The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Field Management
2.2. Source of Bio- and Organic Fertilizers
2.3. Morpho-Physiological Traits
2.4. Yield and Its Attributes
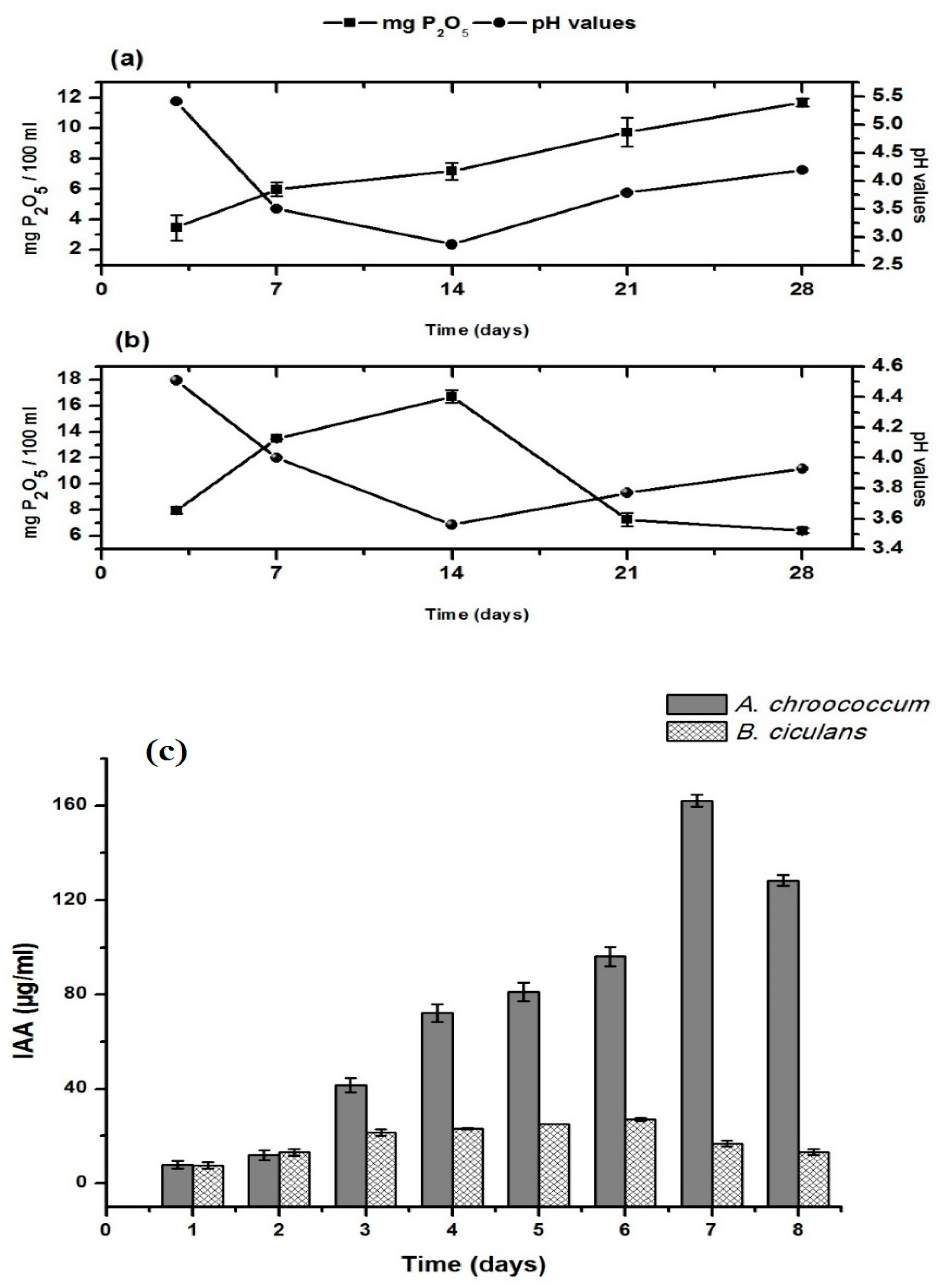
2.5. Efficacy of Bacterial Strains for Phosphate Solubilization and Indole Acetic Acid Production
2.6. The Treatment of Seeds and Soils with the Chemical, Bio-, and Organic Fertilizers
2.7. Preparation and Application of Bio- and Organic Fertilizers
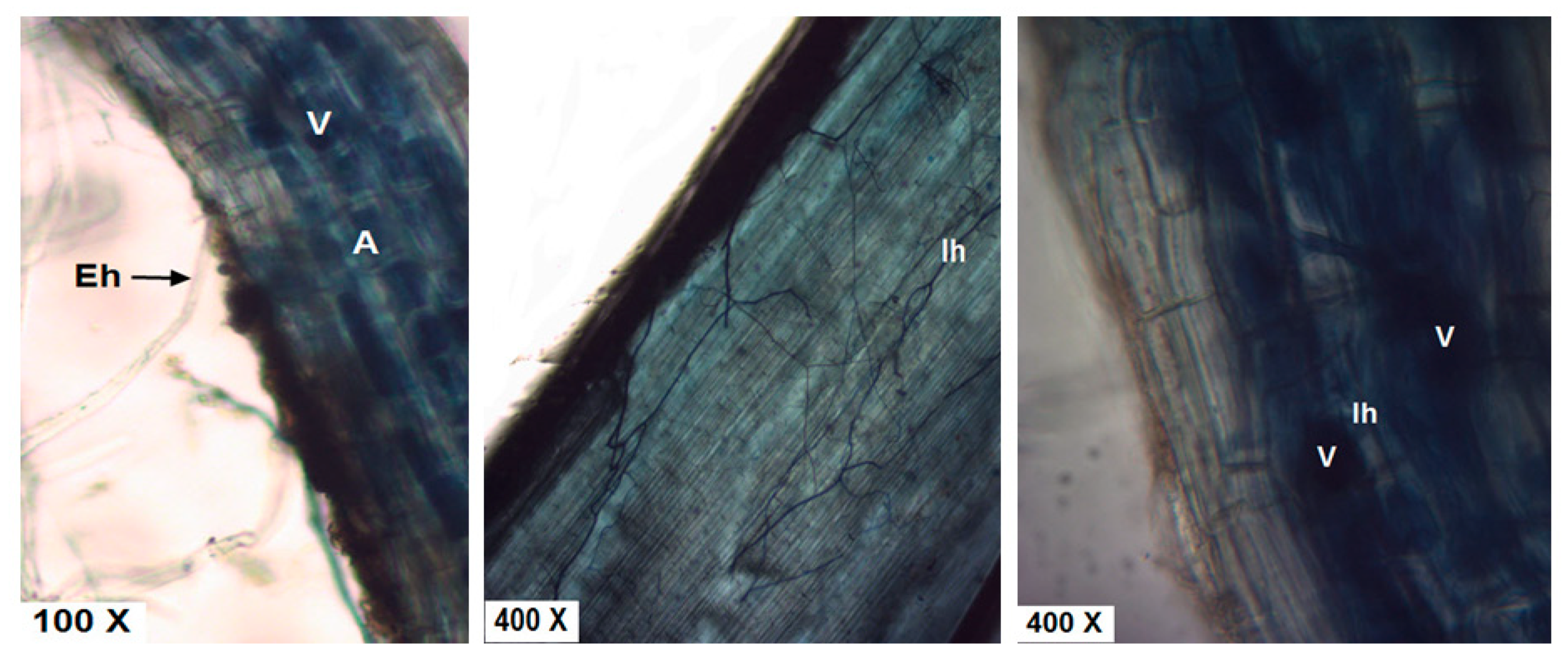
2.8. Staining and Estimation of Mycorrhizal Root Colonization
2.9. Microbial Count Determination
2.10. Determination of Acid Phosphatase and Dehydrogenase Enzymes
2.11. Biochemical Analysis of the Seeds
2.12. Nutrient Uptake
2.13. Plant Hormones, α-Amylase Activities, and Their Transcript Levels
2.14. Statistical Analysis
3. Results
3.1. Plant Growth Promotion Traits
3.2. Effects of Bio- and Organic Fertilizers on Morpho-Physiological Parameters
