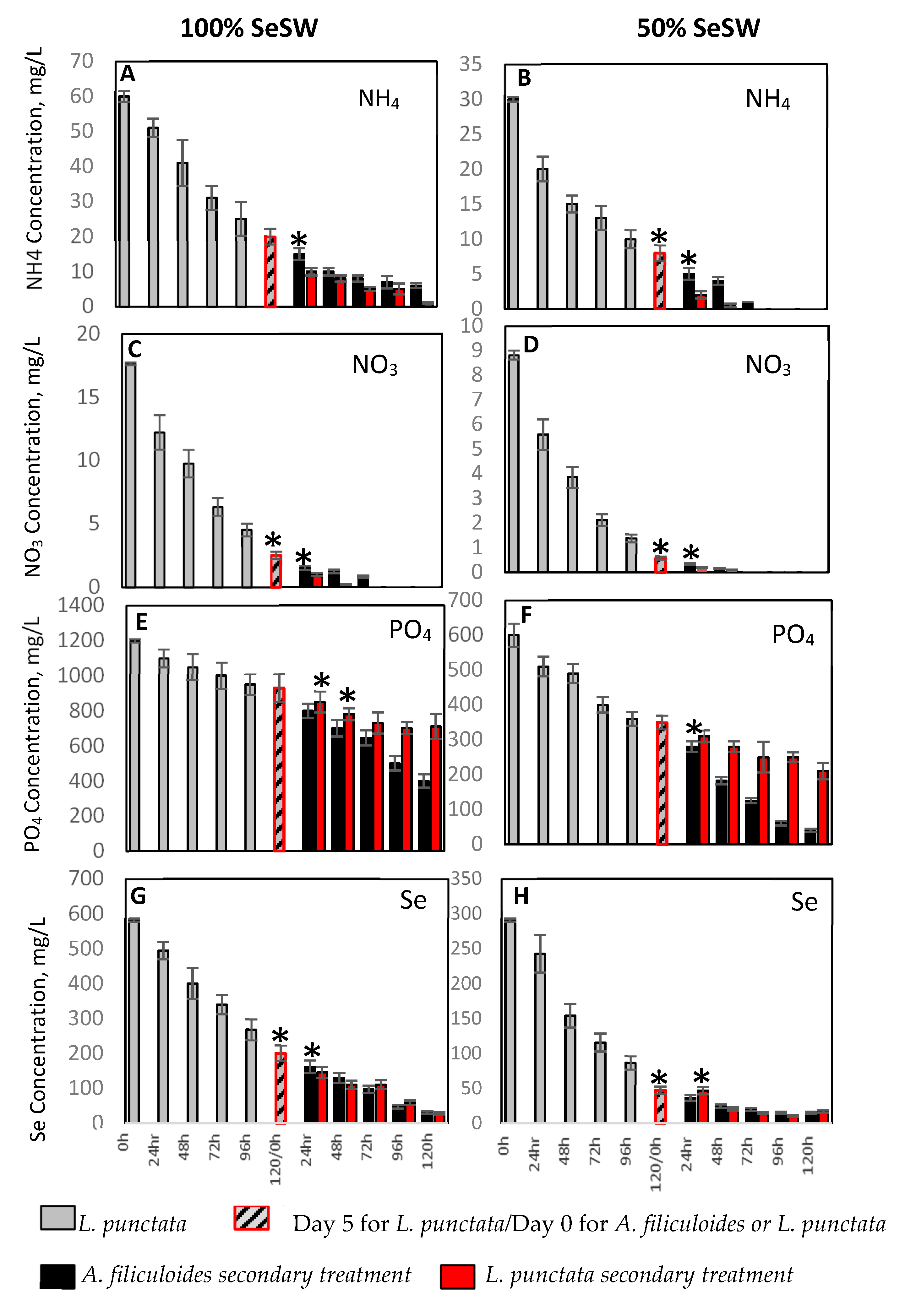
2.1. Wastewater Treatment with L. punctata and A. filiculoides
2.1.1. Removal of Nitrogen and Phosphorus by L. punctata
2.1.2. Secondary Treatment of SeSW by A. filiculoides
2.1.3. Removal of Selenium from by Sequential Treatment with L. punctata and A. filiculoides
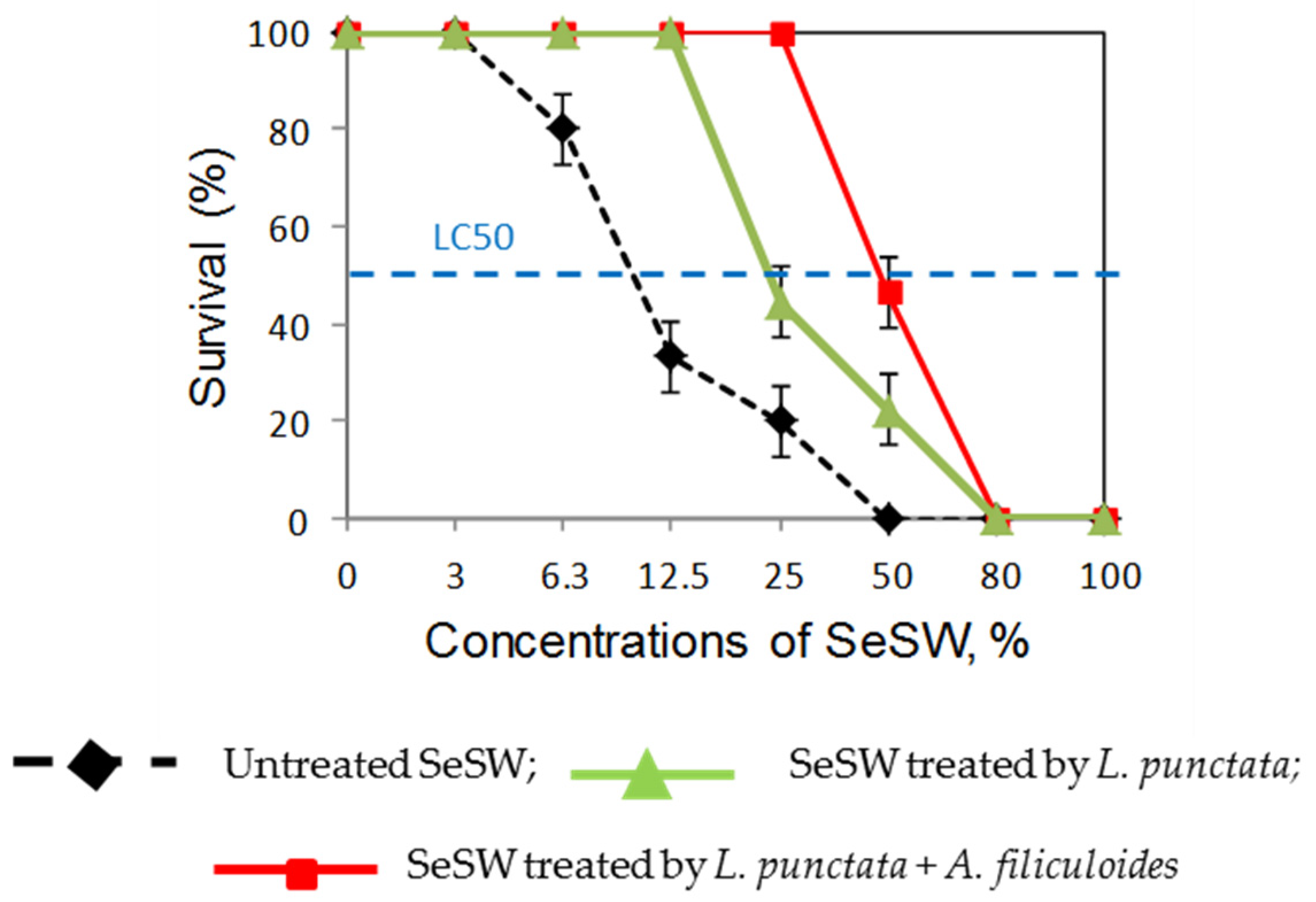
2.1.4. Shrimp Toxicity Tests of Treated Wastewaters
2.1.5. Suppression of Microalgal Growth by L. punctata and A. filiculoides
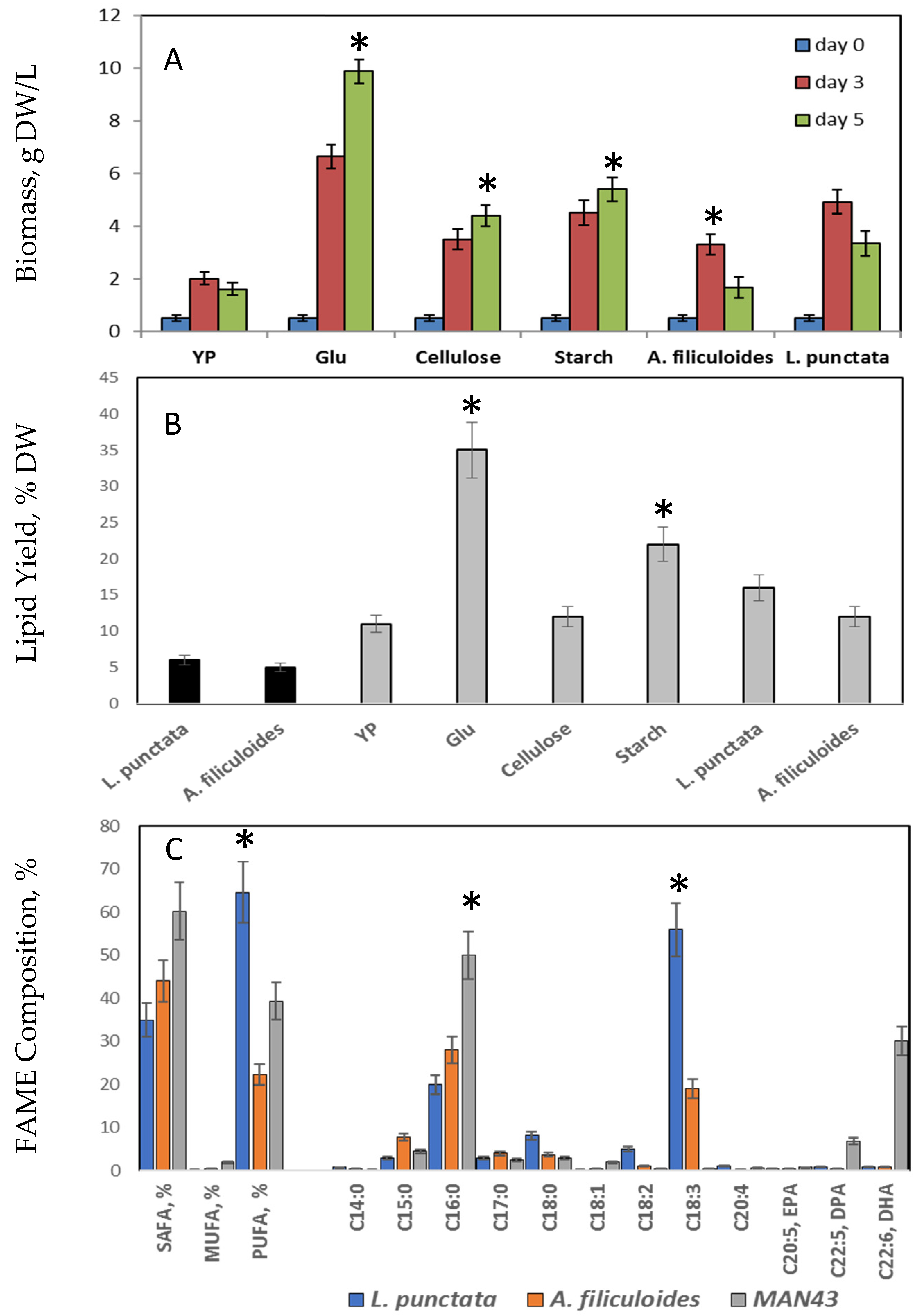
2.2. Biofuel Production from L. punctata and A. filiculoides
2.2.1. L. punctata and A. filiculoides as an Alternative Carbon Source for Lipid Production
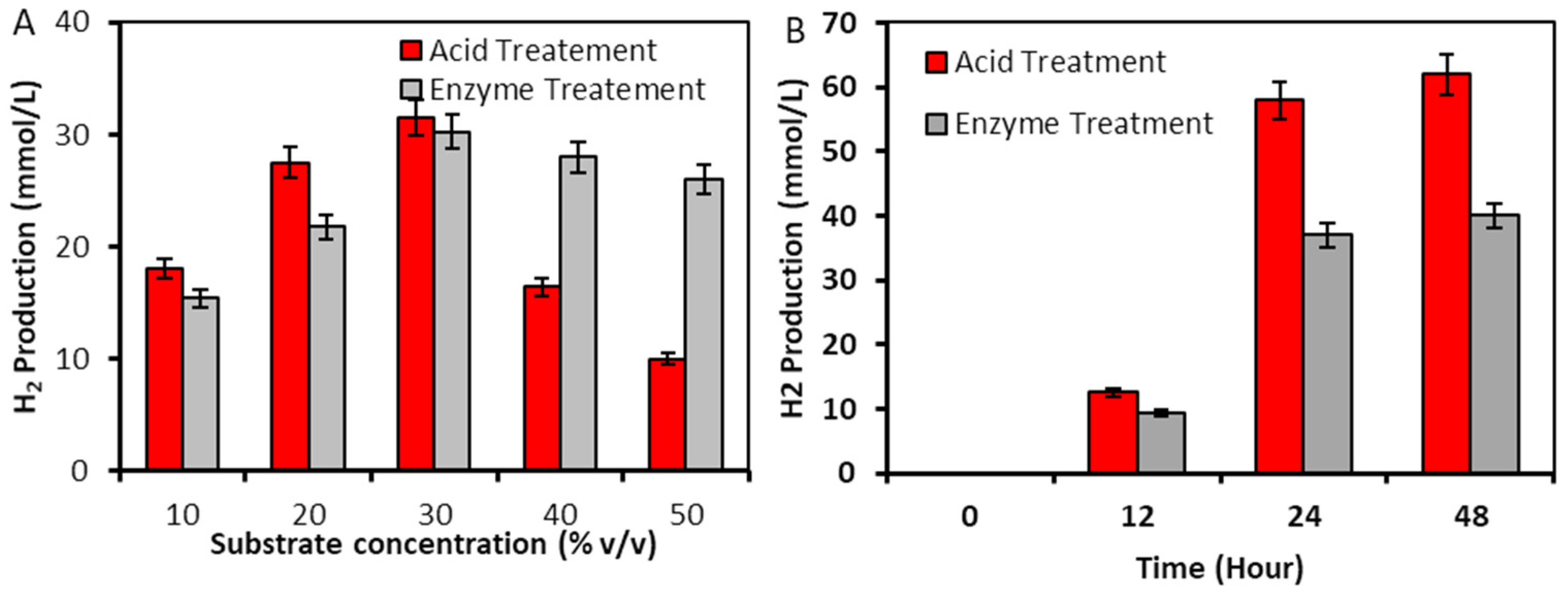
2.2.2. Hydrogen Production from L. punctata
3. Materials and Methods
3.1. Growth of Azolla and Duckweed
3.2. Selenium Extraction and Measurements
3.3. Shrimp Toxicity Test
3.4. Thraustochytrids Isolation and Growth
3.5. Nile Red Staining
3.6. Lipid Extraction and Fatty Acid Composition
3.7. Biohydrogen Production
3.7.1. Microorganism, Media and Growth Condition
3.7.2. Batch Dark Fermentation Experiments
3.7.3. Analytical Methods
3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marmiroli, N.; Marmiroli, M.; Maestri, E. Phytoremediation and Phytotechnologies A Review for the Present and the Future. Soil Water Pollution. Monit. Protect. Remediat. 2006, 69, 403–416. [Google Scholar]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Phys. 1998, 49, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Dushenkov, S. Trends in phytoremediation of radionuclides. Plant Soil 2003, 249, 167–175. [Google Scholar] [CrossRef]
- Miranda, A.F.; Biswas, B.; Ramkumar, N.; Singh, R.; Kumar, J.; James, A.; Roddick, F.; Lal, B.; Subudhi, S.; Bhaskar, T.; et al. Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol. Biofuels 2016, 9, 221. [Google Scholar] [CrossRef]
- Miranda, A.F.; Muradov, N.; Gujar, A.; Stevenson, T.; Nugegoda, D.; Ball, A.S.; Mouradov, A. Application of Aquatic plants for the treatment of selenium-rich mining wastewater and production of renewable fuels and petrochemicals. J. Sustain. Bioenergy Syst. 2014, 4, 97–112. [Google Scholar] [CrossRef][Green Version]
- Muradov, N.; Taha, M.; Miranda, A.F.; Kadali, K.; Gujar, A.; Rochfort, S.; Stevenson, T.; Ball, A.S.; Mouradov, A. Dual application of duckweed and azolla plants for wastewater treatment and renewable fuels and petrochemicals production. Biotechnol. Biofuels 2014, 7, 30. [Google Scholar] [CrossRef]
- Sudiarto, S.I.A.; Renggaman, A.; Choi, H.L. Floating aquatic plants for total nitrogen and phosphorus removal from treated swine wastewater and their biomass characteristics. J. Environ. Manag. 2019, 231, 763–769. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S.K. Aquatic weeds as the next generation feedstock for sustainable bioenergy production. Bioresour. Technol. 2018, 251, 390–402. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Moheimani, N.R. Sustainable biofuels from algae. Mitig. Adapt. Strateg. Glob. 2013, 18, 13–25. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. BioEnergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Rajkumar, R.; Yaakob, Z.; Takriff, M.S. Potential of the micro and macro algae for biofuel production: A brief review. Bioresources 2014, 9, 1606–1633. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Park, J.; Heubeck, S.; Ralph, P.J.; Craggs, R.J. Size matters—Microalgae production and nutrient removal in wastewater treatment high rate algal ponds of three different sizes. Algal Res. 2020, 45, 101734. [Google Scholar] [CrossRef]
