CRISPR/Cas9-mediated Disruption of Fibroblast Growth Factor 5 in Rabbits Results in a Systemic Long Hair Phenotype by Prolonging Anagen
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Construction and in vitro Transcription of Cas9 and sgRNAs
2.3. Microinjection and Embryo Transfer
2.4. Genotyping
2.5. Histopathology and Immunohistochemistry
2.6. Scanning Electron Microscopy
2.7. RT-qPCR
2.8. Western Blot Analysis
2.9. Prediction of the Modified FGF5 Protein Structure
2.10. Phenotypic Data
2.11. Statistical Analyses
2.12. Off-Target Assay
3. Results
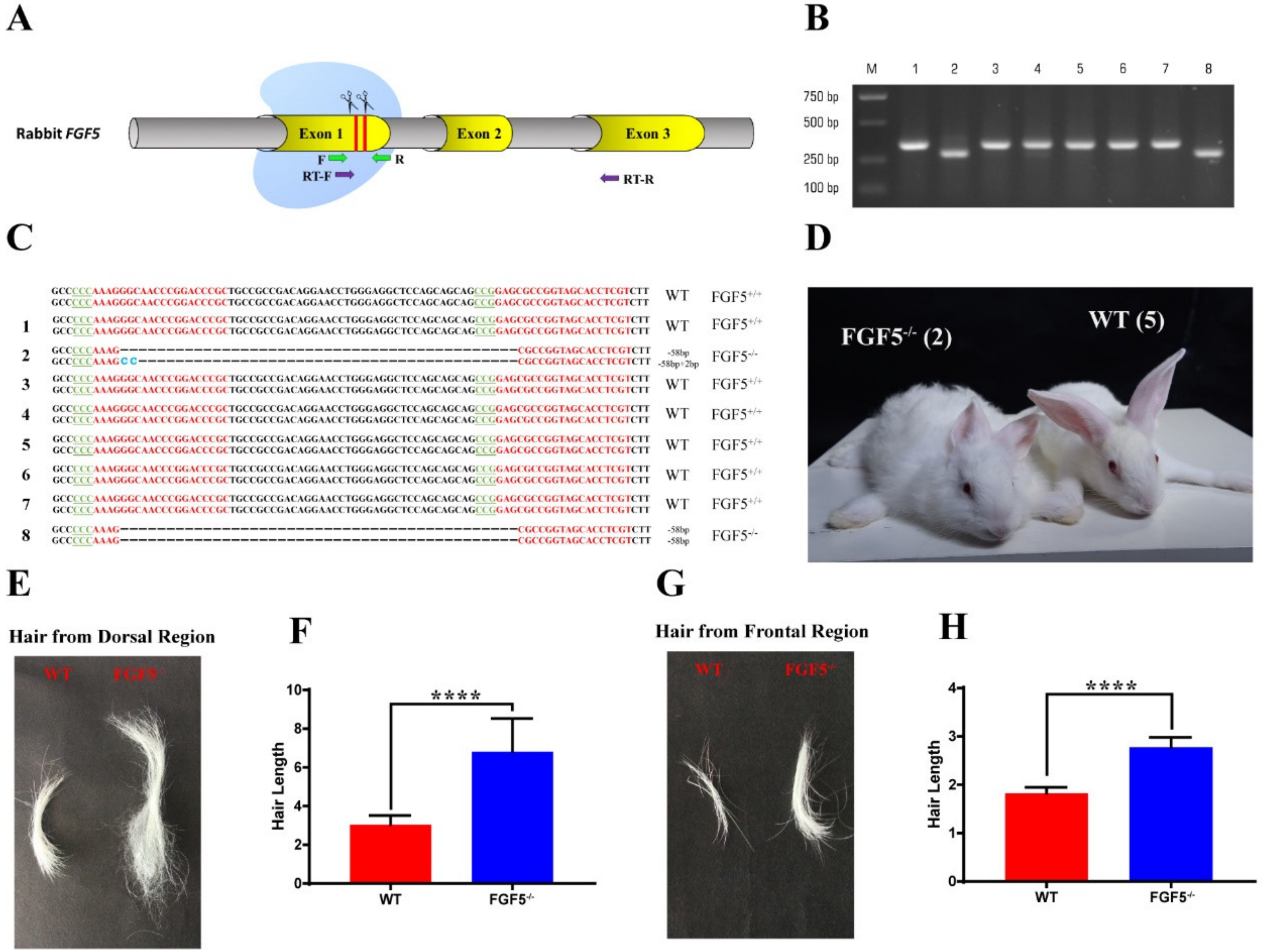
3.1. Generation of FGF5-/- Rabbits by the CRISPR/Cas9 System
3.2. Heritability of the Long Hair Phenotype in FGF5-/- Rabbits
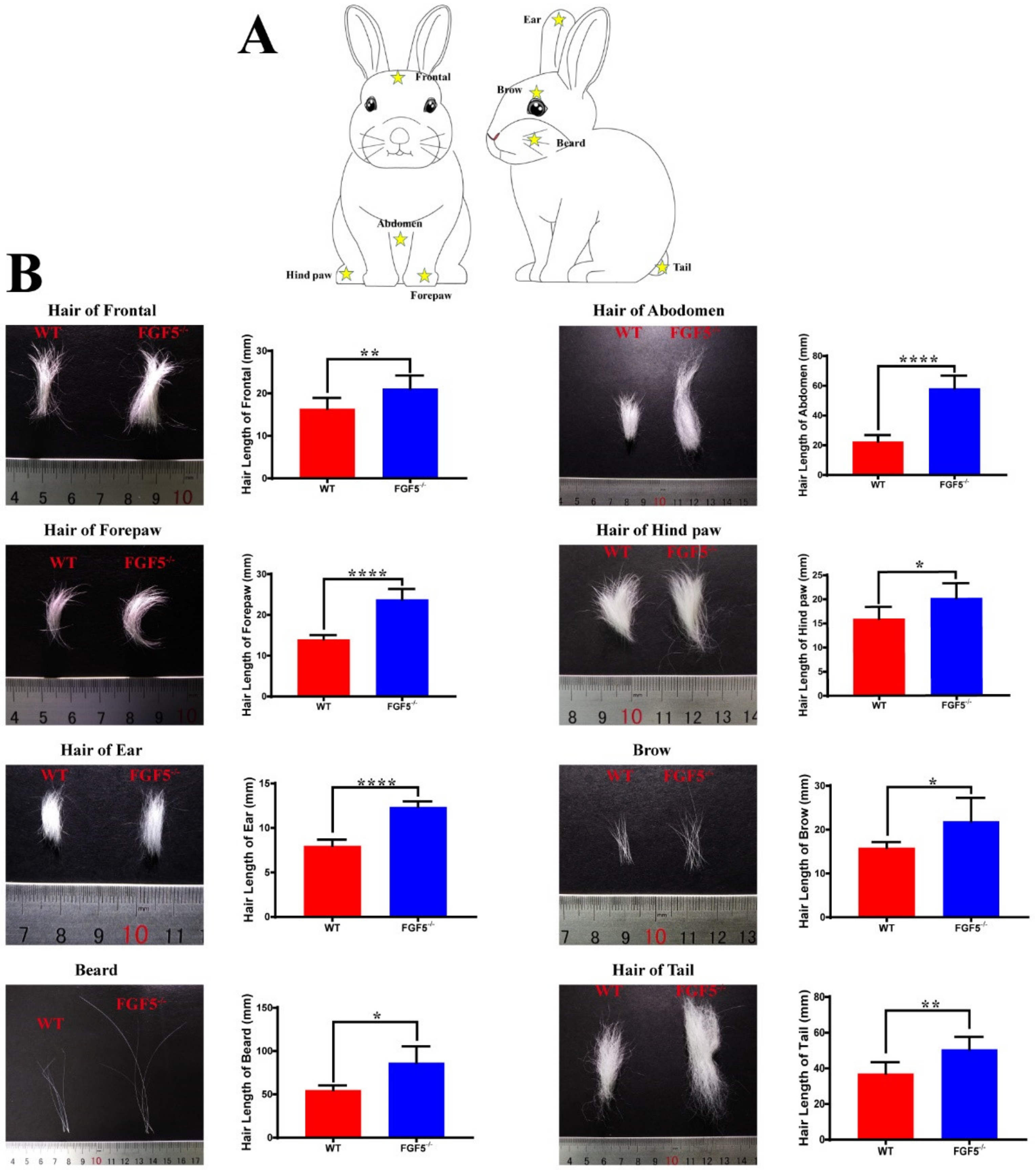
3.3. The Systemic Effect of Disruption of FGF5 in Rabbit Fur
3.4. Sex-Dominant Growth Pattern During Hair Development in FGF5-/- Rabbits
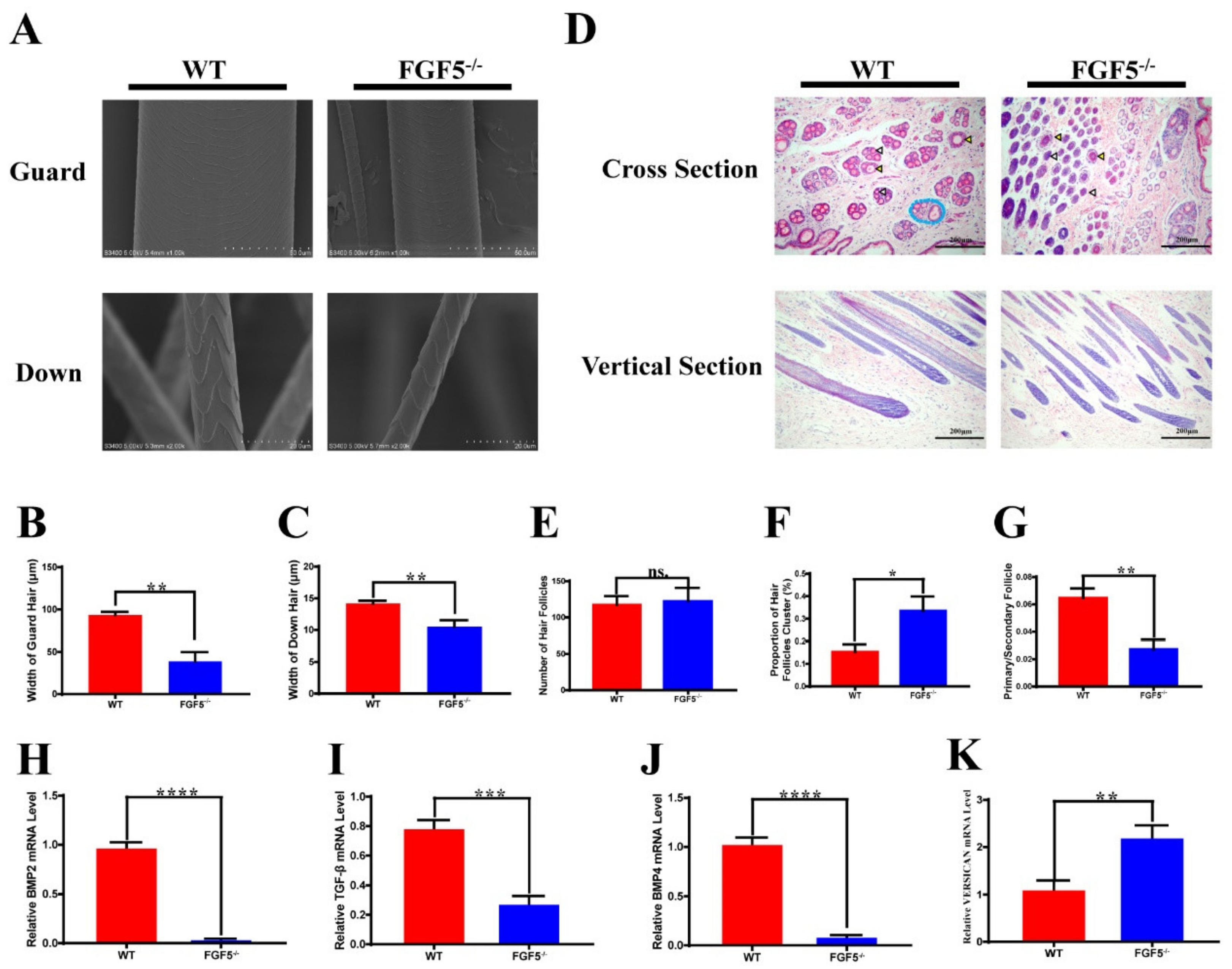
3.5. Morphological Characterization of Hair and Skin in FGF5-/- Rabbits
3.6. Structural Alterations of FGF5 and FGF5s Protein in FGF5-/- and WT Rabbits
3.7. The Long Hair Genotype in FGF5-/- Rabbits Was Caused by a Classical Pathway
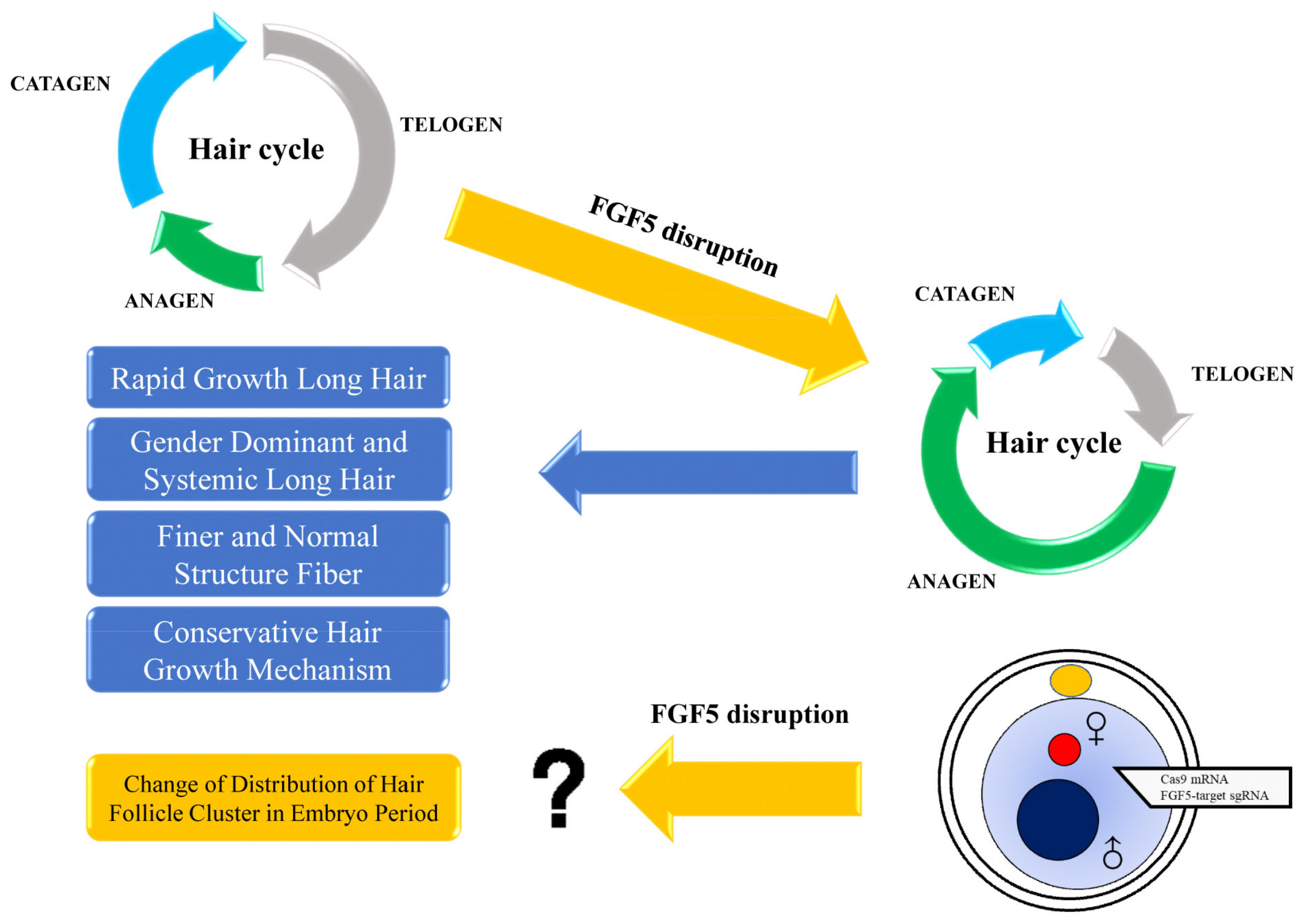
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ding, H.; Zhao, H.; Cheng, G.; Yang, Y.; Wang, X.; Zhao, X.; Qi, Y.; Huang, D. Analyses of histological and transcriptome differences in the skin of short-hair and long-hair rabbits. BMC Genom. 