3.3. Effects of Bio- and Organic Fertilizers on Yield and Their Attributes
3.4. Effects of Bio- and Organic Fertilizers on Grains Quality
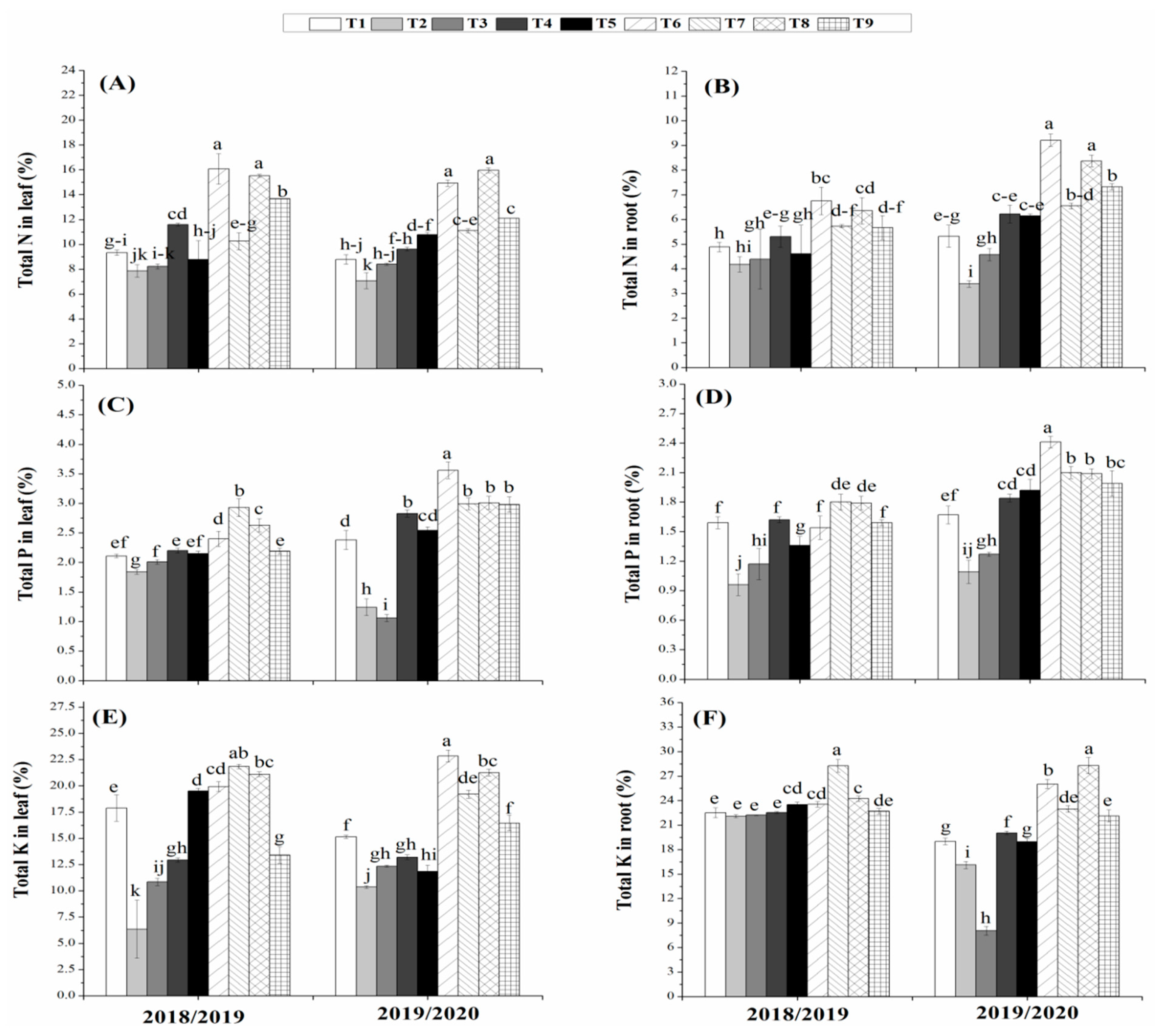
3.5. Effects of Bio- and Organic Fertilizers on Nutrient Uptake
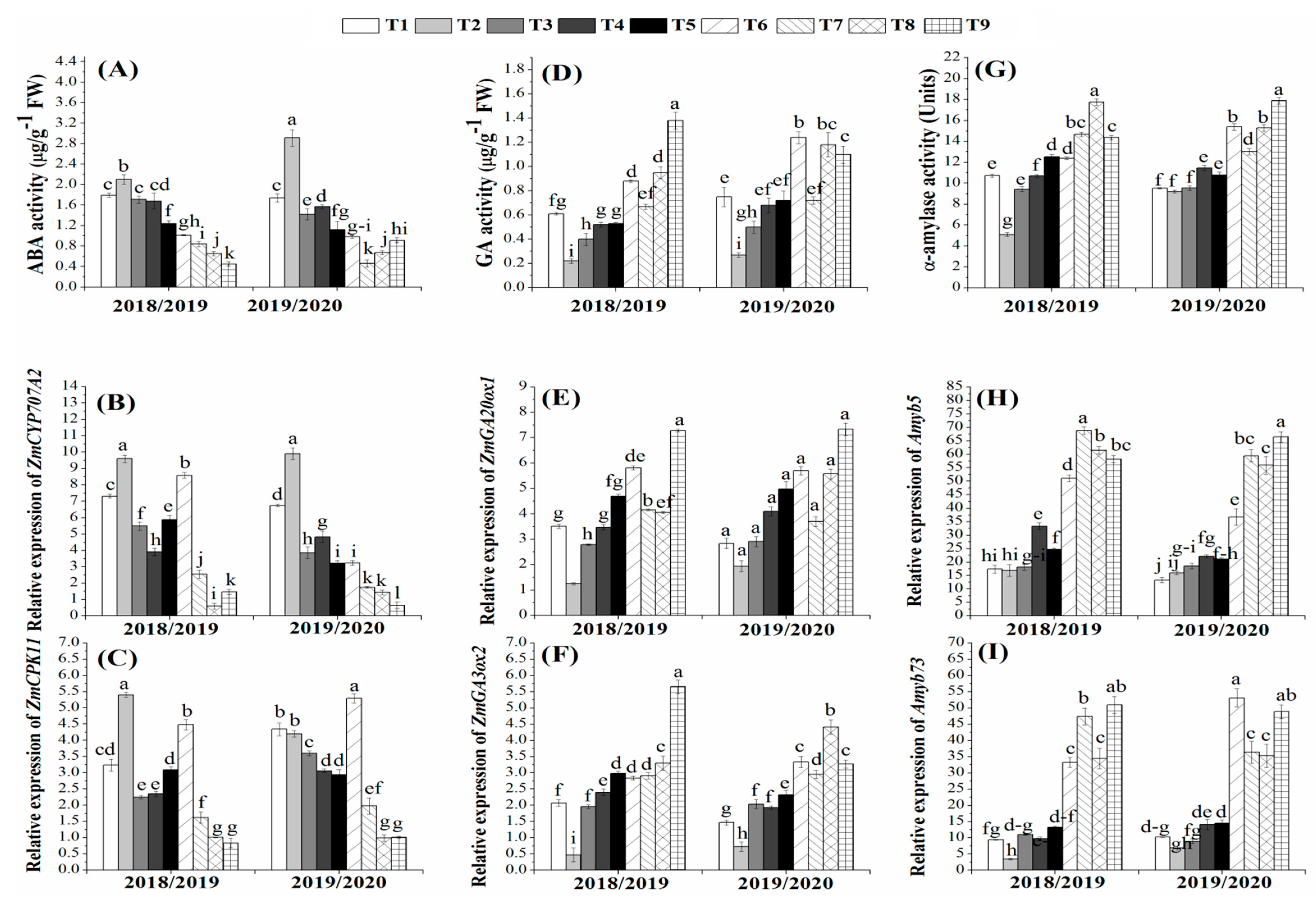
3.6. Effects of Bio- and Organic Fertilizers on Hormone Activities and Their Transcription Levels
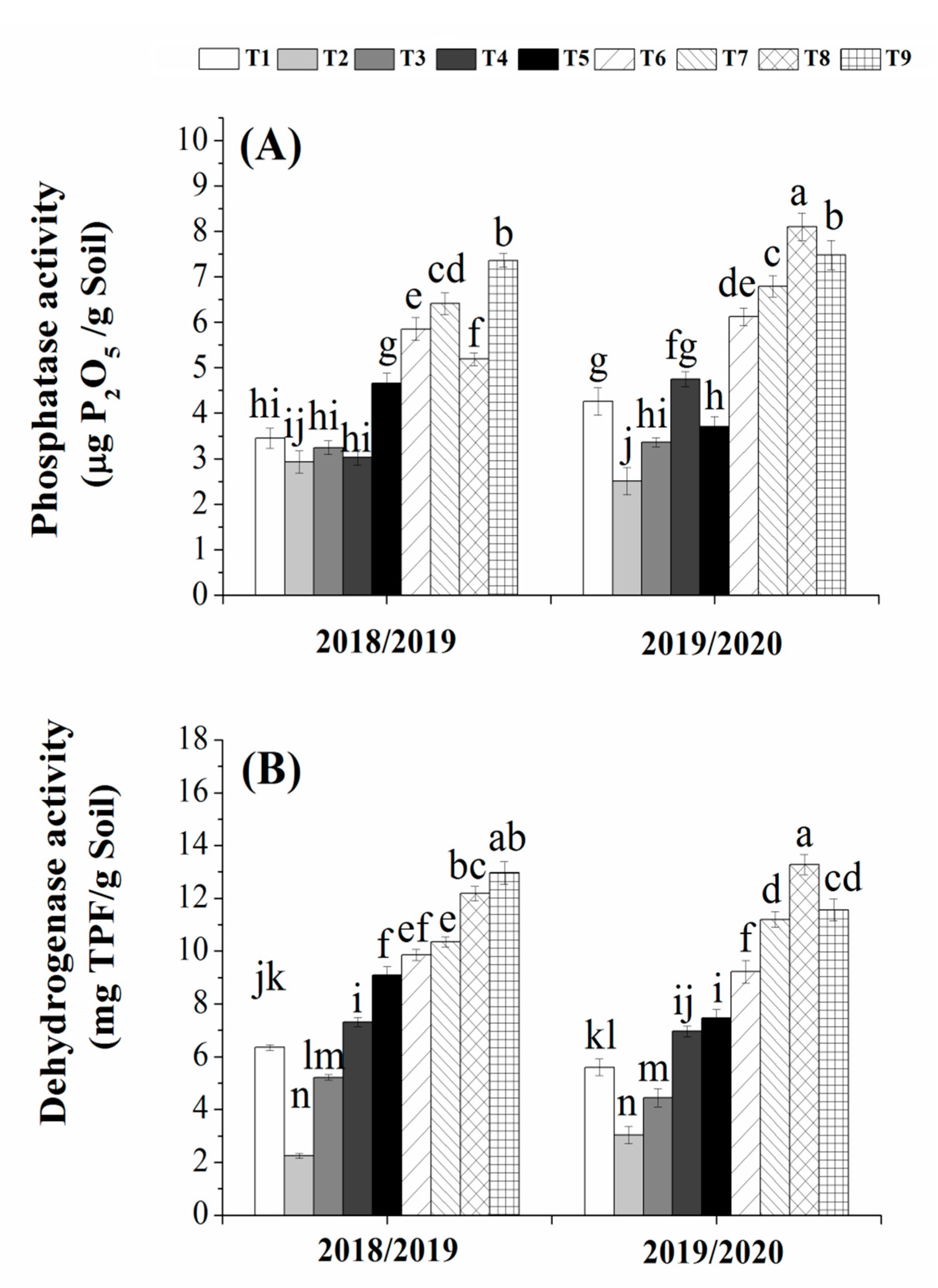
3.7. Effects of Bio- and Organic Fertilizers on Dehydrogenase and Phosphatase Activities
3.8. Effects of Bio- and Organic Fertilizers on Mycorrhizal Colonization Levels
3.9. Effects of Bio- and Organic Fertilizers on Bacterial Counts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohammadi, P.; Castel, S.E.; Brown, A.A.; Lappalainen, T. Quantifying the regulatory effect size of cis-acting genetic variation using allelic fold change. Genome Res. 2017, 27, 1872–1884. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- He, F.; Shen, H.; Lin, C.; Fu, H.; Sheteiwy, M.S.; Guan, Y.; Huang, Y.; Hu, J. Transcriptome analysis of chilling-imbibed embryo revealed membrane recovery related genes in maize. Front. Plant Sci. 2017, 7, 1978. [Google Scholar] [CrossRef]
- Abdoulaye, A.O.; Lu, H.; Zhu, Y.; Alhaj Hamoud, Y.; Sheteiwy, M. The global trend of the net irrigation water requirement of maize from 1960 to 2050. Climate 2019, 7, 124. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Division. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 20 November 2019).
- Holding, D.R. Recent advances in the study of prolamine storage protein organization and function. Front. Plant Sci. 2014, 5, 276. [Google Scholar] [CrossRef] [PubMed]
- Krenz, R.D.; EI Guindy, M.; Ariza-Nino, E.; Siddik, I. Utilization of maize in Egypt. Report Number 72 Proposed to Ministry of Agriculture & Land Reclamation; US Agency for Int. Dev. Agriculture Policy Reform Program. Reform Design and Implementation: Washington, DC, USA, 1999.