- Aguirre, A.M.; Bassi, A.; Saxena, P. Engineering challenges in biodiesel production from microalgae. Crit. Rev. Biotechnol. 2013, 33, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Potential of aquatic macrophytes for removing contaminants from the environment. Crit. Rev. Environ. Sci. Technol. 2009, 39, 754–781. [Google Scholar] [CrossRef]
- Ruan, T.; Zeng, R.; Yin, X.Y.; Zhang, S.X.; Yang, Z.H. Water Hyacinth (Eichhornia crassipes) biomass as a biofuel feedstock by enzymatic hydrolysis. Bioresources 2016, 11, 2372–2380. [Google Scholar] [CrossRef]
- Zhao, F.L.; Yang, W.D.; Zeng, Z.; Li, H.; Yang, X.E.; He, Z.L.; Gu, B.H.; Rafiq, M.T.; Peng, H.Y. Nutrient removal efficiency and biomass production of different bioenergy plants in hypereutrophic water. Biomass Bioenergy 2012, 42, 212–218. [Google Scholar] [CrossRef]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. GCB Bioenergy 2015, 7, 658–672. [Google Scholar] [CrossRef]
- Robles-Pliego, M.; Olguin, E.J.; Hernandez-Landa, J.; Gonzalez-Portela, R.E.; Sanchez-Galvan, G.; Cuervo-Lopez, F. Dual purpose system for water treatment from a polluted river and the production of pistia stratiotes biomass within a biorefinery. CLEAN-Soil Air Water 2015, 43, 1514–1521. [Google Scholar] [CrossRef]
- Mukherjee, B.; Majumdar, M.; Gangopadhyay, A.; Chakraborty, S.; Chaterjee, D. Phytoremediation of parboiled rice mill wastewater using Water lettuce (Pistia Stratiotes). Int. J. Phytoremed. 2015, 17, 651–656. [Google Scholar] [CrossRef]
- Chandanshive, V.V.; Rane, N.R.; Gholave, A.R.; Patil, S.M.; Jeon, B.H.; Govindwar, S.P. Efficient decolorization and detoxification of textile industry effluent by Salvinia molesta in lagoon treatment. Environ. Res. 2016, 150, 88–96. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. Optimization of lipid extraction from Salvinia molesta for biodiesel production using RSM and its FAME analysis. Environ. Sci. Pollut. Res. 2016, 23, 14047–14055. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Santos, M.C.R.; Carrapico, F.; Pereira, A.L. Azolla-Anabaena’s behaviour in urban wastewater and artificial media—Influence of combined nitrogen. Water Res. 2009, 43, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Santos, M.C.; Carrapico, F. Biomass characterization of Azolla filiculoides grown in natural ecosystems and wastewater. Hydrobiologia 1999, 415, 323–327. [Google Scholar] [CrossRef]
- Pereira, A.L.; Martins, M.; Oliveira, M.M.; Carrapico, F. Morphological and genetic diversity of the family Azollaceae inferred from vegetative characters and RAPD markers. Plant Syst. Evol. 2011, 297, 213–226. [Google Scholar] [CrossRef]
- Brouwer, P.; van der Werf, A.; Schluepmann, H.; Reichart, G.J.; Nierop, K.G.J. Lipid yield and composition of Azolla filiculoides and the implications for biodiesel production. Bioenergy Res. 2016, 9, 369–377. [Google Scholar] [CrossRef]