2019, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Diribarne, M.; Mata, X.; Riviere, J.; Bouet, S.; Vaiman, A.; Chapuis, J.; Reine, F.; Fleurot, R.; Auvinet, G.; Deretz, S.; et al. LIPH expression in skin and hair follicles of normal coat and Rex rabbits. PLoS ONE 2012, 7, e30073. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.L.; Ishida, Y.; Nikolaidis, N.; Kolokotronis, S.O.; Fratpietro, S.; Stewardson, K.; Hensley, S.; Tisdale, M.; Boeskorov, G.; Greenwood, A.D. Genetic variation at hair length candidate genes in elephants and the extinct woolly mammoth. BMC Evol. Biol. 2009, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wang, L.; Plikus, M.V.; Jiang, T.X.; Murray, P.J.; Ramos, R.; Guerrero-Juarez, C.F.; Hughes, M.W.; Lee, O.K.; Shi, S.; et al. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 2005, 161, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Oh, J.W.; Lee, H.L.; Dhar, A.; Peng, T.; Ramos, R.; Guerrero-Juarez, C.F.; Wang, X.; Zhao, R.; Cao, X.; et al. A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning. Elife 2017, 6, e22772. [Google Scholar] [CrossRef]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef]
- Sundberg, J.P.; Rourk, M.H.; Boggess, D.; Hogan, M.E.; Sundberg, B.A.; Bertolino, A.P. Angora Mouse Mutation: Altered Hair Cycle, Follicular Dystrophy, Phenotypic Maintenance of Skin Grafts, and Changes in Keratin Expression. Vet. Pathol. 1997, 34, 8. [Google Scholar] [CrossRef]
- Drogemuller, C.; Rufenacht, S.; Wichert, B.; Leeb, T. Mutations within the FGF5 gene are associated with hair length in cats. Anim. Genet. 2007, 38, 218–221. [Google Scholar] [CrossRef]
