Halloysite- and Montmorillonite-Loaded Scaffolds as Enhancers of Chronic Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymeric Blends
2.2.2. Characterization of Polymeric Blends
2.2.3. Preparation of Electrospun Scaffolds
2.2.4. Scaffold Characterizations
Chemico-Physical Characterization
Mechanical Properties
Fibroblasts Biocompatibility and Adhesion
Cytocompatibility of Macrophages and Pro-Inflammatory Immune Response
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Polymeric Blend Characterization
3.2. Scaffold Characterizations
3.2.1. Chemico-Physical Characterization
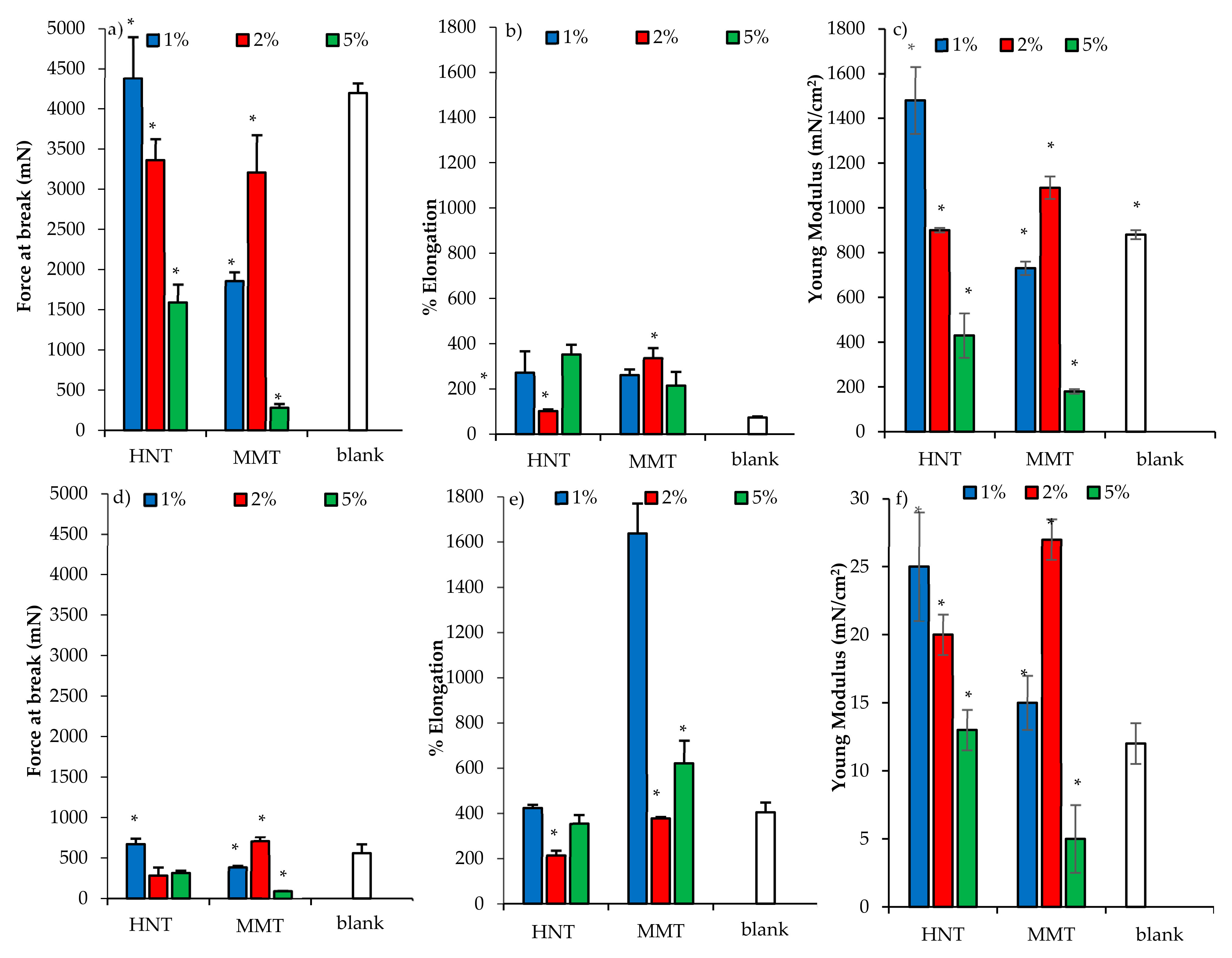
3.2.2. Mechanical Properties
3.2.3. Fibroblasts Biocompatibility and Adhesion
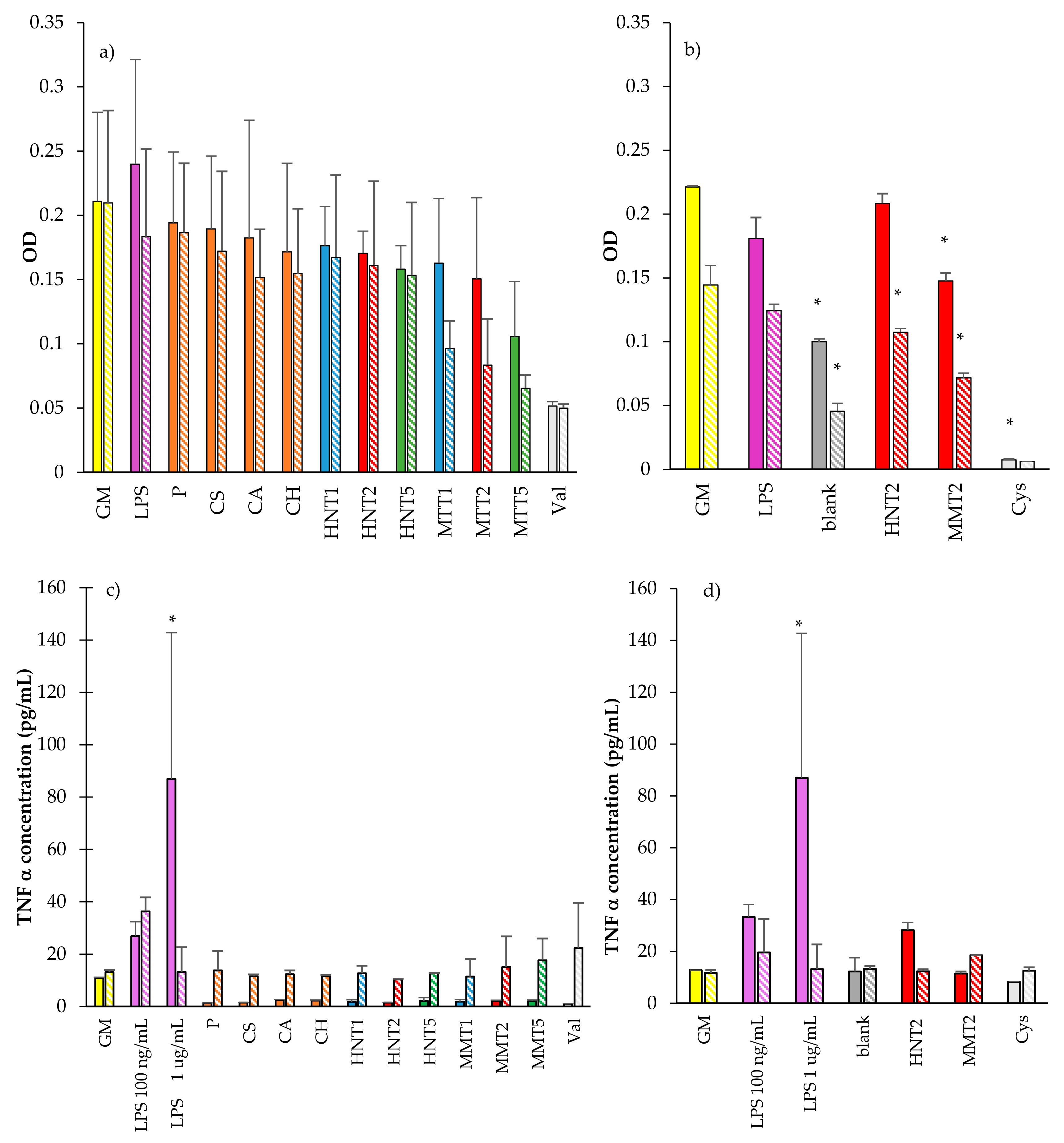
3.2.4. Cytocompatibility of Macrophages and Pro-Inflammatory Immune Response
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Naumenko, E.A.; Guryanov, I.D.; Yendluri, R.; Lvov, Y.M.; Fakhrullin, R.F. Clay nanotube–biopolymer composite scaffolds for tissue engineering. Nanoscale 2016, 8, 7257–7271. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O.C. Clay: New Opportunities for Tissue Regeneration and Biomaterial Design. Adv. Mater. 2013, 25, 4069–4086. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.B.; Metge, D.W.; Eberl, D.D.; Harvey, R.W.; Turner, A.G.; Prapaipong, P.; Poret-Peterson, A.T. What Makes a Natural Clay Antibacterial? Environ. Sci. Technol. 2011, 45, 3768–3773. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Aguzzi, C.; Viseras, C.; Caramella, C. Clay Minerals for Tissue Regeneration, Repair, and Engineering; Ågren, M.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 385–402. [Google Scholar]