- Brouwer, P.; Brautigam, A.; Kulahoglu, C.; Tazelaar, A.O.E.; Kurz, S.; Nierop, K.G.J.; van der Werf, A.; Weber, A.P.M.; Schluepmann, H. Azolla domestication towards a biobased economy? New Phytol. 2014, 202, 1069–1082. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Fakhoorian, T.; Lam, E. Resurgence of duckweed research and applications: Report from the 3rd International Duckweed Conference. Plant Mol. Biol. 2015, 89, 647–654. [Google Scholar] [CrossRef]
- Lam, E.; Appenroth, K.J.; Michael, T.; Mori, K.; Fakhoorian, T. Duckweed in bloom: The 2nd International Conference on Duckweed Research and Applications heralds the return of a plant model for plant biology. Plant Mol. Biol. 2014, 84, 737–742. [Google Scholar] [CrossRef]
- Zhao, H.; Appenroth, K.; Landesman, L.; Salmean, A.A.; Lam, E. Duckweed rising at Chengdu: Summary of the 1st International Conference on Duckweed Application and Research. Plant Mol. Biol. 2012, 78, 627–632. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Utility of duckweeds as source of biomass energy: A review. Bioenergy Res. 2015, 8, 1589–1597. [Google Scholar] [CrossRef]
- Toyama, T.; Hanaoka, T.; Tanaka, Y.; Morikawa, M.; Mori, K. Comprehensive evaluation of nitrogen removal rate and biomass, ethanol, and methane production yields by combination of four major duckweeds and three types of wastewater effluent. Bioresour. Technol. 2018, 250, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Harrigan, T.; Reinhold, D.M. Use of duckweed-based constructed wetlands for nutrient recovery and pollutant reduction from dairy wastewater. Ecol. Eng. 2015, 78, 6–14. [Google Scholar] [CrossRef]
- Cheng, J.; Bergmann, B.A.; Classen, J.J.; Stomp, A.M.; Howard, J.W. Nutrient recovery from swine lagoon water by Spirodela punctata. Bioresour. Technol. 2002, 81, 81–85. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Velho, V.F.; Costa, R.H.; Hofmann, S.M.; Belli Filho, P. Nutrient recovery from swine waste and protein biomass production using duckweed ponds (Landoltia punctata): Southern Brazil. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2012, 65, 2042–2048. [Google Scholar] [CrossRef]
- Korner, S.; Vermaat, J.E.; Veenstra, S. The capacity of duckweed to treat wastewater: Ecological considerations for a sound design. J. Environ. Qual. 2003, 32, 1583–1590. [Google Scholar] [CrossRef]
- Iatrou, E.I.; Stasinakis, A.S.; Aloupi, M. Cultivating duckweed Lemna minor in urine and treated domestic wastewater for simultaneous biomass production and removal of nutrients and antimicrobials. Ecol. Eng. 2015, 84, 632–639. [Google Scholar] [CrossRef]
- Guo, L.; Ding, Y.; Xu, Y.; Li, Z.; Jin, Y.; He, K.; Fang, Y.; Zhao, H. Responses of Landoltia punctata to cobalt and nickel: Removal, growth, photosynthesis, antioxidant system and starch metabolism. Aquat. Toxicol. 2017, 190, 87–93. [Google Scholar] [CrossRef]
- Ding, Y.; Fang, Y.; Guo, L.; Li, Z.; He, K.; Zhao, Y.; Zhao, H. Phylogenic study of Lemnoideae (Duckweeds) through complete chloroplast genomes for eight accessions. PeerJ 2017, 5, e4186. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Colman, B.P.; Schwab, F.; Wiesner, M.R.; Lowry, G.V. Uptake and distribution of silver in the Aquatic plant Landoltia punctata (Duckweed) exposed to silver and silver sulfide nanoparticles. Environ. Sci. Technol. 2017, 51, 4936–4943. [Google Scholar] [CrossRef]