- Housley, D.J.; Venta, P.J. The long and the short of it: Evidence that FGF5 is a major determinant of canine ‘hair’-itability. Anim. Genet. 2006, 37, 309–315. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Wang, H.D.; Shen, Y.; Liu, N.; Cao, J.; Yu, X.J.; Dong, C.S.; He, X.Y. Alpaca fiber growth is mediated by microRNA let-7b via down-regulation of target gene FGF5. Genet. Mol. Res. 2015, 14, 13754–13763. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Wada, K.; Shiomi, G.; Kameyama, Y.; Wakabayashi, Y.; Fukuta, K.; Hashizume, R. A 1-bp deletion in Fgf5 causes male-dominant long hair in the Syrian hamster. Mamm. Genome 2015, 26, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, B.; Zhou, J.; Zhu, H.; Niu, Y.; Ma, B.; Yu, H.; Lei, A.; Yan, H.; Shen, Q.; et al. Disruption of FGF5 in Cashmere Goats Using CRISPR/Cas9 Results in More Secondary Hair Follicles and Longer Fibers. PLoS ONE 2016, 11, e0164640. [Google Scholar]
- Wang, X.; Yu, H.; Lei, A.; Zhou, J.; Zeng, W.; Zhu, H.; Dong, Z.; Niu, Y.; Shi, B.; Cai, B.; et al. Generation of gene-modified goats targeting MSTN and FGF5 via zygote injection of CRISPR/Cas9 system. Sci. Rep. 2015, 5, 13878. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Xu, S.; Zhou, K.; Yang, G. Characterization of hairless (Hr) and FGF5 genes provides insights into the molecular basis of hair loss in cetaceans. BMC Evol. Biol. 2013, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, Q.T.; Sun, T.; Wang, F.; Liu, J.; Leach, R.; Johnson, A.; Puscheck, E.E.; Rappolee, D.A. FGF