- Abdel Monem, M.A.S.; Khalifa, H.E.; Beider, M.; El Ghandour, I.A.; Galal, Y.G.M. Using biofertilizers for maize production: Response and economic return under different irrigation treatments. J. Sust. Agric. 2000, 1–9. [Google Scholar] [CrossRef]
- Subba Roa, N.S. Soil Microbiology, 4th ed.; Science Publishers: Enfield, NH, USA, 1999; p. 407. [Google Scholar]
- Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S.; Guo, X.; Elshaikh, N.A.; Khan, N.; Oumarou, A.; Rahim, S.F. Impact of alternative wetting and soil drying and soil clay content on the morphological and physiological traits of rice roots and their relationships to yield and nutrient use-efficiency. Agric. Water Manag. 2019, 223, 105706. [Google Scholar] [CrossRef]
- Alhaj Hamoud, Y.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.S.; Chen, S.; Qiu, R.; Elbashier, M. Effect of Irrigation Regimes and Soil Texture on the Potassium Utilization Efficiency of Rice. Agronomy 2019, 9, 100. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Kloepper, J.; Schroth, M. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, Berkeley, CA, USA, 20 August 1978; pp. 879–882. [Google Scholar]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. Plant Growth-Promoting. Encyclopedia. Soils Environ. 2005, 1, 103–115. [Google Scholar]
- Zhu, X.C.; Song, F.B.; Xu, H.W. Arbuscular mycorrhizae improve low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients, in Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; Volume 1, pp. 25–55. [Google Scholar]
- Tarnabi, Z.M.; Iranbakhsh, A.; Mehregan, I.; Ahmadvand, R. Impact of arbuscular mycorrhizal fungi (AMF) on gene expression of some cell wall and membrane elements of wheat (Triticum aestivum L.) under water deficit using transcriptome analysis. Physiol. Mol. Biol. Plants 2019, 1–20. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.; Fetene, M.; Bongers, F.; Kuyper, T. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M. Arbuscular mycorrhizal fungi act as bio-stimulants in horticultural crops. Sci. Hort. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Larsen, J.; Pineda-Sánchez, H.; Delgado-Arellano, I.; Castellano-Morales, V.; Carreto-Montoya, L.; Villegas-Moreno, J. Interactions between microbial plant growth promoters and their effects on maize growth performance in different mineral and organic fertilization scenarios. Rhizosphere 2017, 3, 75–81. [Google Scholar] [CrossRef]
- Syamsiyah, J.; Herawati, A.; Mujiyo. The potential of arbuscular mycorrhizal fungi application on aggregrate stability in alfisol soil. In Proceedings of the IOP Conference Series: Earth Environmental Science, Surakarta, Indonesia, 10–12 August 2018; Volume 142, p. 012045. [Google Scholar]
- Zheng, X.; Fan, J.; Cui, J.; Wang, Y.; Zhou, J.; Ye, M.; Sun, M. Effects of biogas slurry application on peanut yield, soil nutrients, carbon storage, and microbial activity in an Ultisol soil in southern China. J. Soils Sediments 2016, 16, 449–460. [Google Scholar] [CrossRef]
- Islam, M.D.R.; Rahman, S.M.E.; Rahman, M.D.M. The effects of biogas slurry on the production and quality of maize fodder. Turk. J. Agric. For. 2010, 34, 91–99. [Google Scholar]
- Tan, F.; Wang, Z.; Zhouyang, S.Y.; Li, H.L.; Xie, Y.P.; Wang, Y.P.; Zheng, Y.M.; Li, Q.B. Nitrogen and phosphorus removal coupled with carbohydrate production by five microalgae cultures cultivated in biogas slurry. Bores. Technol. 2016, 221, 385–393. [Google Scholar] [CrossRef]
- Yu, F.B.; Luo, X.P.; Song, C.F.; Zhang, M.X.; Shan, S.D. Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agric. Scand. Sect. B Soil Plant Sci. 2010, 60, 262–268. [Google Scholar] [CrossRef]
- Lal, C.M.; Shakeel, A.K.; Navindu, G. Impacts of biogas slurry application on soil environment, yield and nutritional quality of baby corn. Vegetos 2015, 28, 194–202. [Google Scholar]
- Niyungeko, C.; Liang, X.; Liu, C.; Liu, Z.; Sheteiwy, M.; Zhang, H.; Zhou, J.; Tian, G. Effect of biogas slurry application rate on colloidal phosphorus leaching in paddy soil: A column study. Geoderma 2018, 325, 117–124. [Google Scholar] [CrossRef]
- Salah, M.S.; Guan, Y.; Cao, D.; Li, J.; Nawaz, A.; Hu, Q.; Hu, W.; Ning, M.; Hu, J. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep. 2015, 5, 14278. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Fu, Y.; Hu, Q.; Nawaz, A.; Guan, Y.; Zhan, L.; Huang, Y.; Hu, J. Seed priming with polyethylene glycol induces antioxidative defense and metabolic performance of rice under nano-ZnO stress. Environ. Sci. Pollut. Res. 2016, 23, 19989–20002. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shen, H.; Xu, J.; Guan, Y.; Song, W.; Hu, J. Seed polyamines metabolism induced by seed priming with Spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017, 137, 58–72. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2018, 256, 1–21. [Google Scholar]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Alhaj Hamoud, Y.; Shaghaleh, H.; Ullah Khan, N.; Yang, R.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef]
- Abou-Aly, H.E.; Mady, M.A. Complemented effect of humic acid and biofertilizers on wheat (Triticum aestivum L.) productivity. Ann. Agric. Sci. Moshtohor 2009, 47, 1–12. [Google Scholar]
- Sheteiwy, M.S.; Dong, Q.; An, J.; Song, W.; Guan, Y.; He, F.; Huang, Y.; Hu, J. Regulation of ZnO nanoparticles-induced physiological and molecular changes by seed priming with humic acid in Oryza sativa seedlings. Plant Growth Regul. 2017, 1–15. [Google Scholar] [CrossRef]
- Moghadam, H.R.T. Humic acid as an ecological pathway to protect corn plants against oxidative stress. Biol. Forum 2013, 7, 1704–1709. [Google Scholar]
- Asli, S.; Neumann, P.M. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant Soil 2010, 336, 313–322. [Google Scholar] [CrossRef]
