Effects on Biodiesel Production Caused by Feed Oil Changes in a Continuous Stirred-Tank Reactor
Abstract
:Featured Application
Abstract
1. Introduction
2. General Aspects of Biodiesel
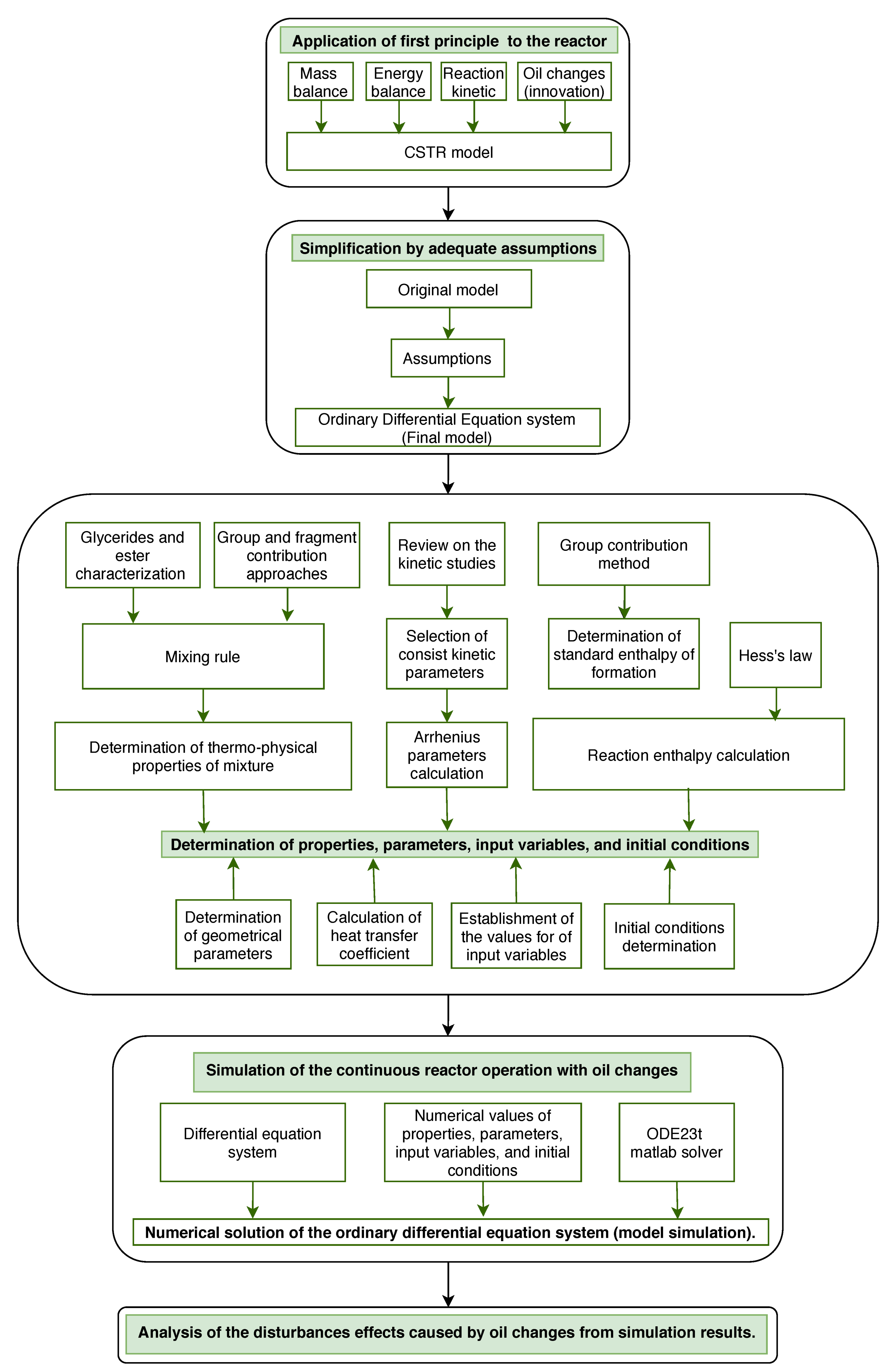
3. Methodology
3.1. Continuous Stirred-Tank Reactor Operation
3.2. Modeling of the Continuous Stirred-Tank Reactor
3.2.1. Transesterification Modeling for Pure Vegetable Oils
3.2.2. Oil Changes Modeling
3.2.3. Mass and Energy Balances for the CSTR
3.3. Determination of the Properties, Parameters, Input Variables and Initial Conditions for the Model
3.3.1. Characterization of Glycerides and Esters