- Zhao, X.; Elliston, A.; Collins, S.R.A.; Moates, G.K.; Coleman, M.J.; Waldron, K.W. Enzymatic saccharification of duckweed (Lemna minor) biomass without thermophysical pretreatment. Biomass Bioenergy 2012, 47, 354–361. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.J. Growing duckweed for biofuel production: A review. Plant Biol. 2015, 17, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Soda, S.; Ohchi, T.; Piradee, J.; Takai, Y.; Ike, M. Duckweed biomass as a renewable biorefinery feedstock: Ethanol and succinate production from Wolffia globosa. Biomass Bioenergy 2015, 81, 364–368. [Google Scholar] [CrossRef]
- Lalau, C.M.; Simioni, C.; Vicentini, D.S.; Ouriques, L.C.; Mohedano, R.A.; Puerari, R.C.; Matias, W.G. Toxicological effects of AgNPs on duckweed (Landoltia punctata). Sci. Total Environ. 2020, 710, 136318. [Google Scholar] [CrossRef]
- Carrapiço, F. Azolla as a Superorganism. Its Implications in Symbiotic Studies; Springer: Dordrecht, The Netherlands, 2010; Volume 17, pp. 227–241. [Google Scholar]
- Zheng, W.W.; Bergman, B.; Chen, B.; Zheng, S.P.; Xiang, G.; Rasmussen, U. Cellular responses in the cyanobacterial symbiont during its vertical transfer between plant generations in the Azolla microphylla symbiosis. New Phytol. 2009, 181, 53–61. [Google Scholar] [CrossRef]
- Pereira, A.L.; Vasconcelos, V. Classification and phylogeny of the cyanobiont Anabaena azollae Strasburger: An answered question? Int. J. Syst. Evol. Microbiol. 2014, 64, 1830–1840. [Google Scholar] [CrossRef]
- Calvert, H.E.; Peters, G.A. The Azolla-anabaena relationship: Morphological analysis of leaf cavity hair populations. New Phytol. 1981, 89, 327–335. [Google Scholar] [CrossRef]
- Hall, D.O.; Markov, S.A.; Watanabe, Y.; Rao, K.K. The potential applications of cyanobacterial photosynthesis for clean technologies. Photosynth. Res. 1995, 46, 159–167. [Google Scholar] [CrossRef]
- Kollah, B.; Patra, A.K.; Mohanty, S.R. Aquatic microphylla Azolla: A perspective paradigm for sustainable agriculture, environment and global climate change. Environ. Sci. Pollut. Res. 2016, 23, 4358–4369. [Google Scholar] [CrossRef]
- Van Hove, C.; Baillonville, T.W.; Diara, H.F.; Godard, P.; Kodomi, Y.M.; Sangima, N. Azolla collection and selection. In Workshop on AzoIla Use; International Rice Research Institute: Fuzhou, China, 1987; pp. 77–87. [Google Scholar]
- Peters, G.A.; Toia, R.E.; Evans, W.R.; Crist, D.K.; Mayne, B.C.; Poole, R.E. Characterization and comparisons of 5 N2-Fixing Azolla-Anabaena associations, 1. Optimization of growth-conditions for biomass increase and N content in a controlled environment. Plant Cell Environ. 1980, 3, 261–269. [Google Scholar]
- Song, U.; Park, H.; Lee, E.J. Ecological responses and remediation ability of Water fern (Azolla japonica) to water pollution. J. Plant Biol. 2012, 55, 381–389. [Google Scholar] [CrossRef]
- Muradov, N.; Fidalgo, B.; Gujar, A.C.; Garceau, N.; T-Raissi, A. Production and characterization of Lemna minor bio-char and its catalytic application for biogas reforming. Biomass Bioenergy 2012, 42, 123–131. [Google Scholar] [CrossRef]