- Amanullah, K.H.; Marwat, K.B.; Shah, P. Nitrogen levels and its time of application influence leaf area, height and biomass of maize planted at low and high density. Pak. J. Bot. 2009, 41, 761–768. [Google Scholar]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Abd-El-Malek, Y.; Ishac, Y.Z. Evaluation of methods used in counting Azotobacter. J. Appl. Bact. 1968, 31, 267–275. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Bio. 2005, 29, 29–34. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du Taux de Mycorhization VA d’un Système Radiculaire Recherche de Methods D’estimation Ayant une Signification Fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Publications: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Setiawati, T.; Mutmainnah, L. Solubilization of potassium containing mineral by microorganisms from sugar cane rhizosphere. Agric. Sci. Procedia 2016, 9, 108–117. [Google Scholar]
- Zhang, X.; Li, F.; Liu, T.; Xu, C.; Duan, D.; Peng, C.; Zhu, S.; Shi, J. The variations in the soil enzyme activity, protein expression, microbial biomass, and community structure of soil contaminated by heavy metals. ISRN Soil Sci. 2013, 2013, 803150. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, D.; Hu, Q.; Guan, Y.; Hu, W.; Nawaz, A.; Hu, J. Physiological changes and sHSPs genes relative transcription in relation to the acquisition of seed germination during maturation of hybrid rice seed. J. Sci. Food Agric. 2015, 96, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Bradford, N.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Cao, F.; Han, Y.; Nadira, U.A.; Zhang, G.; Wu, F. Differential changes in grain ultrastructure, amylase, protein and amino acid profiles between Tibetan wild and cultivated barleys under drought and salinity alone and combined stress. Food Chem. 2013, 141, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substance. Anal. Chem. 1956, 3, 350–356. [Google Scholar] [CrossRef]
- McGill, W.B.; Figueiredo, C.T. Total nitrogen. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 201–211. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: WoodCliff, NY, USA, 1962. [Google Scholar]
- Ashraf, M.Y.; Khan, A.H.; Azmi, A.R. Cell membrane stability and its relation with some physiological process in wheat. Acta Agronomica Hungarica 1992, 41, 182–191. [Google Scholar]
- Chapman, D.H.; Parker, E.R. Determination of NPK Methods of Analysis for Soil, Plant and Waters; Pub. Div. Agri. Univ. of California: Berkeley, CA, USA, 1961; pp. 150–179. [Google Scholar]
- Li, H.S. Principle and Technology of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000; pp. 169–172. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Hauka, F.I.A.; Afify Aida, H.; El-Sawah, A.M. Efficiency Evaluation of some Rhizobacteria isolated from Egyptian Soils, In vitro as Biofertilizers. J. Agric. Chem. Biotechn. Mansoura Univ. 2017, 8, 231–235. [Google Scholar] [CrossRef]
- Walpola, C.B.; Arunakumara, K.; Yoon, M.H. Isolation and characterization of phosphate solubilizing bacteria Klebsiella oxytoca with enhanced tolerant to environmental stress. Afr. J. Microbiol. Res. 2014, 8, 2970–2978. [Google Scholar]
- Hemalatha, N.; Raja, N.; Jayachitra, A.; Rajalakshmi, A.; Valarmathi, N. Isolation and characterization of phosphate solubilizing bacteria and analyzing their effect on Capsicum annum L. Inter. J. Biol. Pharm. Res. 2013, 4, 159–167. [Google Scholar]
- Abdel-fattah, G.M.; Asrar, A.A.; Al-amri, S.M.; Abdel-salam, E.M. Influence of arbuscular mycorrhiza and phosphorus fertilization on the gas exchange, growth and phosphatase activity of soybean (Glycine max L.) plants. Photosynthetica 2014, 52, 581–588. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; Hauka, F.I.A.; Afify Aida, H. Dual inoculation with Azotobacter chroococcum MF135558 and Klebsiella oxytoca MF135559 enhance the growth and yield of wheat plant and reduce N-fertilizers usage. J. Food Dairy Sci. 2018, 10, 67–76. [Google Scholar]
- Afify, A.H.; Hauka, F.I.A.; El-Sawah, A.M. Plant Growth-Promoting Rhizobacteria enhance Onion (Allium cepa L.) productivity and minimize requisite chemical fertilization. Env. Biodiv. Soil Secur. 2018, 2, 119–129. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Evaluation of multispecies plant-growth promoting consortia for the growth promotion of Jatropha curcas L. J. Plant Growth Regul. 2012, 31, 588–598. [Google Scholar] [CrossRef]
- Russo, R.O.; Berlyn, G.P. The use of organic bio-stimulants to help low input sustainable agriculture. J. Sustain. Agric. 1990, 1, 19–42. [Google Scholar] [CrossRef]
- Pukalchik, M.; Kydralieva, K.; Yakimenko, O.; Fedoseeva, E.; Terekhova, V. Outlining the Potential Role of Humic Products in Modifying Biological Properties of the Soil—A Review. Front. Environ. Sci. 2019, 7, 80. [Google Scholar] [CrossRef]
- Du, Z.J.; Chen, X.M.; Qi, X.B.; Li, Z.Y.; Nan, J.K.; Deng, J.Q. The effects of biochar and hoggery biogas slurry on fluvo-aquic soil physical and hydraulic properties: A field study of four consecutive wheat-maize rotations. J. Soils Sediments 2016, 16, 2050–2058. [Google Scholar] [CrossRef]
- Maqshoof, A.; Zahir, A.Z.; Jamil, M.; Latif, F.N.M.; Akhtar, M.F. Integrated use of plant growth promoting rhizobacteria, biogas slurry and chemical nitrogen for sustainable production of maize under salt-affected conditions. Pak. J. Bot. 2014, 46, 375–382. [Google Scholar]