3.3.2. Determination of the Density and Heat Capacity of the Reaction Mixture
3.3.3. Reaction Enthalpies
3.3.4. Kinetic Parameters of the Reactions
3.3.5. Other Parameters, Input Variables, and Initial Conditions for Simulation
4. Results and Discussion
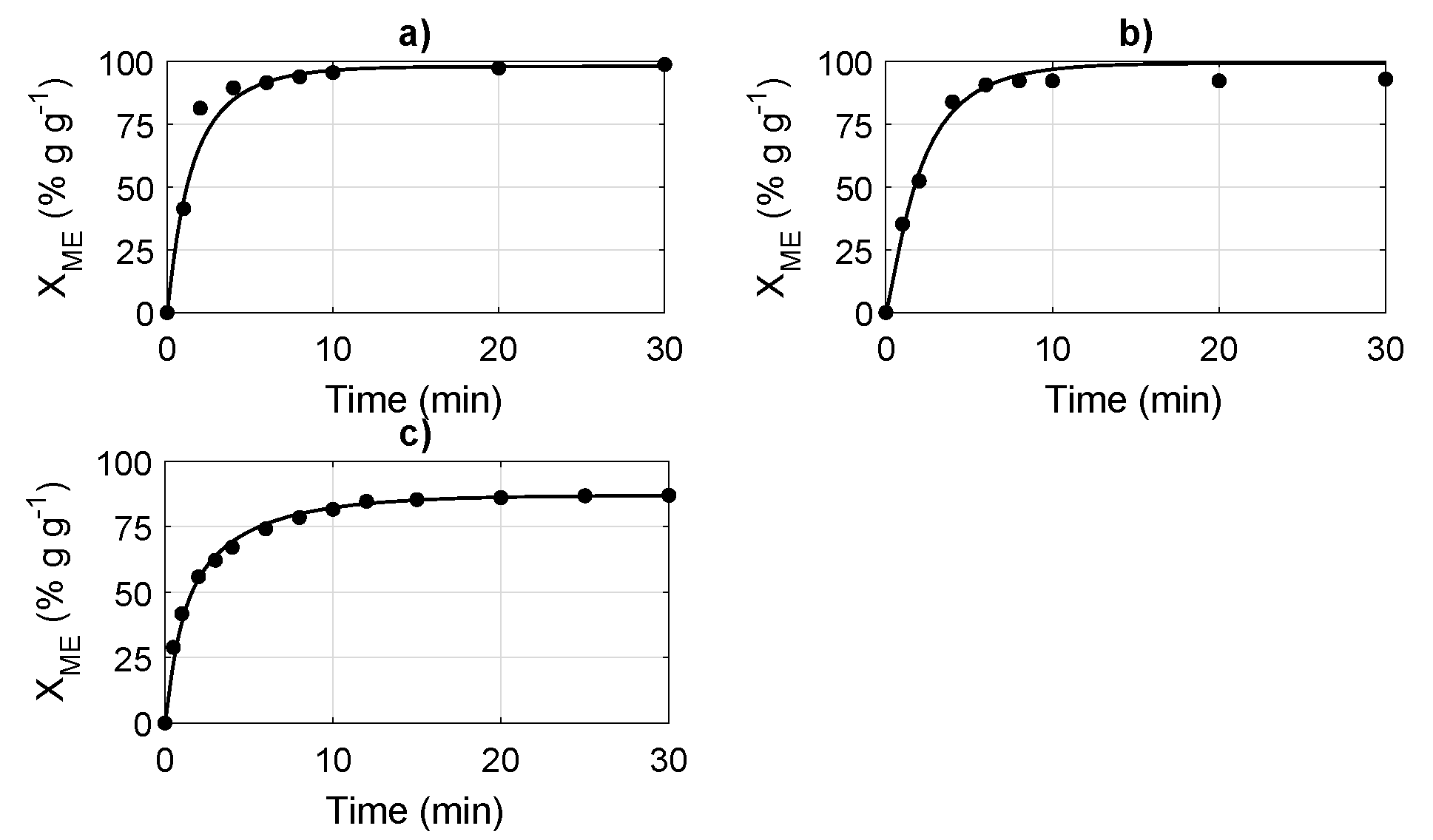
4.1. Simulation and Evaluation of the Transesterification Reaction Models
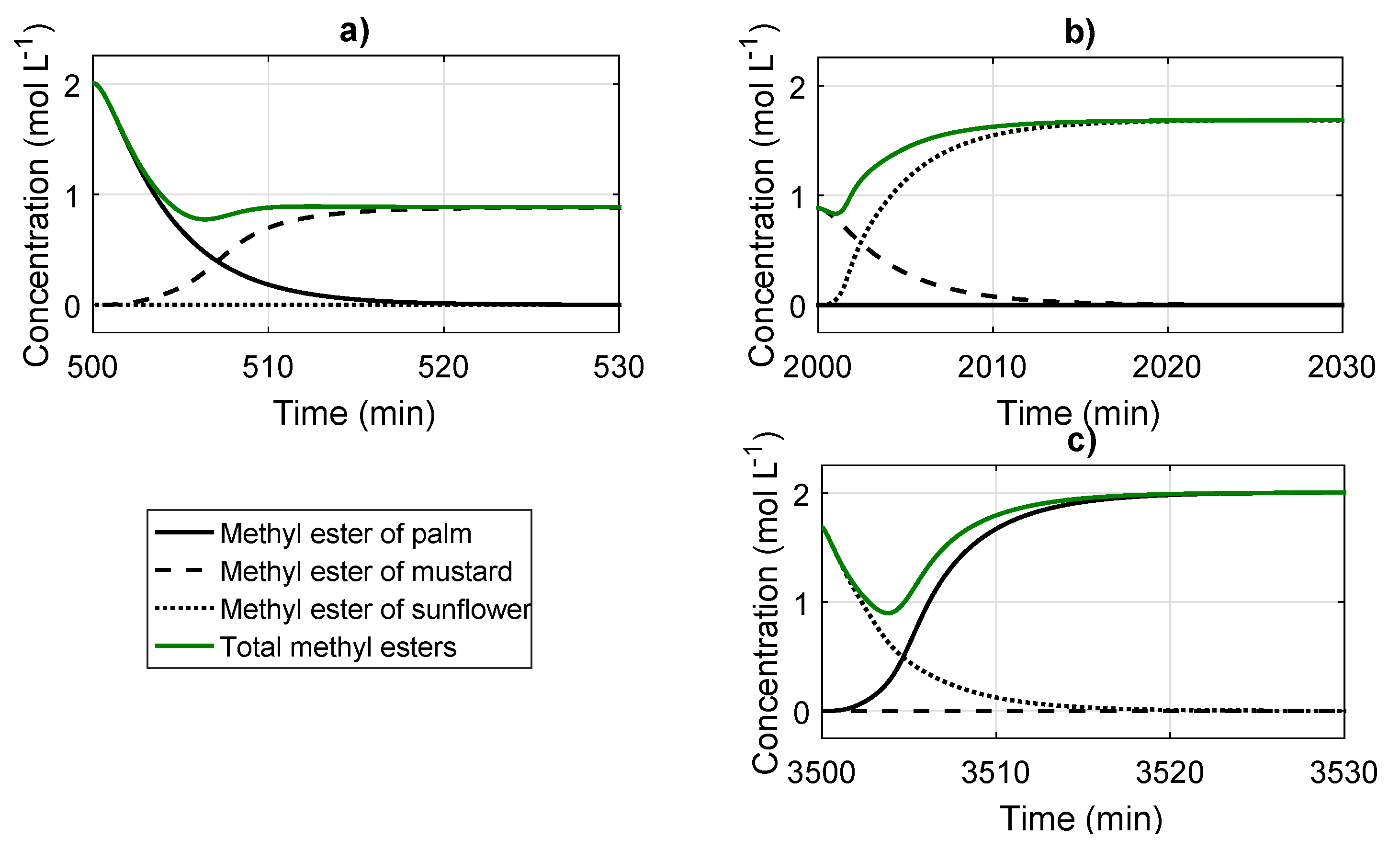
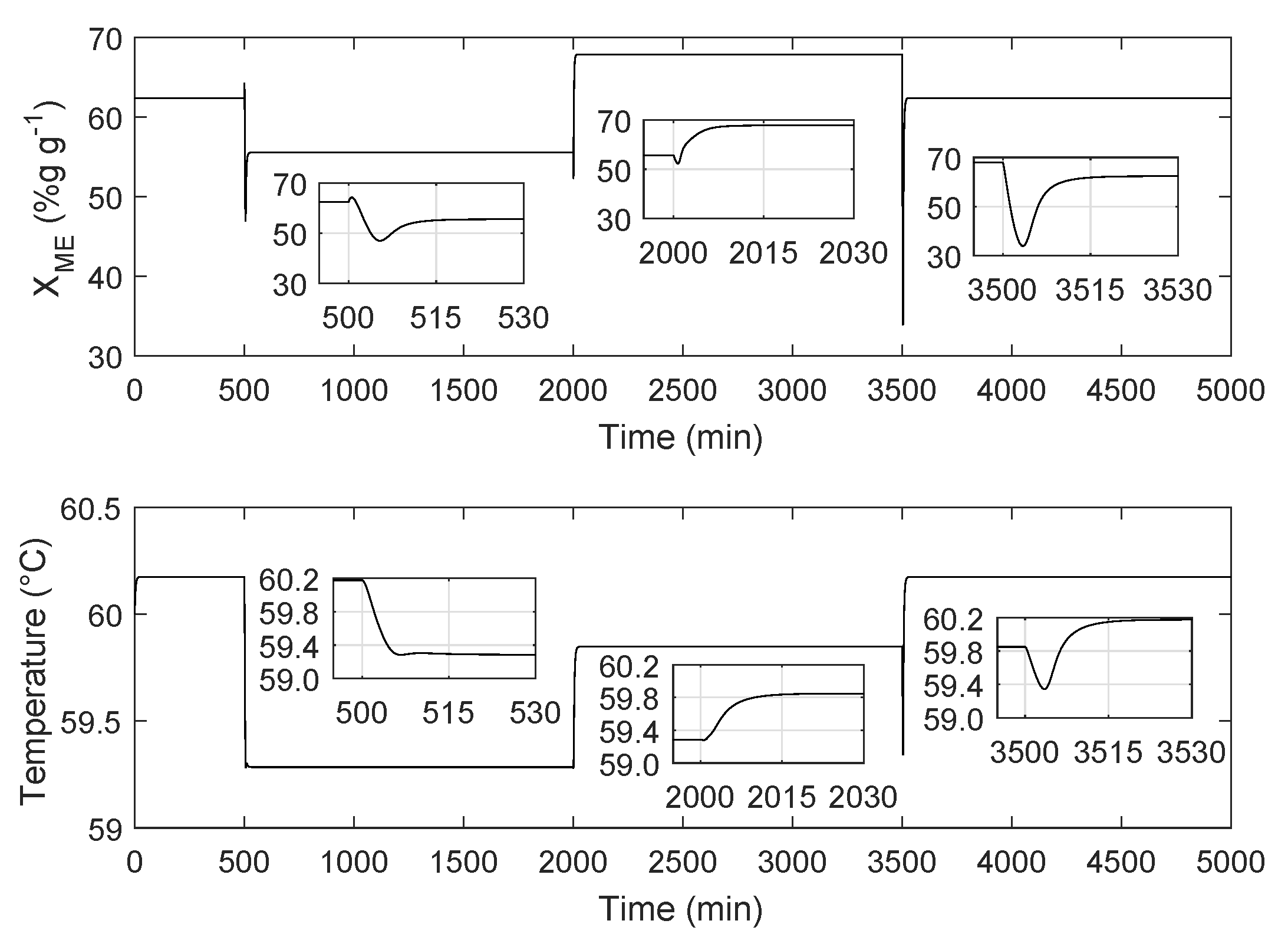
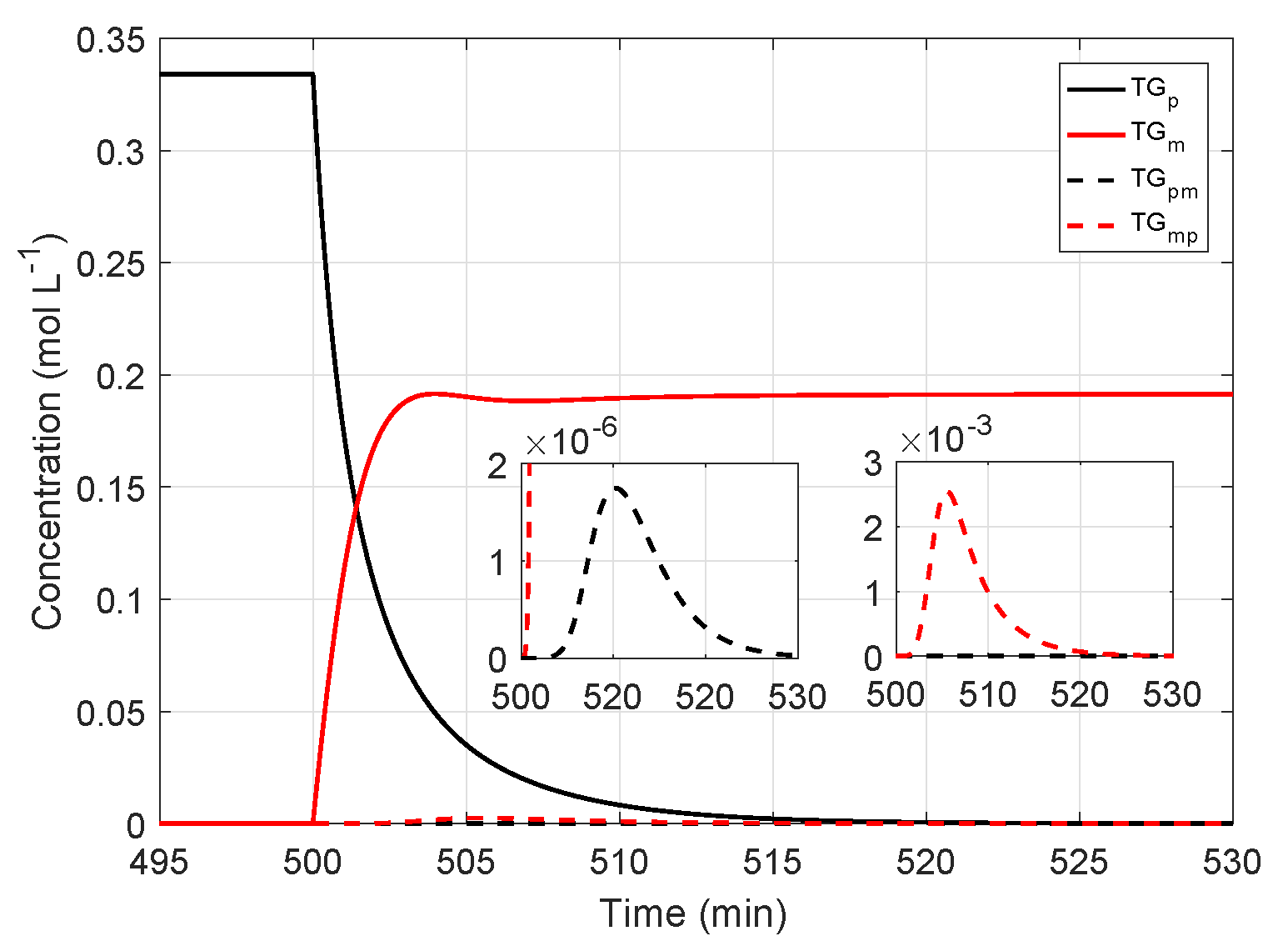
4.2. Reactor Simulation and Analysis of the Effect of the Feed Oil Changes on the System