- Pagliuso, D.; Grandis, A.; Igarashi, E.S.; Lam, E.; Buckeridge, M.S. Correlation of apiose levels and growth rates in Duckweeds. Front. Chem. 2018, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.F.; Liu, Z.Q.; Rochfort, S.; Mouradov, A. Lipid production in aquatic plant Azolla at vegetative and reproductive stages and in response to abiotic stress. Plant Physiol. Biochem. 2018, 124, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.N.; Shahbazi, A.; Croonenberghs, J.; Wang, L.J. Oil production from duckweed by thermochemical liquefaction. Energy Sources Part A 2010, 32, 1293–1300. [Google Scholar] [CrossRef]
- Aslan, M. Optimal operation conditions for bio-hydrogen production from duckweed. Energy Sources Part A 2016, 38, 2072–2078. [Google Scholar] [CrossRef]
- Xu, J.L.; Deshusses, M.A. Fermentation of swine wastewater-derived duckweed for biohydrogen production. Int. J. Hydrogen Energy 2015, 40, 7028–7036. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Dhillon, K.S.; Bañuelos, G.S. Overview and prospects of selenium phytoremediation approaches. In Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 277–321. [Google Scholar]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Kadlec, R.H. Phosphorus removal in emergent free surface wetlands. J. Environ. Sci. Health A 2005, 40, 1293–1306. [Google Scholar] [CrossRef]
- Olguin, E.J.; Sanchez, G.; Mercado, G. Cleaner production and environmentally sound biotechnology for the prevention of upstream nutrient pollution in the Mexican coast of the Gulf of Mexico. Ocean Coast. Manag. 2004, 47, 641–670. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Impact of density loads on performance of duckweed bioreactor: A potential system for synchronized wastewater treatment and energy biomass production. Environ. Prog. Sustain. 2015, 34, 1596–1604. [Google Scholar] [CrossRef]
- Wang, W.G.; Yang, C.; Tang, X.Y.; Zhu, Q.L.; Pan, K.; Cai, D.G.; Hu, Q.C.; Ma, D.W. Carbon and energy fixation of great duckweed Spirodela polyrhiza growing in swine wastewater. Environ. Sci. Pollut. Res. 2015, 22, 15804–15811. [Google Scholar] [CrossRef]
- Akkoyunlu, A.; Bayhan, H.; Akiner, M.E.; Erturk, F. Wastewater treatment in a pilot-scale constructed wetland. Fresen. Environ. Bull. 2015, 24, 4589–4596. [Google Scholar]
- Saha, P.; Banerjee, A.; Sarkar, S. Phytoremediation potential of Duckweed (Lemna minor L.) on steel wastewater. Int. J. Phytoremed. 2015, 17, 589–596. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, Y.; Jin, Y.; Huang, J.; Bao, S.; Fu, T.; He, Z.; Wang, F.; Wang, M.; Zhao, H. Pilot-scale comparison of four duckweed strains from different genera for potential application in nutrient recovery from wastewater and valuable biomass production. Plant Biol. 2015, 17, 82–90. [Google Scholar] [CrossRef]