- Lu, J.; Jiang, L.N.; Chen, D.J.; Toyota, K.; Strong, P.J.; Wang, H.L.; Hirasawa, T. Decontamination of anaerobically digested slurry in a paddy field ecosystem in Jiaxing region of China. Agric. Ecosyst. Environ. 2012, 146, 13–22. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur. J. Soil Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Rosas, S.B.; Avanzin, G.; Carlier, E.; Pasluosta, C.; Pastor, N.; Rovera, M. Root colonization and growth promotion of wheat and maize by Pseudomonas aurantiaca SR1. Soil Biol. Biochem. 2009, 41, 1802–1806. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Orhan, E.; Esitken, A.; Ercisli, S.; Turan, M.; Fikrettin, S. Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci. Hort. 2006, 111, 38–43. [Google Scholar] [CrossRef]
- Çakmakçi, R.; Dönmez, F.; Aydin, A.; Sahin, F. Growth promotion of plants by plant growth promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol. Biochem. 2006, 38, 1482–1487. [Google Scholar] [CrossRef]
- Silva, S.F.; Olivares, F.L.; Canellas, L.P. The biostimulant manufactured using diazotrophic endophytic bacteria and humates is effective to increase sugarcane yield. Chem. Biol. Technol. Agric. 2017, 4, 24. [Google Scholar] [CrossRef]
- Shahid, M.; Hameed, S.; Imran, A.; Ali, S.; Elsas, J.D. Root colonization and growth promotion of sunflower (Helianthus annuus L.) by phosphate solubilizing Enterobacter sp. Fs-11. World J. Microbiol. Biotechnol. 2012, 28, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Balmori, D.M.; Médici, L.O.; Aguiar, N.O.; Campostrini, E.; Rosa, R.C.C.; Façanha, A.R.; Olivares, F.L. A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant Soil 2013, 366, 119–132. [Google Scholar] [CrossRef]
- Thonar, C.; Lekfeldt, J.D.S.; Cozzolino, V.; Kundel, D.; Kulhánek, M.; Mosimann, C.; Neumann, G.; Piccolo, A.; Symanczik, S.; Walder, F. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4, 7. [Google Scholar] [CrossRef]
- Turkmen, O.; Demir, S.; Sensoy, S.; Dursun, A. Effect of arbuscular mycorrhizal fungus and humic acid on the seedling development and nutrient content of pepper growth under saline conditions. J. Biol. Sci. 2005, 5, 568–574. [Google Scholar]
- Tang, Y.; Wen, G.; Li, P.; Dai, C.; Han, J. Effects of Biogas Slurry Application on Crop Production and Soil Properties in a Rice–Wheat Rotation on Coastal Reclaimed Farmland. Water Air Soil Pollut. 2019, 230, 51. [Google Scholar] [CrossRef]
- Cakmakci, R.; Erat, M.; Erdogan, U.; Donmez, M.F. The influence of plant growth-promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J. Plant Nut. Soil Sci. 2007, 170, 288–295. [Google Scholar] [CrossRef]
- Canellas, L.P.; Junior, L.R.L.T.; Dobbss, L.B.; Silva, C.A.; Medici, L.O.; Zandonadi, D.B.; Facanha, A.R. Humic acids crossinteractions with root and organic acids. Ann. Appl. Biol. 2008, 153, 157–166. [Google Scholar] [CrossRef]
- Aguirre, E.; Lemenager, D.; Bacaicoa, E.; Fuentes, M.; Baigorri, R.; Angel, Z.; Jose, G.M. The root application of a purified leonardite humic acid modifies the transcriptional regulation of the main physiological root responses to Fe deficiency in Fe-sufficient cucumber plants. Plant Physiol. Biochem. 2009, 47, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Habashy, N.R.; Abou El-Khair Amal, W.; Zaki, R.N. Effect of organic and biofertilizers on phosphorus and some micronutrients availability in a calcareous soil. Res. J. Agric. Biol. Sci. 2008, 4, 454–552. [Google Scholar]
- Zhang, G.Y.; Zhang, L.P.; Wei, M.F.; Liu, Z.; Fan, Q.L.; Shen, Q.R.; Xu, G.H. Effect of arbuscular mycorrhizal fungi, organic fertilizer and soil sterilization on maize growth. Acta Ecol. Sinica 2011, 31, 192–196. [Google Scholar] [CrossRef]
- Wang, M.Y.; Liu, S.T.; Liu, R.J. P-tolerance of arbuscular mycorrhizal fungi in soil under long-term fertilization. Acta Pedol. Sin. 2006, 143, 1056–1059. [Google Scholar]
- Lioussanne, L.; Perreault, F.; Jolicoeur, M.; St-Arnaud, M. The bacterial community of tomato rhizosphere is modified by inoculation with arbuscular mycorrhizal fungi but unaffected by soil enrichment with mycorrhizal root exudates or inoculation with Phytophthora nicotianae. Soil Biol. Biochem. 2010, 42, 473–483. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Safe. 2007, 67, 75–81. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, J.; He, Y.; Cui, J.; Xu, L.; Zhu, Z.; Zhou, J. Effect of total nitrogen ratio of biogas slurry/chemical fertilizer on microflora and enzyme activities of soil. Trans. Chin. Soc. Agric. Eng. 2015, 31, 142–150. [Google Scholar]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilizers—Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Jamil, M.; Maqshoof, A.; Abbasi, G.H. Evaluation of biofertilizer in combination with organic amendments and rock phosphate. Soil Environ. 2017, 36, 59–69. [Google Scholar]
- Hauka, F.I.A.; Bayoumy, Samia, M.M.; Afify, Aida, H.; Ashour, Eman, H.; El-Awady, M.A. Effect of using compost, mineral nitrogen and biofertilizer on microbial population in the rhizosphere of wheat plants cultivated in sandy soil. J. Agric. Chem. Biotechn. Mansoura Univ. 2010, 6, 307–314. [Google Scholar]
| Year | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|
| Month | Temperature (°C) | RH (%) | Temperature (°C) | RH (%) | ||
| Max | Min | Max | Min | |||
| May | 34 | 19 | 50 | 35 | 20 | 43 |
| June | 34 | 22 | 52 | 37 | 22 | 56 |
| July | 36 | 23 | 57 | 39 | 22 | 59 |
| August | 35 | 22 | 64 | 39 | 22 | 62 |
| September | 34 | 21 | 64 | 34 | 20 | 66 |
| Property | 2018/2019 | 2019/2020 |
|---|---|---|
| pH | 7.66 | 8.80 |
| OM% | 1.86 | 1.95 |
| EC | 1.97 | 1.81 |
| Ca2+ | 15.79 | 11.84 |
| Mg2+ | 7.04 | 9.90 |
| Na+ | 9.03 | 5.13 |
| K+ | 1.05 | 0.34 |
| CO32– | 0.0 | 0.0 |
| HCO3– | 4.01 | 1.42 |
| Cl– | 13.56 | 16.10 |
| SO42– | 15.34 | 9.69 |
| TBC | 1.79 | 1.98 |
| AC | 0.30 | 0.38 |
| PSBC | 4.90 | 5.43 |
| KRC | 15.26 | 21.63 |
| Treatments | Plant Height (cm) | Number of Leaves/Plant | LDW (g) | RDW (g) | LA (cm) | Chl. Content (mg L−1) | |