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TG | Triglycerides |
| DG | Diglycerides |
| MG | Monoglycerides |
| ME | Methyl Esters |
| Al | Alcohol (Methanol) |
| GL | Glycerol |
| CSTR | Continuous Stirred-Tank Reactor |
| FAME | Fatty Acid Methyl ester |
References
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Tapasvi, D.; Wiesenborn, D.; Gustafson, C. Process model for biodiesel production from various feedstocks. Trans. ASAE 2005, 48, 2215–2221. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Kim San, L.; Chai Yin, K.; Azlan Hussain, M. Dynamics and control of a biodiesel transesterification reactor. Chem. Eng. Technol. 2009, 32, 13–26. [Google Scholar] [CrossRef]
- Brásio, A.S.; Romanenko, A.; Fernandes, N.C.; Santos, L.O. First, principle modeling and predictive control of a continuous biodiesel plant. J. Process Control 2016, 47, 11–21. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Q. Novel Design for Simultaneous Production of Biodiesel and Glycerol Carbonate from Soybean Oil. Ind. Eng. Chem. Res. 2018, 57, 16809–16816. [Google Scholar] [CrossRef]
- OECD-FAO. Agricultural Outlook 2018–2027. Number I9166ES, FAO and OECD. 2018. Chapter 9. Available online: http://www.fao.org (accessed on 12 December 2019).
