Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Subjects
2.3. Declarations: Ethics Approval, Consent to Participate, and Consent for Publication
2.4. Eligibility Criteria
2.5. Intervention Protocol
2.6. Measurements
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lattanzio, S.M. Fibromyalgia Syndrome: A Metabolic Approach Grounded in Biochemistry for the Remission of Symptoms. Front. Med. 2017, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marchi, L.; Marzetti, F.; Orrù, G.; Lemmetti, S.; Miccoli, M.; Ciacchini, R.; Hitchcott, P.K.; Bazzicchi, L.; Gemignani, A.; Conversano, C. Alexithymia and Psychological Distress in Patients With Fibromyalgia and Rheumatic Disease. Front. Psychol. 2019, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Rapti, E.; Matsoukas, S.; Kotsa, K. Vitamin D in Fibromyalgia: A Causative or Confounding Biological Interplay? Nutrients 2016, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Aloush, V. Fibromyalgia, obesity and all that lies in between. Harefuah 2019, 158, 587–588. [Google Scholar] [PubMed]
- Gunduz, N.; Üşen, A.; Atar, E.A. The Impact of Perceived Social Support on Anxiety, Depression and Severity of Pain and Burnout Among Turkish Females With Fibromyalgia. Arch. Rheumatol. 2018, 34, 186–195. [Google Scholar] [CrossRef]
- Silber, B.; Schmitt, J. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci. Biobehav. Rev. 2010, 34, 387–407. [Google Scholar] [CrossRef]
- Kösehasanoğullari, M.; Gündüz, N.E.; Akalin, E.; Kösehasanoğulları, M. Is Fibromyalgia Syndrome a Neuropathic Pain Syndrome? Arch. Rheumatol. 2018, 34, 196–203. [Google Scholar] [CrossRef]
- Hulens, M.; Rasschaert, R.; Vansant, G.; Stalmans, I.; Bruyninckx, F.; Dankaerts, W. The link between idiopathic intracranial hypertension, fibromyalgia, and chronic fatigue syndrome: Exploration of a shared pathophysiology. J. Pain Res. 2018, 11, 3129–3140. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed. Pharmacother. 2018, 103, 531–538. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Wittwer, J.; Vargas, K.; Hogan, E.; Holmes, A.; Rogers, P.J.; Goralczyk, R.; Gibson, E.L. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br. J. Nutr. 2015, 113, 350–365. [Google Scholar] [CrossRef]
- Martins, Y.A.; Cardinali, C.A.E.F.; Ravanelli, M.I.; Brunaldi, K. Is hypovitaminosis D associated with fibromyalgia? A systematic review. Nutr. Rev. 2019, 78, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Blankfield, A. A Brief Historic Overview of Clinical Disorders Associated with Tryptophan: The Relevance to Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). Int. J. Tryptophan Res. 2012, 5, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F. The role of diet in the treatment of fibromyalgia. Pain Manag. 2016, 6, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, S.M.; Imbesi, F. Fibromyalgia Syndrome: A Case Report on Controlled Remission of Symptoms by a Dietary Strategy. Front. Med. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional Aspects of Depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef]
- Banerjee, S.; Jones, S. Magnesium as an Alternative or Adjunct to Opioids for Migraine and Chronic Pain: A Review of the Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2017.