- Garcia-Villen, F.; Faccendini, A.; Aguzzi, C.; Cerezo, P.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Ruggeri, M.; Ferrari, F.; Sandri, G.; et al. Montmorillonite-norfloxacin nanocomposite intended for healing of infected wounds. Int. J. Nanomed. 2019, 14, 5051–5060. [Google Scholar] [CrossRef]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Sandri, G.; Viseras, C.; Bonferoni, M.C.; Cerezo, P.; Rossi, S.; Ferrari, F.; Caramella, C. Solid state characterization of silver sulfadiazine loaded on montmorillonite/chitosan nanocomposite for wound healing. Colloid Surf. B 2014, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Aguzzi, C.; Mori, M.; Grisoli, P.; Cerezo, P.; Tenci, M.; Viseras, C.; et al. Montmorillonite-chitosan-silver sulfadiazine nanocomposites for topical treatment of chronic skin lesions: In vitro biocompatibility, antibacterial efficacy and gap closure cell motility properties. Carbohyd. Polym. 2014, 102, 970–977. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Cross, L.M.; Peak, C.W.; Gold, K.; Carrow, J.K.; Brokesh, A.; Singh, K.A. 2D Nanoclay for Biomedical Applications: Regenerative Medicine, Therapeutic Delivery, and Additive Manufacturing. Adv. Mater. 2019, 31, 1900332. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, S.; Schreiber, M.; Mohanty, A.K.; Misra, M. Advanced Electrospun Nanofibers of Layered Silicate Nanocomposites: A Review of Processing, Properties, and Applications; Pandey, J., Reddy, K., Mohanty, A., Misra, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 361–388. [Google Scholar]
- Lvov, Y.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, T.C.; Matthews, P.; Ahmad, R. Dry-heat sterilization of parenteral oil vehicles. Int. J. Pharm. Compd. 2000, 4, 223–224. [Google Scholar] [PubMed]
- Malgarim Cordenonsi, L.; Faccendini, A.; Rossi, S.; Bonferoni, M.C.; Malavasi, L.; Raffin, R.; Scherman Schapoval, E.E.; Del Fante, C.; Vigani, B.; Miele, D.; et al. Platelet lysate loaded electrospun scaffolds: Effect of nanofiber types on wound healing. Eur. J. Pharm. Biopharm. 2019, 142, 247–257. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Del Fante, C.; Vigani, B.; Black, L.; Ferrari, F. Electrospun gelatin-chondroitin sulfate scaffolds loaded with platelet lysate promote immature cardiomyocyte proliferation. Polymers 2018, 10, 208. [Google Scholar] [CrossRef]
