Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Materials
2.3. Cell Culture
2.4. Cell Viability
2.5. Glycerol-3-phosphate Dehydrogenase (GPDH) Measurement
2.6. Measurement of Lipoprotein Lipase (LPL) Activity
2.7. Quantification of Triglyceride (TG) Contents
2.8. Animal Experiments and Diets
2.9. Biochemical Analysis of Serum Samples
2.10. Lipid Contents of Liver and Adipose Tissues
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results
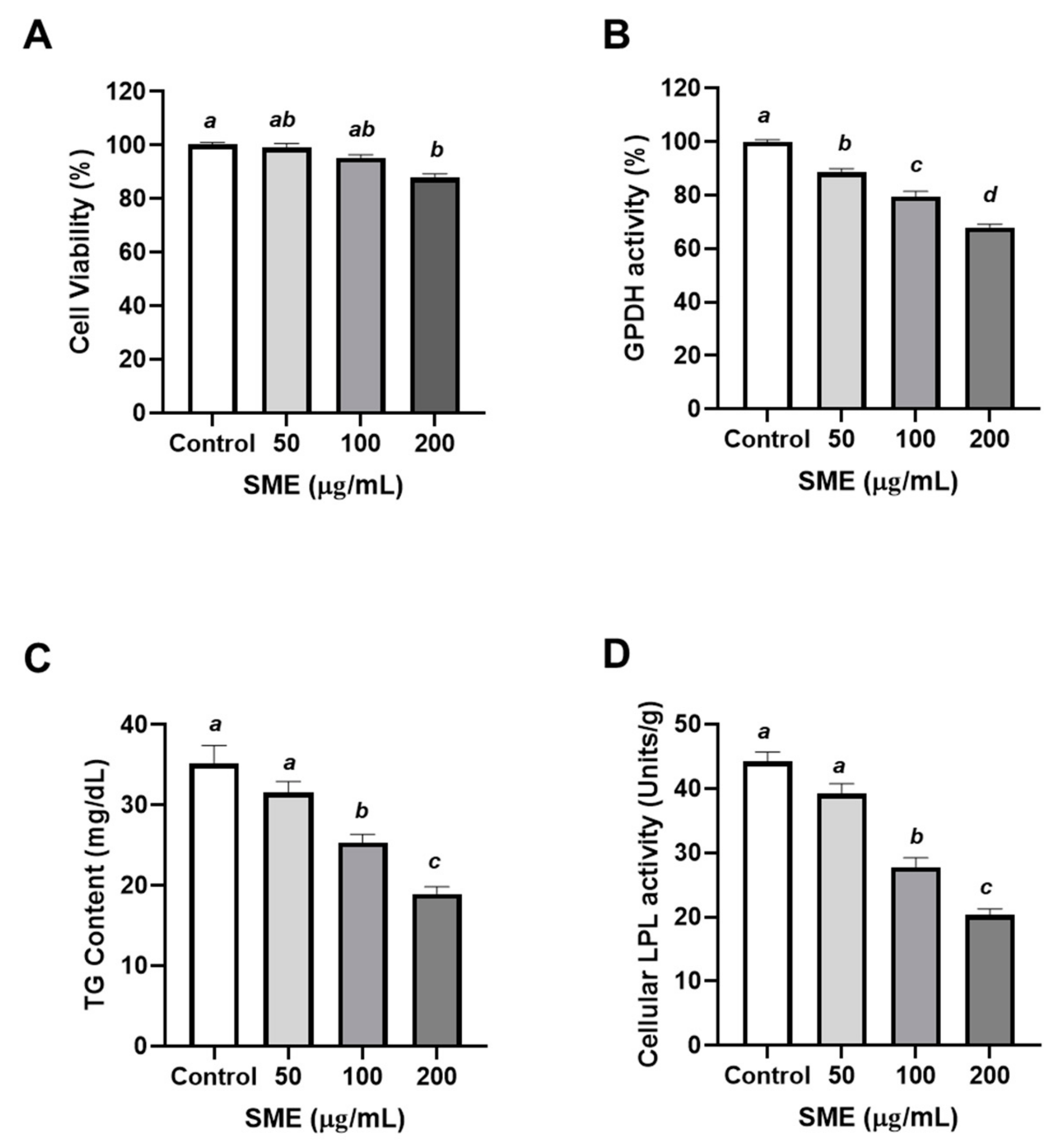