ligand family mRNA expression profile for mouse preimplantation embryos, early gestation human placenta, and mouse trophoblast stem cells. Mol. Reprod. Dev. 2006, 73, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Saitoh, Y.; Suzuki, S.; Ozawa, K.; Kawano, M.; Imamura, T. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem. Biophys. Res. Commun. 2002, 290, 169–176. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Sharov, A.A. BMP signaling in the control of skin development and hair follicle growth. Differentiation 2004, 72, 512–526. [Google Scholar] [CrossRef]
- Nesterova, A.; Nizamutdinov, I.; Golovatenko-Abramov, P.; Konyukhov, B. Fluctuations of BMP signaling pathway during hair cycles in skin of mice with mutant genes we, wal and Fgf5 (go). J. Dermatol. Sci. 2010, 60, 201–203. [Google Scholar] [CrossRef]
- Soma, T.; Tajima, M.; Kishimoto, J. Hair cycle-specific expression of versican in human hair follicles. J. Dermatol. Sci. 2005, 39, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Denda, S.; Soma, T.; Raftery, L.; Momoi, T.; Hibino, T. A potential suppressor of TGF-beta delays catagen progression in hair follicles. J. Investig. Dermatol. Symp. Proc. 2003, 8, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Li, W. Beyond mice: Genetically modifying larger animals to model human diseases. J. Genet. Genom. 2012, 39, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, S.; Vergou, T.; Sterry, W.; Lademann, J.; Patzelt, A. Comparative study of hair follicle morphology in eight mammalian species and humans. Skin Res. Technol. 2014, 20, 147–154. [Google Scholar] [CrossRef]