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Yusuf, N.; Kamarudin, S.K.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Robles-Medina, A.; González-Moreno, P.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef]
- Popp, J.; Harangi-Rákos, M.; Gabnai, Z.; Balogh, P.; Antal, G.; Bai, A. Biofuels and their co-products as livestock feed: Global economic and environmental implications. Molecules 2016, 21, 285. [Google Scholar] [CrossRef] [Green Version]
- Chung, G.F. Effect of Pests and Diseases on Oil Palm Yield. In Palm Oil; Elsevier: Amsterdam, The Netherlands, 2012; pp. 163–210. [Google Scholar]
- Brásio, A.S.; Romanenko, A.; Leal, J.; Santos, L.O.; Fernandes, N.C. Nonlinear model predictive control of biodiesel production via transesterification of used vegetable oils. J. Process Control 2013, 23, 1471–1479. [Google Scholar] [CrossRef] [Green Version]
- Kuen, H.Y.; Mjalli, F.S.; Koon, Y.H. Recursive least squares-based adaptive control of a biodiesel transesterification reactor. Ind. Eng. Chem. Res. 2010, 49, 11434–11442. [Google Scholar] [CrossRef]
- Shi, H.; Wang, D.; Yuan, D.; Wang, T. Two-layer predictive control of a continuous biodiesel transesterification reactor. J. Appl. Math. 2013, 2013, 587841. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.; Mjalli, F.; Yeoh, H. Multivariable adaptive predictive model based control of a biodiesel transesterification reactor. J. Appl. Sci. 2010, 10, 1019–1027. [Google Scholar] [CrossRef]
- Montriwasuwat, N.; Kittisupakorn, P.; Lersbamrungsuk, V. A partial differential equations model predictive control of heterogeneous transesterification process for biodiesel production in tubular reactor. In Proceedings of the MultiConference of Engineers and Computer Scientists, Hong Kong, China, 14–16 March 2012; Volume II, pp. 14–16. [Google Scholar]
- Likozar, B.; Pohar, A.; Levec, J. Transesterification of oil to biodiesel in a continuous tubular reactor with static mixers: Modelling reaction kinetics, mass transfer, scale-up and optimization considering fatty acid composition. Fuel Process. Technol. 2016, 142, 326–336. [Google Scholar] [CrossRef]
- Kern, R.; Shastri, Y. Advanced control with parameter estimation of batch transesterification reactor. J. Process Control 2015, 33, 127–139. [Google Scholar] [CrossRef]
- González, J.C.; Medina, M.; Gutiérrez, P.Á. Predictive control of biodiesel transesterification in a batch reactor. Revista Mexicana de Ingeniería Química 2017, 16, 651–661. [Google Scholar]
- López-Zapata, B.Y.; Adam-Medina, M.; Álvarez-Gutiérrez, P.E.; Castillo-González, J.P.; León, H.R.; Vela-Valdés, L.G. Virtual sensors for biodiesel production in a batch reactor. Sustainability 2017, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- International, A. Standard Specification for Biodiesel Fuel Blend Stock (B100) For Middle Distillate Fuels; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Sanli, H.; Canakci, M. Effects of different alcohol and catalyst usage on biodiesel production from different vegetable oils. Energy Fuels 2008, 22, 2713–2719. [Google Scholar] [CrossRef]
- Bozbas, K. Biodiesel as an alternative motor fuel: Production and policies in the European Union. Renew. Sustain. Energy Rev. 2008, 12, 542–552. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Freedman, B.; Pryde, E.; Mounts, T. Variables affecting the yields of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Noureddini, H.; Zhu, D. Kinetics of transesterification of soybean oil. J. Am. Oil Chem. Soc. 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2019, 262, 116553. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N.; Nabi, M.N. The potential of utilising papaya seed oil and stone fruit kernel oil as non-edible feedstock for biodiesel production in Australia—A review. Energy Rep. 2019, 5, 280–297. [Google Scholar] [CrossRef]
- Ullah, Z.; Khan, A.S.; Muhammad, N.; Ullah, R.; Alqahtani, A.S.; Shah, S.N.; Ghanem, O.B.; Bustam, M.A.; Man, Z. A review on ionic liquids as perspective catalysts in transesterification of different feedstock oil into biodiesel. J. Mol. Liq. 2018, 266, 673–686. [Google Scholar] [CrossRef]
- Akubude, V.; Nwaigwe, K.; Dintwa, E. Production of biodiesel from microalgae via nanocatalyzed transesterification process: A review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Selvaraj, R.; Praveenkumar, R.; Moorthy, I.G. A comprehensive review of biodiesel production methods from various feedstocks. Biofuels 2019, 10, 325–333. [Google Scholar] [CrossRef]