|---|---|---|---|---|---|---|---|
| 2018 | |||||||
| T1 | 100% NPK | 176.8 ± 12gh | 10.6 ± 0.5f | 23.5 ± 1cd | 7.9 ± 0.4g-i | 767.1 ± 29e | 47.4 ± 1e |
| T2 | 50% NPK | 125.3 ± 19i | 8.3 ± 1.5g | 12.8 ± 1.9e | 3.6 ± 0.1i | 516.4 ± 10f | 26.9 ± 3f |
| T3 | Biogas slurry + 50% NPK | 167.0 ± 25h | 11.0 ± 1ef | 22.2 ± 2d | 5.7 ± 0.5ij | 1026.6 ± 14bc | 45.5 ± 2e |
| T4 | Humic acid + 50% NPK | 206.9 ± 5e | 13.0 ± 1cd | 23.2 ± 2cd | 8.8 ± 0.6f–h | 1038.0 ± 42bc | 59.8 ± 1d |
| T5 | Biogas + Humic + 50% NPK | 245.3 ± 12d | 13.6 ± 0.5c | 33.4 ± 2b | 11.3 ± 0.4d–f | 1010.7 ± 17c | 70.8 ± 1c |
| T6 | Biofertilizers + 50% NPK | 276.2 ± 7bc | 15.3 ± 0.5ab | 42.2 ± 5.0a | 15.7 ± 0.6bc | 1199.1 ± 106a | 86.1 ± 3b |
| T7 | Biogas + Biofertilizers + 50% NPK | 269.0 ± 6c | 15.3 ± 0.5ab | 44.8 ± 1a | 17.1 ± 0.7ab | 1238.2 ± 26a | 98.2 ± 4a |
| T8 | Humic + Biofertilizers + 50% NPK | 246.8 ± 13d | 16.6 ± 0.5a | 35.2 ± 2b | 15.1 ± 2bc | 1212.4 ± 72a | 69.9 ± 1c |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 199.0 ± 10f | 11.0 ± 1ef | 26.7 ± 1cd | 10.8 ± 0.5e–g | 995.4 ± 52cd | 52.2 ± 1de |
| 2019 | |||||||
| T1 | 100% NPK | 179.5 ± 12f–h | 11.3 ± 1ef | 25.7 ± 3cd | 8.7 ± 0.5f–h | 893.3 ± 67d | 45.4 ± 4e |
| T2 | 50% NPK | 142.8 ± 18i | 9.0 ± 1g | 15.2 ± 3e | 4.0 ± 0.2j | 618.8 ± 79f | 30.4 ± 7f |
| T3 | Biogas slurry + 50% NPK | 193.3 ± 2e–g | 12.6 ± 1c–e | 23.5 ± 3cd | 7.2 ± 0.7h–i | 1142.9 ± 11ab | 47.1 ± 1e |
| T4 | Humic acid + 50% NPK | 287.1 ± 1a–c | 12.0 ± 1d–f | 25.0 ± 2cd | 12.2 ± 2de | 1136.9 ± 41ab | 56.4 ± 8d |
| T5 | Biogas + Humic + 50% NPK | 293.9 ± 10ab | 12.6 ± 1c–e | 35.6 ± 2b | 13.3 ± 1c–e | 1032.6 ± 19bc | 70.1 ± 3c |
| T6 | Biofertilizers + 50% NPK | 300.1 ± 5a | 14.3 ± 0.5bc | 43.3 ± 3a | 16.9 ± 0.3ab | 1143.7 ± 20ab | 84.8 ± 8b |
| T7 | Biogas+ Biofertilizers + 50% NPK | 295.8 ± 7ab | 14.3 ± 0.5bc | 45.5 ± 4a | 18.9 ± 1a | 1147.7 ± 57ab | 102.2 ± 7a |
| T8 | Humic + Biofertilizers + 50% NPK | 286.3 ± 12a–c | 13.3 ± 0.5cd | 33.9 ± 2b | 15.9 ± 2bc | 1055.5 ± 13bc | 68.8 ± 3c |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 201.5 ± 28e | 11.3 ± 1ef | 27.8 ± 2c | 13.9 ± 4cd | 1046.6 ± 86bc | 51.6 ± 2de |
| Treatments | Cob Weight (g) | Cob Length (cm) | Row Number/Cob | 100 Grain Weight (g) | |
|---|---|---|---|---|---|
| 2018 | |||||
| T1 | 100% NPK | 61.5 ± 3hi | 14.7 ± 2f–h | 11.3 ± 2e–h | 25.8 ± 0.3ef |
| T2 | 50% NPK | 43.4 ± 17j | 12.2 ± 0.2i | 9.6 ± 2.51h | 19.7 ± 5.8h |
| T3 | Biogas slurry + 50% NPK | 78.0 ± 4g | 14.9 ± 0.6f–h | 11.0 ± 1.0f–h | 25.6 ± 1.2f |
| T4 | Humic acid + 50% NPK | 126.2 ± 5f | 15.5 ± 0.2fg | 11.3 ± 0.5e–h | 26.3 ± 1.3ef |
| T5 | Biogas + Humic + 50% NPK | 162.1 ± 23d | 16.1 ± 0.5ef | 11.6 ± 0.5d–h | 33.4 ± 0.5ab |
| T6 | Biofertilizers + 50% NPK | 219.8 ± 5bc | 20.5 ± 0.2a | 13.3 ± 1.1a–e | 33.0 ± 1.3ab |
| T7 | Biogas + Biofertilizers + 50% NPK | 227.5 ± 7ab | 20.5 ± 0.9a | 13.6 ± 0.5a–d | 35.4 ± 1.1a |
| T8 | Humic + Biofertilizers + 50% NPK | 211.1 ± 7c | 18.5 ± 0.2b–d | 12.0 ± 0.1c–g | 33.7 ± 2.6ab |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 71.3 ± 7gh | 14.0 ± 0.3g–h | 11.3 ± 0.5e–h | 22.7 ± 0.6g |
| 2019 | |||||
| T1 | 100% NPK | 72.9 ± 7gh | 17.4 ± 0.9 c–f | 12.3 ± 1.5b–g | 28.5 ± 0.2d–f |
| T2 | 50% NPK | 50.3 ± 2ij | 13.6 ± 0.7hi | 10.3 ± 0.5gh | 20.1 ± 0.2gh |
| T3 | Biogas slurry + 50% NPK | 85.1 ± 8g | 17.4 ± 1.9c–e | 12.6 ± 1.1a–f | 28.9 ± 0.8c–e |
| T4 | Humic acid + 50% NPK | 140.9 ± 3e | 18.7 ± 0.9b–d | 13.3 ± 1.1a–e | 28.9 ± 0.8c–e |
| T5 | Biogas + Humic + 50% NPK | 167.0 ± 9d | 18.6 ± 0.3b–d | 14.0 ± 0.1a–c | 31.4 ± 1.3b–d |
| T6 | Biofertilizers + 50% NPK | 235.0 ± 6a | 19.0 ± 0.9a–d | 14.3 ± 0.5ab | 29.9 ± 0.6cd |
| T7 | Biogas + Biofertilizers + 50% NPK | 237 ± 2a | 19.7 ± 0.4ab | 14.6 ± 0.5a | 31.9 ± 0.7bc |
| T8 | Humic + Biofertilizers + 50% NPK | 214.6 ± 7bc | 19.1 ± 0.9a–c | 13.6 ± 1.5a–d | 29.0 ± 0.6c–e |
| T9 | Biogas + Humic+ Biofertilizers + 50% NPK | 78.8 ± 8g | 17.3 ± 0.5de | 12.6 ± 1.1a–f | 26.3 ± 0.4ef |
| Treatments | Soluble Sugars (µgg−1 FW) | Protein Content (mgg−1 FW) | Starch Content (%) | Carbohydrates (%) | Amino Acids (mgg−1) | |
|---|---|---|---|---|---|---|
| 2018 | ||||||
| T1 | 100% NPK | 12.8 ± 0.5g | 114.0 ± 3.5ij | 23.1 ± 0.7i | 37.4 ± 0.9gh | 10.2 ± 0.1k |
| T2 | 50% NPK | 9.0 ± 0.5j | 94.4 ± 3.1kl | 17.9 ± 1.6k | 29.1 ± 0.71k | 9.4 ± 0.3l |
| T3 | Biogas slurry + 50% NPK | 12.5 ± 0.2g | 107.1 ± 1.6jk | 20.9 ± 0.3j | 35.7 ± 1.2hi | 12.3 ± 0.1gh |
| T4 | Humic acid + 50% NPK | 11.8 ± 0.3gh | 113.9 ± 1.4ij | 23.4 ± 1.1i | 40.3 ± 0.8f | 11.0 ± 0.2ij |
| T5 | Biogas + Humic + 50% NPK | 13.8 ± 0.5f | 133.0 ± 11.0h | 47.6 ± 0.9a | 46.4 ± 0.8d | 13.1 ± 0.5g |
| T6 | Biofertilizers + 50% NPK | 17.1 ± 0.5e | 342.6 ± 19.3a | 40.9 ± 0.6b | 64.4 ± 3.5a | 18.9 ± 0.5c |
| T7 | Biogas + Biofertilizers + 50% NPK | 20.5 ± 0.5c | 190.5 ± 5.0f | 35.7 ± 0.8c | 51.4 ± 1.1c | 15.5 ± 0.7f |
| T8 | Humic + Biofertilizers + 50% NPK | 18.3 ± 0.3d | 243.0 ± 4.2d | 29.0 ± 0.4fg | 55.5 ± 1.2b | 16.41 ± 0.2e |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 23.3 ± 0.7b | 282.7 ± 5.8b | 33.2 ± 0.5d | 52.9 ± 0.5c | 17.9 ± 0.2d |
| 2019 | ||||||
| T1 | 100% NPK | 14.2 ± 0.3f | 128.1 ± 2.4hi | 19.1 ± 0.8k | 32.0 ± 1.6j | 11.2 ± 0.2i |
| T2 | 50% NPK | 10.1 ± 0.7i | 85.8 ± 5.4l | 14.3 ± 0.9l | 22.3 ± 0.8m | 9.2 ± 0.1l |
| T3 | Biogas slurry + 50% NPK | 11.4 ± 0.2h | 110.4 ± 4.7jk | 15.5 ± 0.7l | 25.3 ± 1.0l | 10.4 ± 0.7jk |
| T4 | Humic acid + 50% NPK | 12.0 ± 0.2gh | 130.7 ± 1.6hi | 19.5 ± 0.8jk | 32.8 ± 1.1j | 12.3 ± 0.3h |
| T5 | Biogas + Humic + 50% NPK | 14.6 ± 0.5f | 128.7 ± 1.4hi | 26.3 ± 1.5h | 33.8 ± 0.6ij | 12.4 ± 0.1gh |
| T6 | Biofertilizers + 50% NPK | 18.1 ± 0.4d | 260.7 ± 16.7c | 35.5 ± 0.9c | 53.4 ± 2.6bc | 21.3 ± 1.0a |