- Deng, J.; Chen, M.; Liu, Z.; Song, Y.; Sui, T.; Lai, L.; Li, Z. The disrupted balance between hair follicles and sebaceous glands in Hoxc13-ablated rabbits. FASEB J. 2019, 33, 1226–1234. [Google Scholar] [CrossRef]
- Sui, T.; Yuan, L.; Liu, H.; Chen, M.; Deng, J.; Wang, Y.; Li, Z.; Lai, L. CRISPR/Cas9-mediated mutation of PHEX in rabbit recapitulates human X-linked hypophosphatemia (XLH). Hum. Mol. Genet. 2016, 25, 2661–2671. [Google Scholar]
- Chen, M.; Yao, B.; Yang, Q.; Deng, J.; Song, Y.; Sui, T.; Zhou, L.; Yao, H.; Xu, Y.; Ouyang, H.; et al. Truncated C-terminus of fibrillin-1 induces Marfanoid-progeroid-lipodystrophy (MPL) syndrome in rabbit. Dis. Model. Mech. 2018, 11, dmm031542. [Google Scholar] [CrossRef]
- Caputi, M. A nonsense mutation in the fibrillin-1 gene of a Marfan syndrome patient induces NMD and disrupts an exonic splicing enhancer. Genes Dev. 2002, 16, 1754–1759. [Google Scholar] [CrossRef]
- Pavone, P.; Praticò, A.D.; Falsaperla, R.; Ruggieri, M.; Zollino, M.; Corsello, G.; Neri, G. Congenital generalized hypertrichosis: The skin as a clue to complex malformation syndromes. Ital. J. Pediatrics 2015, 41, 55. [Google Scholar] [CrossRef]
- Jean, M.; Hébert, T.; Rosenquist, J.; Götz, R.M. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar]