- Cheng, J.; Landesman, L.; Bergmann, B.A.; Classen, J.J.; Howard, J.W.; Yamamoto, Y.T. Nutrient removal from swine lagoon liquid by Lemna minor 8627. Trans. ASAE 2002, 45, 1003–1010. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Knecht, M.F.; Goransson, A. Terrestrial plants require nutrients in similar proportions. Tree Physiol. 2004, 24, 447–460. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Körner, S.; Vermaat, J.E. The relative importance of Lemna gibba L., bacteria and algae for the nitrogen and phosphorus removal in duckweed-covered domestic wastewater. Water Res. 1998, 32, 3651–3661. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Khalid Hanif, M. Performance of common duckweed species (Lemnaceae) and the waterfern Azolla filiculoides on different types of waste water. Water Res. 1998, 32, 2569–2576. [Google Scholar] [CrossRef]
- Zirschky, J.; Reed, S.C. The use of duckweed for wastewater treatment. J. Water Pollut. Control Fed. 1988, 60, 1253–1258. [Google Scholar]
- Weres, O.; Jaouni, A.O.; Tsao, L. The distribution, speciation and geochemical cycling of selenium in a sedimentary environment. Appl. Geochem. 1989, 4, 543–563. [Google Scholar] [CrossRef]
- Ohlendorf, H.M.; Hothem, R.L.; Bunck, C.M.; Aldrich, T.W.; Moore, J.F. Relationships between selenium concentrations and avian reproduction. Trans. N. Am. Wildl. Nat. Resour. Conf. 1986, 51, 330–342. [Google Scholar]
- Presser, T.S.; Ohlendorf, H.M. Biogeochemical cycling of selenium in the San-Joaquin valley. Environ. Manag. 1987, 11, 805–821. [Google Scholar] [CrossRef]
- Conde, J.E.; Alaejos, M.S. Selenium concentrations in natural and environmental waters. Chem. Rev. 1997, 97, 1979–2003. [Google Scholar] [CrossRef]
- Zhong, Y.; Cheng, J.J. Effects of selenium on biological and physiological properties of the duckweed Landoltia punctata. Plant Biol. 2016, 18, 797–804. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y.; Cheng, J.J. Effects of selenite on chlorophyll fluorescence, starch content and fatty acid in the duckweed Landoltia punctata. J. Plant Res. 2016, 129, 997–1004. [Google Scholar] [CrossRef]
- Ornes, W.H.; Sajwan, K.S.; Dosskey, M.G.; Adriano, D.C. Bioaccumulation of selenium by floating Aquatic plants. Water Air Soil Pollut. 1991, 57–58, 53–57. [Google Scholar] [CrossRef]
- Yan, Y.H.; Candreva, J.; Shi, H.; Ernst, E.; Martienssen, R.; Schwender, J.; Shanklin, J. Survey of the total fatty acid and triacylglycerol composition and content of 30 duckweed species and cloning of a Delta 6-desaturase responsible for the production of gamma-linolenic and stearidonic acids in Lemna gibba. BMC Plant Biol. 2013, 13, 201. [Google Scholar] [CrossRef]
- Xu, J.L.; Cui, W.H.; Cheng, J.J.; Stomp, A.M. Production of high-starch duckweed and its conversion to bioethanol. Biosyst. Eng. 2011, 110, 67–72. [Google Scholar] [CrossRef]
- Cheng, J.J.; Stomp, A.M. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. CLEAN-Soil Air Water 2009, 37, 17–26. [Google Scholar] [CrossRef]
- Bowles, R.D.; Hunt, A.E.; Bremer, G.B.; Duchars, M.G.; Eaton, R.A. Long-chain n-3 polyunsaturated fatty acid production by members of the marine protistan group the thraustochytrids: Screening of isolates and optimisation of docosahexaenoic acid production. In Progress in Industrial Microbiology; Osinga, R., Tramper, J., Burgess, J.G., Wijffels, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 35, pp. 193–202. [Google Scholar]