| T7 | Bioga s+ Biofertilizers + 50% NPK | 20.3 ± 0.8c | 167.7 ± 17.6g | 27.6 ± 1.0gh | 39.5 ± 1.9fg | 16.4 ± 0.4e |
| T8 | Humic + Biofertilizers + 50% NPK | 25.5 ± 0.7a | 247.1 ± 17.6cd | 30.8 ± 1.6e | 46.4 ± 1.8d | 18.5 ± 0.7cd |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 19.8 ± 0.4c | 216.6 ± 5.0e | 30.4 ± 0.6ef | 43.4 ± 1.0e | 19.7 ± 0.5b |
| Treatments | Mycorrhizal Colonization Levels (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 DAP | 60 DAP | 90 DAP | ||||||||
| F | M | A | F | M | A | F | M | A | ||
| 2018 | ||||||||||
| T1 | 100% NPK | – | – | – | – | – | – | – | – | – |
| T2 | 50% NPK | – | – | – | – | – | – | – | – | – |
| T3 | Biogas slurry + 50% NPK | – | – | – | – | – | – | – | – | – |
| T4 | Humic acid + 50% NPK | – | – | – | – | – | – | – | – | – |
| T5 | Biogas + Humic + 50% NPK | – | – | – | – | – | – | – | – | – |
| T6 | Biofertilizers + 50% NPK | 70.0d | 35.0bc | 16.6de | 75.0d | 45.3d | 24.7c | 90.0d | 70.0e | 40.5e |
| T7 | Biogas + Biofertilizers + 50% NPK | 75.0c | 38.0a | 17.6cd | 85.0b | 55.7b | 29.6b | 100.0a | 82.0b | 52.8b |
| T8 | Humic + Biofertilizers + 50% NPK | 65.0e | 32.0d | 15.0f | 70.0e | 41.2f | 22.6e | 85.0e | 67.0f | 34.4g |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 60.0f | 28.0e | 12.5g | 63.0 | 38.0g | 18.6f | 81.0f | 55.0h | 31.8h |
| 2019 | ||||||||||
| T1 | 100% NPK | – | – | – | – | – | – | – | – | – |
| T2 | 50% NPK | – | – | – | – | – | – | – | – | – |
| T3 | Biogas slurry + 50% NPK | – | – | – | – | – | – | – | – | – |
| T4 | Humic acid + 50% NPK | – | – | – | – | – | – | – | – | – |
| T5 | Biogas + Humic + 50% NPK | – | – | – | – | – | – | – | – | – |
| T6 | Biofertilizers + 50% NPK | 80.0b | 36.3b | 22.0b | 85.0b | 49.0c | 29.0b | 95.0b | 80.0c | 47.9c |
| T7 | Biogas + Biofertilizers + 50% NPK | 86.0a | 39.0a | 24.0a | 91.0a | 59.0a | 30.7a | 100.0a | 94.0a | 57.4a |
| T8 | Humic + Biofertilizers + 50% NPK | 75.0c | 34.0c | 19.0c | 80.0c | 46.0d | 24.0cd | 93.0c | 75.0d | 43.0d |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 65.0e | 31.0d | 15.7ef | 72.0e | 43.0e | 23.0de | 86.0e | 65.0g | 37.4f |
| Treatments | Total Bacterial Counts (106 cfu g−1 Dry Soil) | Counts of Azotobacter spp. (104 cfu g−1 Dry Soil) | Counts of Phosphate-Solubilizing Bacteria (104 cfu g−1 Dry Soil) | Counts of Potassium-Releasing Bacteria (104 cfu g−1 Dry Soil) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 DAP | 60 DAP | 90 DAP | 30 DAP | 60 DAP | 90 DAP | 30 DAP | 60 DAP | 90 DAP | 30 DAP | 60 DAP | 90 DAP | ||
| 2018 | |||||||||||||
| T1 | 100% NPK | 9.35 | 81.14 | 52.21 | 0.57 | 5.02 | 4.57 | 50.84 | 88.42 | 68.22 | 98.84 | 143.74 | 102.90 |
| T2 | 50% NPK | 5.93 | 61.49 | 40.78 | 0.41 | 4.25 | 3.31 | 40.17 | 74.96 | 54.11 | 81.06 | 105.89 | 87.27 |
| T3 | Biogas slurry + 50% NPK | 17.60 | 143.37 | 99.85 | 1.28 | 10.04 | 7.08 | 76.80 | 126.27 | 84.99 | 134.75 | 298.03 | 187.89 |
| T4 | Humic acid + 50% NPK | 16.96 | 175.40 | 80.03 | 0.86 | 12.00 | 8.23 | 82.13 | 130.64 | 92.61 | 119.11 | 277.29 | 195.13 |
| T5 | Biogas + Humic + 50% NPK | 18.02 | 179.03 | 97.18 | 1.81 | 15.28 | 9.26 | 88.53 | 179.03 | 81.56 | 158.22 | 265.64 | 165.02 |
| T6 | Biofertilizers + 50% NPK | 13.90 | 152.47 | 94.13 | 0.74 | 9.17 | 6.74 | 86.04 | 127.00 | 94.90 | 186.66 | 268.19 | 182.55 |
| T7 | Biogas + Biofertilizers + 50% NPK | 17.45 | 179.76 | 109.76 | 1.17 | 10.37 | 7.88 | 106.66 | 169.94 | 79.27 | 171.02 | 245.99 | 165.40 |
| T8 | Humic + Biofertilizers + 50% NPK | 17.13 | 150.29 | 95.28 | 3.73 | 22.92 | 16.00 | 87.82 | 121.90 | 97.56 | 163.55 | 257.64 | 158.54 |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 18.48 | 190.32 | 106.71 | 5.76 | 30.56 | 19.43 | 135.82 | 200.14 | 100.99 | 166.75 | 283.47 | 216.47 |
| 2019 | |||||||||||||
| T1 | 100% NPK | 8.85 | 121.52 | 80.78 | 0.79 | 6.49 | 4.82 | 55.66 | 107.88 | 63.06 | 112.84 | 173.66 | 117.06 |
| T2 | 50% NPK | 5.68 | 83.42 | 65.12 | 0.61 | 4.81 | 3.46 | 41.00 | 93.85 | 49.87 | 86.48 | 126.73 | 92.32 |
| T3 | Biogas slurry + 50% NPK | 19.93 | 202.94 | 94.39 | 1.35 | 14.43 | 7.91 | 92.66 | 175.67 | 100.98 | 154.02 | 298.80 | 219.28 |
| T4 | Humic acid + 50% NPK | 20.26 | 173.66 | 115.82 | 1.17 | 18.04 | 8.53 | 81.66 | 204.54 | 103.87 | 168.02 | 292.78 | 224.22 |
| T5 | Biogas + Humic + 50% NPK | 16.80 | 157.22 | 119.12 | 2.71 | 21.65 | 11.74 | 79.00 | 180.08 | 122.00 | 190.67 | 331.28 | 196.19 |
| T6 | Biofertilizers + 50% NPK | 15.64 | 153.61 | 112.52 | 1.03 | 11.43 | 10.38 | 102.33 | 187.30 | 111.28 | 179.96 | 315.24 | 183.00 |
| T7 | Biogas + Biofertilizers + 50% NPK | 18.36 | 208.55 | 117.88 | 1.72 | 13.23 | 9.39 | 95.33 | 211.76 | 128.18 | 189.02 | 273.53 | 210.62 |
| T8 | Humic + Biofertilizers + 50% NPK | 17.66 | 173.26 | 116.23 | 4.07 | 30.08 | 21.02 | 86.33 | 173.66 | 123.65 | 183.67 | 316.00 | 174.76 |
| T9 | Biogas + Humic + Biofertilizers + 50% NPK | 20.50 | 222.99 | 117.47 | 5.31 | 42.11 | 22.25 | 106.00 | 208.55 | 129.42 | 221.56 | 323.66 | 157.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. https://doi.org/10.3390/agronomy10030319
Gao C, El-Sawah AM, Ali DFI, Alhaj Hamoud Y, Shaghaleh H, Sheteiwy MS. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy. 2020; 10(3):319. https://doi.org/10.3390/agronomy10030319
Chicago/Turabian StyleGao, Canhong, Ahmed M. El-Sawah, Dina Fathi Ismail Ali, Yousef Alhaj Hamoud, Hiba Shaghaleh, and Mohamed S. Sheteiwy. 2020. "The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.)" Agronomy 10, no. 3: 319. https://doi.org/10.3390/agronomy10030319
APA StyleGao, C., El-Sawah, A. M., Ali, D. F. I., Alhaj Hamoud, Y., Shaghaleh, H., & Sheteiwy, M. S. (2020). The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy, 10(3), 319. https://doi.org/10.3390/agronomy10030319