- He, X.; Chao, Y.; Zhou, G.; Chen, Y. Fibroblast growth factor 5-short (FGF5s) inhibits the activity of FGF5 in primary and secondary hair follicle dermal papilla cells of cashmere goats. Gene 2016, 575, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Liu, C.X.; Zhang, X.M.; Chen, L.; Peng, X.R.; He, S.G.; Lin, J.P.; Han, B.; Wang, L.Q.; Huang, J.C.; et al. CRISPR/Cas9-mediated loss of FGF5 function increases wool staple length in sheep. FEBS J. 2017, 284, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.M.; Zhang, S.B.; Lei, X.H.; Deng, Z.L.; Guo, W.X.; Qiu, Z.F.; Liu, S.; Wang, X.Y.; Zhang, H.; Duan, E.K. Estrogen leads to reversible hair cycle retardation through inducing premature catagen and maintaining telogen. PLoS ONE 2012, 7, e40124. [Google Scholar] [CrossRef] [PubMed]
- Jahoda Colin, A.B.; Christiano Angela, M. Niche Crosstalk: Intercellular Signals at the Hair Follicle. Cell 2011, 146, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Bullough, W.S. Growth control in mammalian skin. Nature 1962, 193, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Legrand, R.; Tiret, L.; Abitbol, M. Two recessive mutations in FGF5 are associated with the long-hair phenotype in donkeys. Genet. Sel. Evol. 2014, 46, 65. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Elder, M.J. Anatomy and physiology of eyelash follicles: Relevance to lash ablation procedures. Ophthalmic Plast. Reconstr. Surg. 1997, 13, 21–25. [Google Scholar] [CrossRef]
- Rook, A. Endocrine Influences on Hair Growth. Br. Med. J. 1965, 1, 609–614. [Google Scholar] [CrossRef]
- Seago, S.V.; Ebling, F.J. The hair cycle on the human thigh and upper arm. Br. J. Dermatol. 1985, 113, 9–16. [Google Scholar] [CrossRef]