- Fan, K.-W.; Jiang, Y.; Faan, Y.-W.; Chen, F. Lipid Characterization of Mangrove Thraustochytrid − Schizochytrium mangrovei. J. Agric. Food Chem. 2007, 55, 2906–2910. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, S. Thraustochytrid marine protists: Production of PUFAs and other emerging technologies. Mar. Biotechnol. 2008, 10, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Manikan, V.; Nazir, M.Y.M.; Kalil, M.S.; Isa, M.H.M.; Kader, A.J.A.; Yusoff, W.M.W.; Hamid, A.A. A new strain of docosahexaenoic acid producing microalga from Malaysian coastal waters. Algal Res. 2015, 9, 40–47. [Google Scholar] [CrossRef]
- Jaseera, K.V.; Kaladharan, P.; Vijayan, K.K.; Sandhya, S.V.; Antony, M.L.; Pradeep, M.A. Isolation and phylogenetic identification of heterotrophic thraustochytrids from mangrove habitats along the southwest coast of India and prospecting their PUFA accumulation. J. Appl. Phycol. 2019, 31, 1057–1068. [Google Scholar] [CrossRef]
- Juntila, D.J.; Yoneda, K.; Suzuki, I. Identification of extracellular proteins from Aurantiochytrium sp 18W-13a. J. Appl. Phycol. 2018, 30, 63–69. [Google Scholar] [CrossRef]
- Taoka, Y.; Nagano, N.; Okita, Y.; Izumida, H.; Sugimoto, S.; Hayashi, M. Extracellular enzymes produced by marine eukaryotes, thraustochytrids. Biosci. Biotechnol. Biochem. 2009, 73, 180–182. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, D.; Barrow, C.J.; Puri, M. Exploring potential use of Australian thraustochytrids for the bioconversion of glycerol to omega-3 and carotenoids production. Biochem. Eng. J. 2013, 78, 11–17. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Fang, Y.; Jin, Y.L.; Huang, J.; Bao, S.; Fu, T.; He, Z.M.; Wang, F.; Zhao, H. Potential of duckweed in the conversion of wastewater nutrients to valuable biomass: A pilot-scale comparison with water hyacinth. Bioresour. Technol. 2014, 163, 82–91. [Google Scholar] [CrossRef] [PubMed]
- McAloon, A.; Taylor, F.; Yee, W.; Ibsen, K.; Wooley, R. Determining the Cost of Producing Ethanol from Corn Starch and Lignocellulosic Feedstocks; National Renewable Energy Laboratory: Golden, CO, USA, 2000. [Google Scholar]
- Chen, Q.; Jin, Y.L.; Zhang, G.H.; Fang, Y.; Xiao, Y.; Zhao, H. Improving production of bioethanol from Duckweed (Landoltia punctata) by pectinase pretreatment. Energies 2012, 5, 3019–3032. [Google Scholar] [CrossRef]
- Subudhi, S.; Nayak, T.; Kumar, N.R.; Vijayananth, P.; Lal, B. Impact of regulated pH on proto scale hydrogen production from xylose by an alkaline tolerant novel bacterial strain, Enterobacter cloacae DT-1. Int. J. Hydrogen Energy 2013, 38, 2728–2737. [Google Scholar] [CrossRef]
- Junghare, M.; Subudhi, S.; Lal, B. Improvement of hydrogen production under decreased partial pressure by newly isolated alkaline tolerant anaerobe, Clostridium butyricum TM-9A: Optimization of process parameters. Int. J. Hydrogen Energy 2012, 37, 3160–3168. [Google Scholar] [CrossRef]
- Mandal, B.; Nath, K.; Das, D. Improvement of biohydrogen production under decreased partial pressure of H-2 by Enterobacter cloacae. Biotechnol. Lett. 2006, 28, 831–835. [Google Scholar] [CrossRef]
- Pioneer Hi-Bred Australia, P.L. Growth Potential Corn Growers Workshop. Available online: http://www.pioneer.com/CMRoot/International/Australia_Intl/Publications/Corn_Workshop_Book.pdf (accessed on 16 June 2016).