- Falcone, P.M.; Lopolito, A.; Sica, E. Policy mixes towards sustainability transition in the Italian biofuel sector: Dealing with alternative crisis scenarios. Energy Res. Soc. Sci. 2017, 33, 105–114. [Google Scholar] [CrossRef]
- Falcone, P.M.; Lopolito, A.; Sica, E. Instrument mix for energy transition: A method for policy formulation. Technol. Forecast. Soc. Chang. 2019, 148, 119706. [Google Scholar] [CrossRef]
- Glensor, K.; Muñoz, B.; Rosa, M. Life-Cycle Assessment of Brazilian Transport Biofuel and Electrification Pathways. Sustainability 2019, 11, 6332. [Google Scholar] [CrossRef] [Green Version]
- Luyben, W.L. Process Modeling, Simulation and Control for Chemical Engineers; McGraw-Hill Higher Education: Singapore, 1989. [Google Scholar]
- Bequette, B.W. Process Dynamics: Modeling, Analysis, and Simulation; Prentice hall PTR: Upper saddle river, NJ, USA, 1998. [Google Scholar]
- Zapata, B.Y.L.; Medina, M.A.; Gutiérrez, P.Á.; de León, H.H.; Beltrán, C.G.; Gordillo, R.M. Different approaches for the dynamic model for the production of biodiesel. Chem. Eng. Res. Des. 2018, 132, 536–550. [Google Scholar] [CrossRef]
- Halim, S.F.A.; Kamaruddin, A.H.; Fernando, W. Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: Optimization using response surface methodology (RSM) and mass transfer studies. Bioresour. Technol. 2009, 100, 710–716. [Google Scholar] [CrossRef]
- Hernández, P.N.B.; Santamaría, J.R.A.; Rios, L.A. Biodiesel: Producción, Calidad y Caracterización; Universidad de Antioquia: Antioquia, Colombia, 2009. [Google Scholar]
- Issariyakul, T.; Dalai, A.K. Comparative kinetics of transesterification for biodiesel production from palm oil and mustard oil. Can. J. Chem. Eng. 2012, 90, 342–350. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J.; Esteban, A. Kinetics of sunflower oil methanolysis. Ind. Eng. Chem. Res. 2005, 44, 5447–5454. [Google Scholar] [CrossRef]
- Likozar, B.; Levec, J. Effect of process conditions on equilibrium, reaction kinetics and mass transfer for triglyceride transesterification to biodiesel: Experimental and modeling based on fatty acid composition. Fuel Process. Technol. 2014, 122, 30–41. [Google Scholar] [CrossRef]
- Chang, A.F.; Liu, Y. Integrated process modeling and product design of biodiesel manufacturing. Ind. Eng. Chem. Res. 2009, 49, 1197–1213. [Google Scholar] [CrossRef]
- Antoniosi Filho, N.; Mendes, O.; Lanças, F. Computer prediction of triacylglycerol composition of vegetable oils by HRGC. Chromatographia 1995, 40, 557–562. [Google Scholar] [CrossRef]
- Su, Y.C.; Liu, Y.; Diaz Tovar, C.A.; Gani, R. Selection of prediction methods for thermophysical properties for process modeling and product design of biodiesel manufacturing. Ind. Eng. Chem. Res. 2011, 50, 6809–6836. [Google Scholar] [CrossRef] [Green Version]
- Canakci, M.; Van Gerpen, J. A pilot plant to produce biodiesel from high free fatty acid feedstocks. In Proceedings of the 2001 ASAE Annual Meeting. American Society of Agricultural and Biological Engineers, Sacramento, CA, USA, 30 July–1 August 2001; p. 1. [Google Scholar]
- Ceriani, R.; Gani, R.; Meirelles, A.J. Prediction of heat capacities and heats of vaporization of organic liquids by group contribution methods. Fluid Phase Equilibria 2009, 283, 49–55. [Google Scholar] [CrossRef]
- Ihmels, E.C.; Gmehling, J. Extension and revision of the group contribution method GCVOL for the prediction of pure compound liquid densities. Ind. Eng. Chem. Res. 2003, 42, 408–412. [Google Scholar] [CrossRef]
- Righetti, M.; Salvetti, G.; Tombari, E. Heat capacity of glycerol from 298 to 383 K. Thermochimica Acta 1998, 316, 193–195. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. The Properties of Gases and Liquids; Mcgraw-Hill: New York, NY, USA, 2001; Volume 5. [Google Scholar]
- Perry Robert, H.; Green Don, W.; Maloney James, O. Perry’s Chemical Engineers’ Handbook; Mc Graw-Hills: New York, NY, USA, 1997; pp. 56–64. [Google Scholar]
- Zong, L.; Ramanathan, S.; Chen, C.C. Fragment-based approach for estimating thermophysical properties of fats and vegetable oils for modeling biodiesel production processes. Ind. Eng. Chem. Res. 2009, 49, 876–886. [Google Scholar] [CrossRef]
- Zong, L.; Ramanathan, S.; Chen, C.C. Predicting thermophysical properties of mono-and diglycerides with the chemical constituent fragment approach. Ind. Eng. Chem. Res. 2010, 49, 5479–5484. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Estimation of the thermodynamic properties of C-H-N-O-S-halogen compounds at 298.15 K. J. Phys. Chem. Ref. Data 1993, 22, 805–1159. [Google Scholar] [CrossRef] [Green Version]