- Lu, C.P.; Polak, L.; Keyes, B.E.; Fuchs, E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 2016, 354, 6319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chapman, S.C. Cloning and expression analysis of Fgf5, 6 and 7 during early chick development. Gene Expr. Patterns 2012, 12, 245–253. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liu, H.; Pan, H.; Wang, X.; Zhang, Y.; Yao, B.; Li, N.; Lai, L.; Li, Z. CRISPR/Cas9-mediated Disruption of Fibroblast Growth Factor 5 in Rabbits Results in a Systemic Long Hair Phenotype by Prolonging Anagen. Genes 2020, 11, 297. https://doi.org/10.3390/genes11030297
Xu Y, Liu H, Pan H, Wang X, Zhang Y, Yao B, Li N, Lai L, Li Z. CRISPR/Cas9-mediated Disruption of Fibroblast Growth Factor 5 in Rabbits Results in a Systemic Long Hair Phenotype by Prolonging Anagen. Genes. 2020; 11(3):297. https://doi.org/10.3390/genes11030297
Chicago/Turabian StyleXu, Yuxin, Hongmei Liu, Huilin Pan, Xinyue Wang, Yuxin Zhang, Bing Yao, Nannan Li, Liangxue Lai, and Zhanjun Li. 2020. "CRISPR/Cas9-mediated Disruption of Fibroblast Growth Factor 5 in Rabbits Results in a Systemic Long Hair Phenotype by Prolonging Anagen" Genes 11, no. 3: 297. https://doi.org/10.3390/genes11030297
APA StyleXu, Y., Liu, H., Pan, H., Wang, X., Zhang, Y., Yao, B., Li, N., Lai, L., & Li, Z. (2020). CRISPR/Cas9-mediated Disruption of Fibroblast Growth Factor 5 in Rabbits Results in a Systemic Long Hair Phenotype by Prolonging Anagen. Genes, 11(3), 297. https://doi.org/10.3390/genes11030297