- Ping, L.; Xu, J.; Zhou, C.; Lu, J.; Lu, Y.; Shen, Z.; Jiang, L.; Dai, N.; Xu, X.; Cheng, Y. Tryptophan hydroxylase-2 polymorphism is associated with white matter integrity in first-episode, medication-naïve major depressive disorder patients. Psychiatry Res. Neuroimaging 2019, 286, 4–10. [Google Scholar] [CrossRef]
- Tanya, S.; Chutima, R.; Andre, F.C.; Michel, B.; Michael, M. Anxiety disorders: Sex differences in serotonin and tryptophan metabolism. Curr. Top. Med. Chem. 2018, 18, 1704–1715. [Google Scholar]
- Rezende, R.M.; Pelúzio, M.d.C.G.; de Jesus Silva, F.; Lucia, E.M.D.; Favarato, L.S.C.; Martino, H.S.D. Does aerobic exercise associated with tryptophan supplementation attenuates hyperalgesia and inflammation in female rats with experimental fibromyalgia? PLoS ONE 2019, 14, e0211824. [Google Scholar] [CrossRef]
- Batista, E.D.; Andretta, A.; De Miranda, R.C.; Nehring, J.; Paiva, E.D.S.; Schieferdecker, M.E.M. Food intake assessment and quality of life in women with fibromyalgia. Rev. Bras. de Reum. (English Ed.) 2016, 56, 105–110. [Google Scholar] [CrossRef]
- Barron, L.J.; Barron, R.F.; Johnson, J.C.S.; Wagner, I.; Ward, C.J.B.; Ward, S.R.B.; Barron, F.M.; Ward, W.K. A retrospective analysis of biochemical and haematological parameters in patients with eating disorders. J. Eat. Disord. 2017, 5, 32. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2019, 59, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Boatella, J.; Rafecas, M.; Rafecas, M. Nuts: Source of energy and macronutrients. Br. J. Nutr. 2006, 96, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Latorre Román, P.Á.; López Munera, R.; Izquierdo Rus, T.; García Pinillos, F. La Satisfacción Corporal en Adultos Españoles, Influencia del Sexo, Edad y Estado Ponderal. Rev. Iberoam Diagnóstico y Evaluación e Avaliação Psicológica 2018, 47, 83–94. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Carneiro, A.V. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition 2020, 69, 110559. [Google Scholar] [CrossRef] [PubMed]
- Adjibade, M.; Assmann, K.; Andreeva, V.; Lemogne, C.; Hercberg, S.; Galán, P.; Kesse-Guyot, E. Prospective association between adherence to the Mediterranean diet and risk of depressive symptoms in the French SU.VI.MAX cohort. Eur. J. Nutr. 2017, 57, 1225–1235. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Mantzorou, M.; Serdari, A.; Bonotis, K.; Vasios, G.; Pavlidou, E.; Trifonos, C.; Vadikolias, K.; Petridis, D.; Giaginis, C. Evaluating Mediterranean diet adherence in university student populations: Does this dietary pattern affect students’ academic performance and mental health? Int. J. Heal. Plan. Manag. 2019, 35, 5–21. [Google Scholar] [CrossRef]
- Suresh, K.P. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, M. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Agarwal, S.; Fulgoni, V.L. Tryptophan Intake in the US Adult Population Is Not Related to Liver or Kidney Function but Is Associated with Depression and Sleep Outcomes. J. Nutr. 2016, 146, 2609S–2615S. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/?query=ndbNumber:12155 (accessed on 1 February 2019).