- Bala, D.D.; Chidambaram, D. Enhancing kinetics of biodiesel production using morpholine. Catal. Commun. 2016, 83, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Esonye, C.; Onukwuli, O.D.; Ofoefule, A.U. Chemical kinetics of a two-step transesterification of dyacrodes edulis seed oil using acid-alkali catalyst. Chem. Eng. Res. Des. 2019, 145, 245–257. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M. Kinetics of acid base catalyzed transesterification of Jatropha curcas oil. Bioresour. Technol. 2010, 101, 7701–7706. [Google Scholar] [CrossRef]
- Kumar, G.R.; Ravi, R.; Chadha, A. Kinetic studies of base-catalyzed transesterification reactions of non-edible oils to prepare biodiesel: The effect of co-solvent and temperature. Energy Fuels 2011, 25, 2826–2832. [Google Scholar] [CrossRef]
- Noriega, M.; Narváez, P.; Heinz, C. Kinetics of Jatropha oil methanolysis. Fuel 2014, 134, 244–249. [Google Scholar] [CrossRef]
- Yunus, R.; Syam, A.M. Kinetics of transesterification of Jatropha curcas-based triglycerides with an alcohol in the presence of alkaline catalyst. In Proceedings of the 2010 1st International Nuclear &Renewable Energy Conference (INREC), Amman, Jordan, 21–24 March 2010; pp. 1–4. [Google Scholar]
- Syam, A.M.; Hamid, H.A.; Yunus, R.; Rashid, U. Dynamic modeling of reversible methanolysis of Jatropha curcas oil to biodiesel. Sci. World J. 2013, 2013, 268385. [Google Scholar] [CrossRef] [PubMed]
- Tapanes, N.C.O.; Aranda, D.A.G.; de Mesquita Carneiro, J.W.; Antunes, O.A.C. Transesterification of Jatropha curcas oil glycerides: Theoretical and experimental studies of biodiesel reaction. Fuel 2008, 87, 2286–2295. [Google Scholar] [CrossRef]
- Tiwari, P.; Garg, S. Study of reversible kinetic models for alkali-catalyzed Jatropha curcas transesterification. Biomass Convers. Biorefinery 2016, 6, 61–70. [Google Scholar] [CrossRef]
- Sarve, A.; Varma, M.N.; Sonawane, S.S. Optimization and kinetic studies on biodiesel production from kusum (schleichera triguga) oil using response surface methodology. J. Oleo Sci. 2015, 64, 987–997. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Tiwari, P.; Garg, S. Alkali transesterification of linseed oil for biodiesel production. Fuel 2013, 104, 553–560. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Kinetics of Brassica c arinata Oil Methanolysis. Energy Fuels 2006, 20, 1722–1726. [Google Scholar] [CrossRef]
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Leevijit, T.; Wisutmethangoon, W.; Prateepchaikul, G.; Tongurai, C.; Allen, M. A second order kinetics of palm oil transesterification. In Proceedings of the Joint International Conference on “Sustainable Energy and Environment (SEE), Hua Hin, Thailand, 1–3 December 2004. [Google Scholar]
- Narváez, P.C.; Rincón, S.; Sánchez, F. Kinetics of palm oil methanolysis. J. Am. Oil Chem. Soc. 2007, 84, 971–977. [Google Scholar] [CrossRef]
- Klofutar, B.; Golob, J.; Likozar, B.; Klofutar, C.; Žagar, E.; Poljanšek, I. The transesterification of rapeseed and waste sunflower oils: Mass-transfer and kinetics in a laboratory batch reactor and in an industrial-scale reactor/separator setup. Bioresour. Technol. 2010, 101, 3333–3344. [Google Scholar] [CrossRef]
- Pecha, J.; Šánek, L.; Fürst, T.; Kolomazník, K. A kinetics study of the simultaneous methanolysis and hydrolysis of triglycerides. Chem. Eng. J. 2016, 288, 680–688. [Google Scholar] [CrossRef]
- Bashiri, H.; Pourbeiram, N. Biodiesel production through transesterification of soybean oil: A kinetic Monte Carlo study. J. Mol. Liq. 2016, 223, 10–15. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Tubino, M.; Junior, J.G.R.; Bauerfeldt, G.F. Biodiesel synthesis: A study of the triglyceride methanolysis reaction with alkaline catalysts. Catal. Commun. 2016, 75, 6–12. [Google Scholar] [CrossRef]
- Wu, L.; Wei, T.; Lin, Z.; Zou, Y.; Tong, Z.; Sun, J. Bentonite-enhanced biodiesel production by NaOH-catalyzed transesterification: Process optimization and kinetics and thermodynamic analysis. Fuel 2016, 182, 920–927. [Google Scholar] [CrossRef]
- Bambase, M.E.; Nakamura, N.; Tanaka, J.; Matsumura, M. Kinetics of hydroxide-catalyzed methanolysis of crude sunflower oil for the production of fuel-grade methyl esters. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 273–280. [Google Scholar] [CrossRef]

| Compound | Charac. | ||
|---|---|---|---|
| Triglyceride | Simple-molecule | Fragments-based [54] | |
| Diglyceride | Simple-molecule | Fragments-based [55] | |
| Monoglyceride | Simple-molecule | Fragments-based [55] | |
| Methyl ester | Pseudo-molecule | Group Contribution [50] | Group contribution [49] |
| Glycerol | - | Group contribution [50] | Algebraic eq. [51] |
| Alcohol | - | Rackett eq. [52] | Algebraic eq. [53] |