- Cravero, F.; Churchman, G.J. The origin of spheroidal halloysites: A review of the literature. Clay Miner. 2016, 51, 417–427. [Google Scholar] [CrossRef]
- Faccendini, A.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Aguzzi, C.; Grisoli, P.; Viseras, C.; Sandri, G.; Ferrari, F. Norfloxacin loaded electrospun scaffolds: Montmorillonite nanocomposite vs. free drug. Pharmaceutics 2020, in press. [Google Scholar]
- Habibi, S.; Saket, M.; Nazockdast, H.; Hajinasrollah, K. Fabrication and characterization of exfoliated chitosan–gelatin–montmorillonite nanocomposite nanofibers. J. Text. I 2019, 110, 1672–1677. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Caramella, C.; Ferrari, F. Electrospinning Technologies in Wound Dressing Applications. Ther. Dress. Wound Health Appl. 2020, 14, 315–366. [Google Scholar]
- Falcón, J.M.; Sawczen, T.; Aoki, I.V. Dodecylamine-Loaded Halloysite Nanocontainers for Active Anticorrosion Coatings. Front. Mater. 2015, 2, 69. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer–clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Deb Nath, S.; Abueva, C.; Kim, B.; Taek Lee, B. Chitosan–hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr. Polym. 2015, 115, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Sayyari, N.; Shaabani, A.; Niknejad, H.; Tahereh, T. Novel biocompatible zinc-curcumin loaded coaxial nanofibers for bone tissue engineering application. Polymers 2018, 142, 244–255. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocoll. 2017, 17, 726–735. [Google Scholar] [CrossRef]
- Islam, S.; Rahaman, S.; Yeum, J.H. Electrospun novel super-absorbent based on polysaccharide–polyvinyl alcohol–montmorillonite clay nanocomposites. Carbohydr. Polym. 2015, 115, 69–77. [Google Scholar] [CrossRef]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef]









| % w/w | MMT | HNT | P | CH | CA | CS | H2O/CH3COOH |