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. Neuroimaging 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Córdoba, F.E.; Eslava-Schmalbach, J. Validación colombiana del índice de calidad de sueño de Pittsburgh. Revista de Neurología 2005, 40, 150. [Google Scholar] [CrossRef]
- Raich, R.M.; Mora, M.; Soler, A.; Avila, C.; Clos, I.; Zapater, L. Adaptación de un instrumento de evaluación de la insatisfacción corporal. Adaptation of a body dissatisfaction assessment instrument. Clínica y Salud 1996, 7, 51–66. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Buela-Casal, G.; Guillén, A.; Nicolás, R. Cuestionario de Ansiedad Estado-Rasgo Adaptación Española; Manual: San Francisco, CA, USA, 1970. [Google Scholar]
- Avargues-Navarro, M.L.; Borda-Mas, M.; Asuero-Fernández, R.; Pérez-San-Gregorio, M.Á.; Martín-Rodríguez, A.; Beato-Fernández, L. Conductas purgativas y pronóstico terapéutico en mujeres con trastornos alimentarios tratadas en el contexto sanitario. Int. J. Clin. Heal. Psychol. 2017, 17, 120–127. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States. Educational and Industrial Testing Services: San Diego, CA, USA, 1971. [Google Scholar]
- Garner, D.M.; Bohr, Y.; Garfinkel, P.E. The Eating Attitudes Test: Psychometric Features and Clinical Correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef]
- Rivas, T.; Bersabé, R.; Jiménez, M.; Berrocal, C. The Eating Attitudes Test (EAT-26): Reliability and validity in Spanish female samples. Span. J. Psychol. 2010, 13, 1044–1056. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Gibson, E.L. Tryptophan supplementation and serotonin function: Genetic variations in behavioural effects. Proc Nutr Soc. 2018, 77, 174–188. [Google Scholar] [CrossRef]
- Song, C.; Lin, A.; Bonaccorso, S.; Heide, C.; Verkerk, R.; Kenis, G.; Bosmans, E.; Scharpe, S.; Whelan, A.; Cosyns, P.; et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J. Affect. Disord. 1998, 49, 211–219. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997.
- Nielsen, F.H.; Johnson, L.; Zeng, H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res. 2011, 23, 158–168. [Google Scholar]
- Bernaras, E.; Jaureguizar, J.; Garaigordobil, M. Child and Adolescent Depression: A Review of Theories, Evaluation Instruments, Prevention Programs, and Treatments. Front. Psychol. 2019, 10, 543. [Google Scholar] [CrossRef]