- Abbas, C.A.; Binder, T.P.; Solheim, L.P.; Matlock, M. Process for Hydrogen Gas Production from Carbohydrate Feedstocks. U.S. Patent 7,998,455, 16 August 2011. [Google Scholar]
- Voca, N.; Varga, B.; Kricka, T.; Curic, D.; Jurisic, V.; Matin, A. Progress in ethanol production from corn kernel by applying cooking pre-treatment. Bioresour. Technol. 2009, 100, 2712–2718. [Google Scholar] [CrossRef]
- Kim, Y.; Mosier, N.S.; Hendrickson, R.; Ezeji, T.; Blaschek, H.; Dien, B.; Cotta, M.; Dale, B.; Ladisch, M.R. Composition of corn dry-grind ethanol by-products: DDGS, wet cake, and thin stillage. Bioresour. Technol. 2008, 99, 5165–5176. [Google Scholar] [CrossRef]
- Singh, S.S.; Singh, S.K.; Mishra, A.K. Na+ regulation by combined nitrogen in Azolla pinnata-Anabaena azollae symbiotic association during salt toxicity. Ecotoxicol. Environ. Saf. 2008, 69, 32–38. [Google Scholar] [CrossRef]
- Gupta, A.; Wilkens, S.; Adcock, J.L.; Puri, M.; Barrow, C.J. Pollen baiting facilitates the isolation of marine thraustochytrids with potential in omega-3 and biodiesel production. J. Ind. Microbiol. Biotechnol. 2013, 40, 1231–1240. [Google Scholar] [CrossRef]
- Burja, A.M.; Radianingtyas, H.; Windust, A.; Barrow, C.J. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: Screening of strains and optimization of omega-3 production. Appl. Microbiol. Biotechnol. 2006, 72, 1161–1169. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
| Species | Biomass, gDW/L | NH4 Uptake | PO4 Uptake | NO3 Uptake | Se Uptake | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | NH4 *, % | NH4 ** Total, % | PO4-P *, % | PO4-P ** Total, % | NO3 *, % | NO3 ** Total, % | Se *, % | Se ** total, % | |
| 100% SeSW | ||||||||||
| L. p # | 0.44 ± 0.1 | 0.66 ± 0.1 | 66.7 ± 9 | NA | 22.5 ± 8.8 | NA | 86.7 ± 11.7 | NA | 65 ± 9.2 | NA |
| A. f ## | 0.37 ± 0.1 | 0.5 ± 0.1 | 70.4 ± 11.2 | 90 ± 7.9 | 57.0 ± 11.3 | 67.0 ± 11.3 | 100 | 100 | 85.1 ± 7.3 | 94 ± 12.2 |
| L. p ## | 0.32 ± 0.1 | 0.7 ± 0.1 | 90.4 ± 9.3 | 96.5 ± 6.8 | 24.1 ± 5.3 | 43.1 ± 8.3 | 100 | 100 | 86.5 ± 4.7 | 98 ± 10.2 |
| 50% SeSW | ||||||||||
| L. p # | 0.44 ± 0.1 | 0.96 ± 0.1 | 73.9 ± 8.4 | NA | 42.5 ± 13.3 | NA | 93.5 ± 21.7 | NA | 84 ± 12.2 | NA |
| A. f ## | 0.3 ± 0.1 | 0.6 ± 0.1 | 100 | 100 | 89.2 ± 14.5 | 93.2 ± 15.4 | 100 | 100 | 70.5 ± 1.8 | 95 ± 12.3 |
| L. p ## | 0.31 ± 0.1 | 0.8 ± 0.1 | 100 | 100 | 41.2 ± 9.9 | 65.2 ± 8.4 | 100 | 100 | 65.5 ± 3.9 | 94 ± 13.4 |
| Wastewater Dilutions | Se, mg/g DW |
|---|---|
| L. punctata (Primary Treatment) | |
| Control | 0.042 ± 0.01 |
| 50% * | 0.25 ± 0.1 |
| 100% * | 0.56 ± 0.1 |
| A. filiculoides (Secondary Treatment) | |
| Control | 0.02 ± 0.01 |
| 50% ** | 0.18 ± 0.09 |
| 100% ** | 0.42 ± 0.01 |
| L. punctata (Secondary Treatment) | |
| Control | 0.01 ± 0.03 |
| 50% ** | 0.16 ± 0.09 |
| 100% ** | 0.38 ± 0.01 |
| Strains | Starch | Cellulose | L. punctata | A. filiculoides | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amylase, Units min−1 mL−1 | Cellulase, Units min−1 mL−1 | Amylase, Units min−1 mL−1 | Cellulase, Units min−1 mL−1 | Amylase, Units min−1 mL−1 | Cellulase, Units min−1 mL−1 | |||||||
| t = 0 | t = 72 h | t = 0 | t = 72 h | t = 0 | t = 72 h | t = 0 | t = 72 h | t = 0 | t = 72 h | t = 0 | t = 72 h | |
| MAN43 | 10.2 ± 3.1 | 29.3 ± 6.7 | 2.0 ± 0.7 | 18.1 ± 4.3 | 13.4 ± 4.1 | 33.4 ± 11.2 | 6.2 ± 2.1 | 9.0 ± 0.9 | 10.2 ± 3.4 | 28.7 ± 8.1 | 6.3 ± 2.2 | 18.4 ± 6.3 |
| Reducing Sugars | Acid Treatment | Enzymatic Treatment |
|---|---|---|
| Concentration (g/L) | Concentration (g/L) | |
| Glucose | 12 ± 0.04 | 11.80 ± 0.04 |
| Xylose | 4.36 ± 0.05 | 0.924 ± 0.03 |
| Arabinose | 0.04 ± 0.02 | 0 |
| Biomass Treatment | H2 Production, (mmol/L) | Volatile Fatty Acid | B/A Ratio | H2 Yield * | |
|---|---|---|---|---|---|
| Acetic Acid, (g/L) | Butyric Acid, (g/L) | ||||
| Acid Treated Prehydrolysate | 62 ± 0.08 | 0.712 ± 0.03 | 0.302 ± 0.03 | 0.42 | 2.14 ± 0.04 |
| Enzymatic Saccharified Hydrolysate | 40 ± 0.05 | 0.726 ± 0.02 | 0.302 ± 0.04 | 0.55 | 1.8 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, A.F.; Kumar, N.R.; Spangenberg, G.; Subudhi, S.; Lal, B.; Mouradov, A. Aquatic Plants, Landoltia punctata, and Azolla filiculoides as Bio-Converters of Wastewater to Biofuel. Plants 2020, 9, 437. https://doi.org/10.3390/plants9040437
Miranda AF, Kumar NR, Spangenberg G, Subudhi S, Lal B, Mouradov A. Aquatic Plants, Landoltia punctata, and Azolla filiculoides as Bio-Converters of Wastewater to Biofuel. Plants. 2020; 9(4):437. https://doi.org/10.3390/plants9040437
Chicago/Turabian StyleMiranda, Ana F., N. Ram Kumar, German Spangenberg, Sanjukta Subudhi, Banwari Lal, and Aidyn Mouradov. 2020. "Aquatic Plants, Landoltia punctata, and Azolla filiculoides as Bio-Converters of Wastewater to Biofuel" Plants 9, no. 4: 437. https://doi.org/10.3390/plants9040437
APA StyleMiranda, A. F., Kumar, N. R., Spangenberg, G., Subudhi, S., Lal, B., & Mouradov, A. (2020). Aquatic Plants, Landoltia punctata, and Azolla filiculoides as Bio-Converters of Wastewater to Biofuel. Plants, 9(4), 437. https://doi.org/10.3390/plants9040437