|---|---|---|---|---|---|---|---|
| Blank | - | - | 10 | 2.5 | 2.5 | 0.5 | 55/45 |
| MMT1 | 1 | - | |||||
| MMT2 | 2 | - | |||||
| MMT5 | 5 | - | |||||
| HNT1 | - | 1 | |||||
| HNT2 | - | 2 | |||||
| HNT5 | - | 5 |
| Sample | Conductivity (µS/cm) | Surface Tension (N/m) | Consistency (mN × mm) |
|---|---|---|---|
| Blank | 1363 ± 11 | 36.6 ± 0.2 | 188 ± 2 |
| HNT1s | 1271 ± 3 | 37.7 ± 0.2 | 155 ± 3 |
| HNT2s | 1303 ± 4 | 38.1 ± 0.1 | 175 ± 2 |
| HNT5s | 1352 ± 9 | 38.6 ± 0.2 | 203 ± 5 |
| MMT1s | 1255 ± 23 | 38.7 ± 0.4 | 171 ± 2 |
| MMT2s | 1527 ± 17 | 40.8 ± 0.2 | 308 ± 8 |
| MMT5s | 1663 ± 9 | 41.3 ± 0.1 | 338 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandri, G.; Faccendini, A.; Longo, M.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Prina-Mello, A.; Aguzzi, C.; Viseras, C.; et al. Halloysite- and Montmorillonite-Loaded Scaffolds as Enhancers of Chronic Wound Healing. Pharmaceutics 2020, 12, 179. https://doi.org/10.3390/pharmaceutics12020179
Sandri G, Faccendini A, Longo M, Ruggeri M, Rossi S, Bonferoni MC, Miele D, Prina-Mello A, Aguzzi C, Viseras C, et al. Halloysite- and Montmorillonite-Loaded Scaffolds as Enhancers of Chronic Wound Healing. Pharmaceutics. 2020; 12(2):179. https://doi.org/10.3390/pharmaceutics12020179
Chicago/Turabian StyleSandri, Giuseppina, Angela Faccendini, Marysol Longo, Marco Ruggeri, Silvia Rossi, Maria Cristina Bonferoni, Dalila Miele, Adriele Prina-Mello, Carola Aguzzi, Cesar Viseras, and et al. 2020. "Halloysite- and Montmorillonite-Loaded Scaffolds as Enhancers of Chronic Wound Healing" Pharmaceutics 12, no. 2: 179. https://doi.org/10.3390/pharmaceutics12020179
APA StyleSandri, G., Faccendini, A., Longo, M., Ruggeri, M., Rossi, S., Bonferoni, M. C., Miele, D., Prina-Mello, A., Aguzzi, C., Viseras, C., & Ferrari, F. (2020). Halloysite- and Montmorillonite-Loaded Scaffolds as Enhancers of Chronic Wound Healing. Pharmaceutics, 12(2), 179. https://doi.org/10.3390/pharmaceutics12020179