- Chen, G.; Hu, X.; Li, L.; Huang, X.; Lui, S.; Kuang, W.; Ai, H.; Bi, F.; Gu, Z.; Gong, Q. Disorganization of white matter architecture in major depressive disorder: A meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci. Rep. 2016, 6, 21825. [Google Scholar] [CrossRef]
- Zhang, X.; Beaulieu, J.M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan Hydroxylase-2 Controls Brain Serotonin Synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef] [PubMed]
- Alciati, A.; Atzeni, F.; Grassi, M.; Caldirola, D.; Sarzi-Puttini, P.; Angst, J.; Perna, G. Features of mood associated with high body weight in females with fibromyalgia. Compr. Psychiatry 2018, 80, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Marsá, M.; Alberdi-Páramo, Í.; Niell-Galmés, L. Suplementos nutricionales en trastornos de la conducta alimentaria. Actas esp Psiquiatr 2017, 45, 16–36. [Google Scholar]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. AGE 2012, 35, 1277–1285. [Google Scholar] [CrossRef]
- Samad, N.; Yasmin, F.; Naheed, S.; Bari, A.Z.; Ayaz, M.M.; Zaman, A. Serum levels of leptin, zinc and tryptophan in obese subjects with sleep deficits. Pak J. Pharm. Sci. 2017, 30, 1431–1438. [Google Scholar]
- Brown, C.C.; Horrom, N.J.; Wagman, A.M. Effects of L-tryptophan on sleep onset insomniacs. Waking Sleep. 1979, 3, 101–108. [Google Scholar]
- Spinweber, C. L-Tryptophan administered to chronic sleep-onset insomniacs: Late-appearing reduction of sleep latency. Psychopharmacology 1986, 90, 151–155. [Google Scholar] [CrossRef]
- Cao, Y.; Zhen, S.; Taylor, A.W.; Appleton, S.L.; Atlantis, E.; Shi, Z. Magnesium Intake and Sleep Disorder Symptoms: Findings from the Jiangsu Nutrition Study of Chinese Adults at Five-Year Follow-Up. Nutrients 2018, 10, 1354. [Google Scholar] [CrossRef]
- Murphy, S.E.; Longhitano, C.; Ayres, R.E.; Cowen, P.J.; Harmer, C. Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology 2006, 187, 121–130. [Google Scholar] [CrossRef]
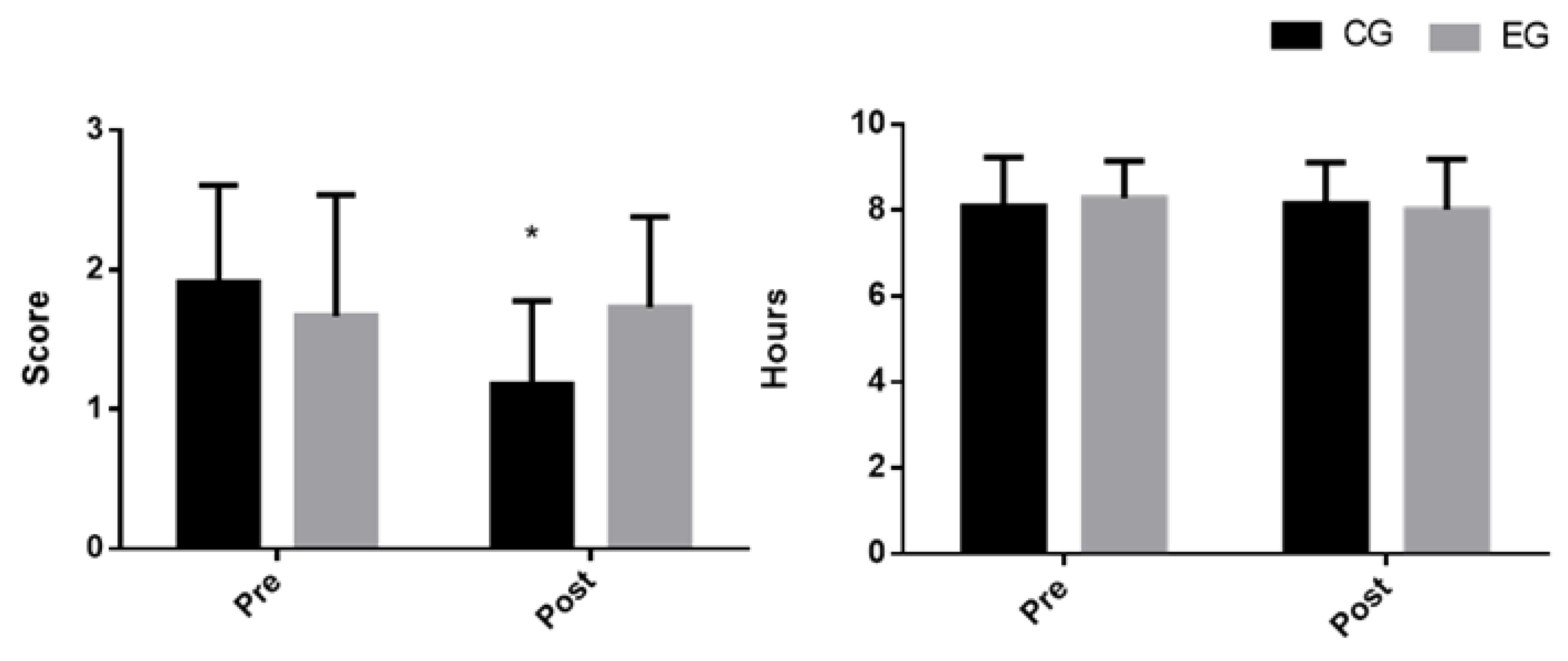
| Group | n | Mean | SD | Mean Post | SD Post | Time | Time × Group | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | ES η² | F | p | ES η² | ||||||
| PITTSBURG | |||||||||||
| Subjective sleep quality rating | |||||||||||
| Control | 11 | 1.55 | 1.51 | 1.18 | 1.33 | 1.67 | 0.211 | 0.013 | 0.034 | 0.856 | 0 |
| Experimental | 11 | 1.55 | 1.44 | 1.27 | 1.49 | ||||||
| Latency | |||||||||||
| Control | 11 | 1.55 | 0.93 | 1.55 | 0.82 | 0.132 | 0.721 | 0 | 0.132 | 0.721 | 0 |
| Experimental | 11 | 2 | 1 | 2.09 | 1.04 | ||||||
| Sleep Duration rating | |||||||||||
| Control | 11 | 1.91 | 0.7 | 1.18 | 0.6 | 7.62 | 0.013 | 0.061 | 7.62 | 0.013 | 0.061 |