| Seed | Reference | Agitation (rpm) | Catalyst | (wt%) | Ratio (wt%/wt%) | Mechanism Steep | T (°C) |
|---|---|---|---|---|---|---|---|
| Canola | Bala and Chidambaram (2016) [57] | 225 | NaOH | 1.5 | 6:1 | Single | 65 |
| Coffee | Bala and Chidambaram (2016) [57] | 225 | NaOH | 1.5 | 6:1 | Single | 65 |
| Corn | Bala and Chidambaram (2016) [57] | 225 | NaOH | 1.5 | 6:1 | Single | 65 |
| Dyacrodes edulis | Esonye et al. (2019) [58] | 140 | NaOH | 0.1 | 6:1 | Three | 55, 60, 65 |
| Jatropha | Jain and Sharma (2010) [59] | 400 | NaOH | 1 | 3:7 | Single | 50 |
| Kumar et al. (2011) [60] | Omitted | KOH | 1 | 6:1 | Single | 28, 45 | |
| Noriega et al. (2014) [61] | 600 | NaOH | 0.2, 0.4, 0.6, 1 | 3:1, 4.5:1, 6:1, 9:1, 12:1 | Three | 40, 50, 60 | |
| Yunus and Syam (2010) [62] | Omitted | KOH | 1 | 6:1 | Two | 50, 55, 60, 65 | |
| Syam et al. (2013) [63] | Omitted | KOH | 1 | 6:1 | Three | 60 | |
| Tapanes et al. (2008) [64] | 300 | KOH | 0.8 | 9:1 | Single | 45 | |
| Tiwari and Garg (2016) [65] | 300, 750 | NaOH | 0.5 | 6:1 | Single | 30, 40, 50, 60 | |
| Kusum | Sarve et al. (2015) [66] | Omitted | CHONa | 0.55, 0.7 0.85, 1, 1.15 | Single | 4, 6, 8, 10, 12 | 45, 50, 55, 60, 65 |
| Linseed | Kumar et al. (2013) [67] | 750 | NaOH | 0.5,1 | 6:1, 9:1 | Single | 40, 50, 60 |
| Mahua | Kumar et al. (2011) [60] | Omitted | KOH | 1 | 6:1 | Single | 28, 45 |
| Mustard | Issariyakul and Dalai (2012) [42] | 600 | KOH | 1 | 6:1 | Three | 40, 50, 60 |
| Vicente et al. (2006) [68] | 600 | KOH | 0.5, 1, 1.5 | 6:1 | Three | 25, 35, 45, 55, 65 | |
| Palm | Darnoko and Cheryan (2000) [69] | Omitted | KOH | 1 | 6:1 | Three | 50, 55, 60, 65 |
| Issariyakul and Dalai (2012) [42] | 600 | KOH | 1 | 6:1 | Three | 40, 50, 60 | |
| Leevijit et al. (2004) [70] | 2000 () | NaOH | 1 | 6:1 | Three | 60 | |
| Narváez et al. (2007) [71] | 400 | NaOH | 0.20 | 6:1 | Three | 50 | |
| Rapeseed | Klofutar et al. (2010) [72] | 500 | KOH | 1 | 6:1 | Three | 40, 50 |
| Pecha et al. (2016) [73] | 2000 | TMAH | 0.5, 1, 1.5 | 6:1 | Three | 40, 50, 60 | |
| Soybean | Bashiri and Pourbeiram (2016) [74] | 300 | NaOH | 0.2 | 6:1 | Three | 50 |
| Freedman et al. (1985) [75] | Omitted | NaOH | 0.5 | 6:1 | Three | 20, 30, 40, 50, 60 | |
| Nourenddini and Zhu (1997) [26] | 300 | NaOH | 0.2 | 6:1 | Three | 50 | |
| Tubino et al. (2016) [76] | 400 | NaOCH, KOCH, NaOH, KOH | 0.0157 (mol) | 6:1 | Single | 30, 40, 50, 60 | |
| Wu et al. (2018) [77] | 500, 600, 700, 800 | NaOH | 0, 0.6, 0.7, 0.8, 0.9 | 4.5:1, 6:1, 7.5:1, 9:1 | Single | 55, 60, 65 | |
| Sunflower | Bambase et al. (2007) [78] | 400 | NaOH | 0.25-1 | 6:1 | Three | 60 |
| Klofutar et al. (2010) [72] | 500 | KOH | 1 | 6:1 | Three | 40, 50 | |
| Vicente et al. (2005) [43] | 600 | KOH | 0.5, 1, 1.5 | 6:1 | Three | 25, 35, 45, 55, 65 |
| Variable | Value | Unities | Variable | Value | Unities |
|---|---|---|---|---|---|
| 7140 | L/min | 0.97 | mol/L | ||
| 160.8 | L/min | 0.52 | mol/L | ||
| 60 | C | 0.82 | mol/L | ||
| 30 | C | 0.15 | mol/L | ||
| 5.82 | mol/L | 0.01 | mol/L | ||
| 3.12 | mol/L | 0 | mol/L | ||
| 4.92 | mol/L |
| Variable | Change | Transitory State | Staady State | ||||
|---|---|---|---|---|---|---|---|
| Duration | Min. Value | Before | After | Value | Percentage | ||
| (min) | (% g/g) | (% g/g) | (% g/g) | (% g/g) | |||
| P-M | 19.14 | 46.96 | 62.40 | 55.56 | −6.84 | −10.90 | |
| M-G | 10.00 | 52.31 | 55.6 | 67.87 | 12.27 | 22.07 | |
| G-P | 21.27 | 33.94 | 67.87 | 62.40 | −5.62 | −9.00 | |
| T | P-M | 5.72 | NA | 60.17 | 59.28 | −0.89 | −1.48 |
| M-G | 16.10 | NA | 59.28 | 59.85 | 0.57 | 0.95 | |
| G-P | 22.60 | 59.24 | 59.85 | 60.17 | 0.32 | 0.53 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo Gónzalez, J.P.; Álvarez Gutiérrez, P.E.; Adam Medina, M.; López Zapata, B.Y.; Ramírez Guerrero, G.V.; Vela Valdés, L.G. Effects on Biodiesel Production Caused by Feed Oil Changes in a Continuous Stirred-Tank Reactor. Appl. Sci. 2020, 10, 992. https://doi.org/10.3390/app10030992
Castillo Gónzalez JP, Álvarez Gutiérrez PE, Adam Medina M, López Zapata BY, Ramírez Guerrero GV, Vela Valdés LG. Effects on Biodiesel Production Caused by Feed Oil Changes in a Continuous Stirred-Tank Reactor. Applied Sciences. 2020; 10(3):992. https://doi.org/10.3390/app10030992
Chicago/Turabian StyleCastillo Gónzalez, Juan P., Peggy E. Álvarez Gutiérrez, Manuel Adam Medina, Betty Y. López Zapata, Gerardo V. Ramírez Guerrero, and Luis G. Vela Valdés. 2020. "Effects on Biodiesel Production Caused by Feed Oil Changes in a Continuous Stirred-Tank Reactor" Applied Sciences 10, no. 3: 992. https://doi.org/10.3390/app10030992