| Experimental | 11 | 1.67 | 0.87 | 1.73 | 0.65 | ||||||
| Sleep Duration (h) | |||||||||||
| Control | 11 | 8.09 | 1.14 | 8.15 | 0.96 | 2.81 | 0.111 | 0.012 | 4.57 | 0.047 | 0.019 |
| Experimental | 11 | 8.28 | 0.87 | 8.01 | 1.18 | ||||||
| Sleep efficiency, % | |||||||||||
| Control | 11 | 73.65 | 16.21 | 82.43 | 12.58 | 9.3 | 0.007 | 0.059 | 0.57 | 0.461 | 0.004 |
| Experimental | 11 | 71.7 | 17.55 | 75.27 | 11.44 | ||||||
| Habitual sleep efficiency rating | |||||||||||
| Control | 11 | 1.36 | 1.29 | 0.91 | 1.04 | 4.35 | 0.052 | 0.034 | 0.1 | 0.752 | 0 |
| Experimental | 11 | 1.56 | 1.01 | 1.36 | 1.03 | ||||||
| Mean sleep disturbances rating | |||||||||||
| Control | 11 | 1.73 | 0.65 | 0.91 | 0.47 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 |
| Experimental | 11 | 1.91 | 0.3 | 1.91 | 0.54 | ||||||
| Mean sleep medication rating | |||||||||||
| Control | 11 | 0.55 | 0.82 | 0.27 | 0.65 | 3.5 | 0.076 | 0.059 | 0.07 | 0.792 | 0.001 |
| Experimental | 11 | 0.64 | 0.5 | 0.27 | 0.65 | ||||||
| Mean daytime dysfunction rating | |||||||||||
| Control | 11 | 1.82 | 0.75 | 1 | 0 | 7.62 | 0.019 | 0.272 | 0.85 | 0.377 | 0.03 |
| Experimental | 11 | 1.91 | 0.54 | 1.38 | 0.74 | ||||||
| Global sleep quality index | |||||||||||
| Control | 11 | 10.18 | 3.63 | 7.55 | 2.46 | 5.15 | 0.034 | 0.042 | 3.4 | 0.08 | 0.028 |
| Experimental | 11 | 10.27 | 4.13 | 10 | 3.55 | ||||||
| Group | n | Mean | SD | Mean Post | SD Post | Time | Time × Group | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | ES η² | F | p | ES η² | ||||||
| BSQ | |||||||||||
| Control | 11 | 66.64 | 39.09 | 60.00 | 41.05 | 5.53 | 0.029 | 0.017 | 0.37 | 0.550 | 0.001 |
| Experimental | 11 | 54.09 | 32.10 | 42.82 | 23.65 | ||||||
| STAI | |||||||||||
| Trait Anxiety | |||||||||||
| Control | 11 | 9.73 | 3.55 | 5.55 | 2.58 | 14.5 | 0.001 | 0.181 | 0.13 | 0.721 | 0.001 |
| Experimental | 11 | 11.55 | 5.84 | 8.09 | 3.70 | ||||||
| POMS Depression | |||||||||||
| Control | 11 | 7.64 | 4.20 | 5.09 | 4.93 | 18.63 | <0.001 | 0.062 | 0.88 | 0.359 | 0.003 |
| Experimental | 11 | 4.73 | 3.50 | 3.09 | 3.27 | ||||||
| POMS Anger | |||||||||||
| Control | 11 | 11.82 | 5.98 | 7.73 | 4.10 | 2.58 | 0.124 | 0.0435 | 0.866 | 0.363 | 0.001 |
| Experimental | 11 | 11.82 | 6.06 | 10.73 | 8.24 | ||||||
| POMS Vigor | |||||||||||
| Control | 11 | 4.91 | 2.17 | 5.18 | 4.83 | 0.003 | 0.959 | 0 | 0.1315 | 0.721 | 0.000 |
| Experimental | 11 | 10.09 | 5.30 | 9.73 | 4.43 | ||||||
| POMS Fatigue | |||||||||||
| Control | 11 | 13.64 | 3.01 | 10.18 | 4.47 | 12.36 | 0.002 | 0.073 | 2.63 | 0.120 | 0.016 |
| Experimental | 11 | 11.18 | 4.51 | 9.91 | 5.01 | ||||||
| POMS Tension | |||||||||||
| Control | 11 | 36.45 | 8.64 | 38.27 | 10.45 | 0.365 | 0.553 | 0.003 | 2.467 | 0.132 | 0.024 |
| Experimental | 11 | 31.64 | 7.32 | 27.55 | 9.52 | ||||||
| Total Score | |||||||||||
| Control | 11 | 137.91 | 13.15 | 123.36 | 12.15 | 14.03 | 0.001 | 0.105 | 1.66 | 0.212 | 0.013 |
| Experimental | 11 | 129.18 | 19.13 | 122.09 | 19.10 | ||||||
| Eating Disorders (EAT-26) | |||||||||||
| Diet | |||||||||||
| Control | 11 | 12.55 | 8.29 | 10.18 | 9.61 | 10.61 | 0.004 | 0.025 | 0.054 | 0.818 | 0.000 |
| Experimental | 11 | 7.00 | 6.83 | 4.27 | 5.85 | ||||||
| Bulimia | |||||||||||
| Control | 11 | 5.00 | 4.54 | 5.27 | 3.10 | 0.045 | 0.833 | 0 | 0.045 | 0.883 | 0.000 |
| Experimental | 11 | 3.91 | 2.51 | 3.91 | 2.66 | ||||||
| Oral control | |||||||||||
| Control | 11 | 4.36 | 3.23 | 3.73 | 3.41 | 1.107 | 0.305 | 0.012 | 0.005 | 0.945 | 0.000 |
| Experimental | 11 | 3.00 | 3.19 | 2.27 | 2.45 | ||||||
| Total Score | |||||||||||
| Control | 11 | 21.91 | 13.25 | 17.73 | 15.01 | 9.273 | 0.006 | 0.063 | 0.01 | 0.919 | 0.000 |
| Experimental | 11 | 13.91 | 10.38 | 10.00 | 9.47 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodríguez, A.; Rubio-Arias, J.Á.; Ramos-Campo, D.J.; Reche-García, C.; Leyva-Vela, B.; Nadal-Nicolás, Y. Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 2227. https://doi.org/10.3390/ijerph17072227
Martínez-Rodríguez A, Rubio-Arias JÁ, Ramos-Campo DJ, Reche-García C, Leyva-Vela B, Nadal-Nicolás Y. Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia. International Journal of Environmental Research and Public Health. 2020; 17(7):2227. https://doi.org/10.3390/ijerph17072227
Chicago/Turabian StyleMartínez-Rodríguez, Alejandro, Jacobo Á. Rubio-Arias, Domingo J. Ramos-Campo, Cristina Reche-García, Belén Leyva-Vela, and Yolanda Nadal-Nicolás. 2020. "Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia" International Journal of Environmental Research and Public Health 17, no. 7: 2227. https://doi.org/10.3390/ijerph17072227
APA StyleMartínez-Rodríguez, A., Rubio-Arias, J. Á., Ramos-Campo, D. J., Reche-García, C., Leyva-Vela, B., & Nadal-Nicolás, Y. (2020). Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia. International Journal of Environmental Research and Public Health, 17(7), 2227. https://doi.org/10.3390/ijerph17072227