3.1. Effects of SME on Proliferation and Differentiation of 3T3-L1 Cells
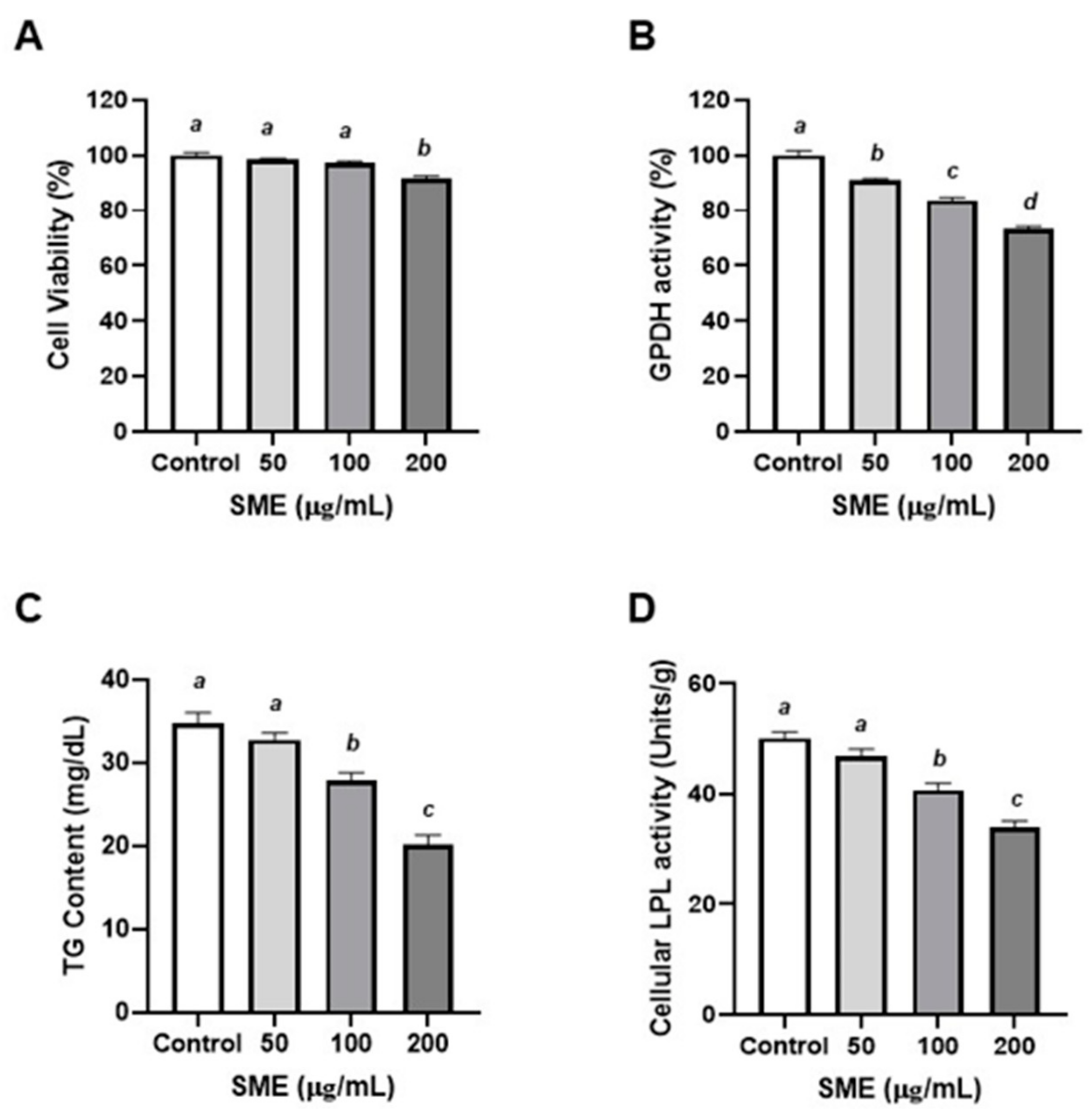
3.2. Effects of SME on Proliferation and Differentiation of Porcine Pre-Adipocytes
3.3. SMP Ameliorated HFD-Induced Obesity
3.4. ALT, AST, ALP, and LDH Activities in Serum
3.5. Lipids Profiles in Serum
3.6. TG and TC Levels in Liver and WAT
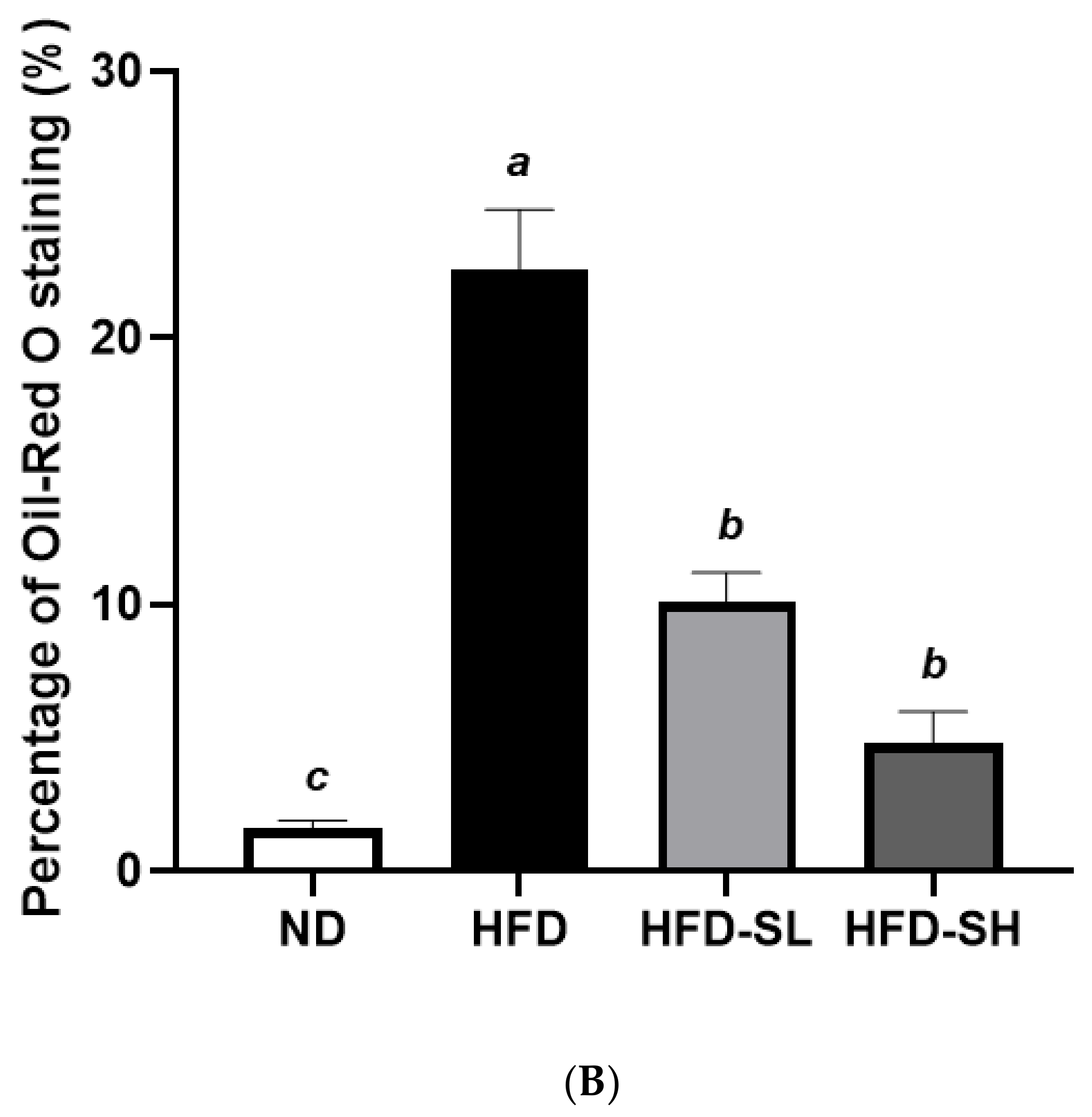
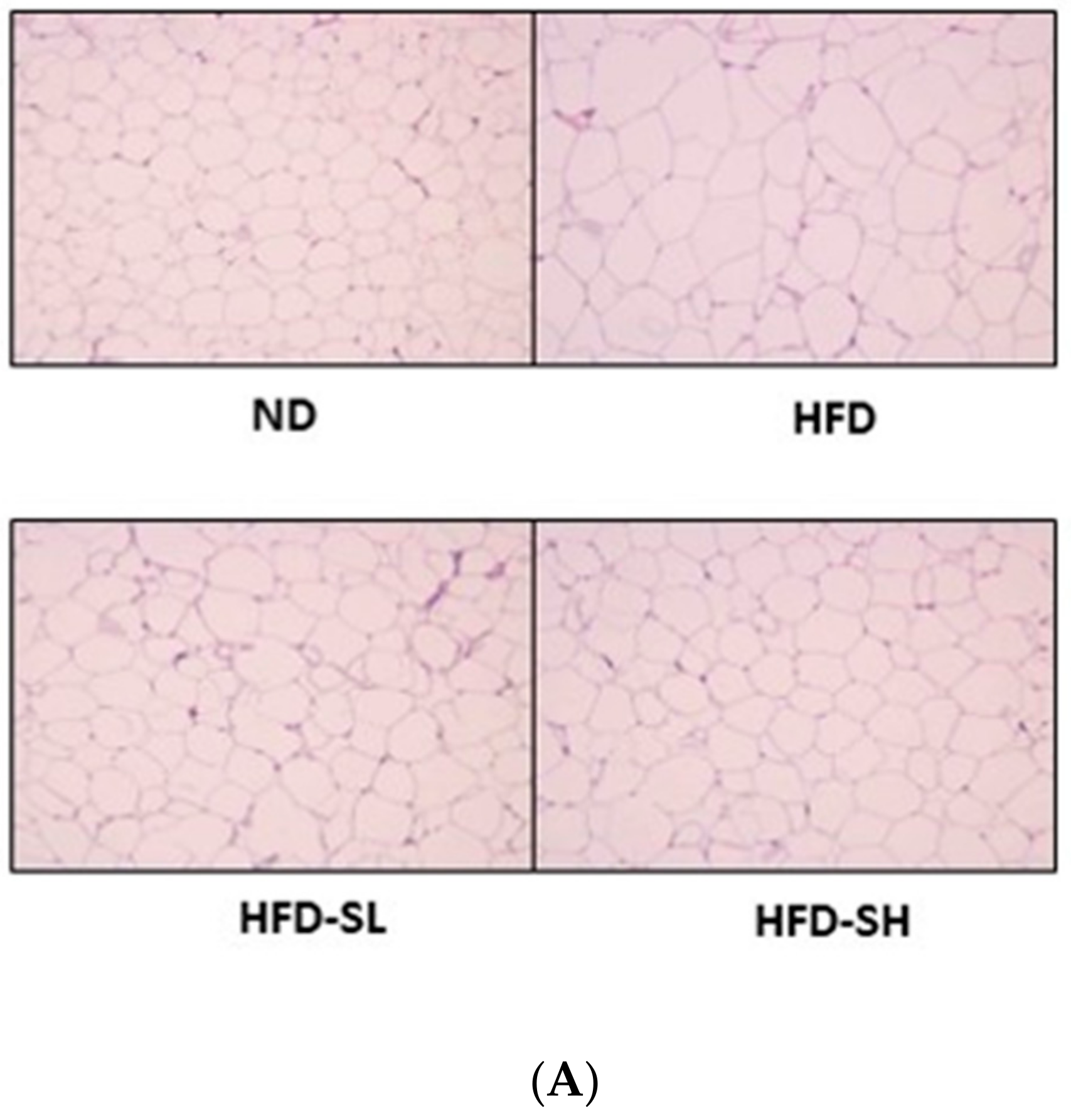
3.7. Histological Analysis in Liver and Epididymal Adipose Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bjorntorp, P. The associations between obesity, adipose tissue distribution and disease. Acta Med. Scand. Suppl. 1988, 723, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. Korea Health Statistics 2014 Korean National Health and Nutrition Examination Survey; Korea Centers for Disease Control and Prevention: Seoul, Korea, 2014.
- World Health Organization. Global status report on noncommunicable disease 2014. Geneva: WHO Global Strategy on Diet, Physical Activity and Health. 2015. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 10 December 2019).
- Albu, J.; Allison, D.; Boozer, C.D.; Heymsfield, S.; Kissileff, H.; Kretser, A.; Krumhar, K.; Leibel, R.; Nonas, C.; Pi-Sunyer, X.; et al. Obesity solutions: report of a meeting. Nutr. Rev. 1997, 55, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.E.; Choi, Y.J.; Lee, Y.H.; Kim, D.J.; Park, S.W.; Huh, B.H.; Lee, E.J.; Jee, S.H.; Hur, K.Y.; Choi, S.H.; et al. ApoB/ApoA-I ratio is independently associated with carotid atherosclerosis in type 2 diabetes mellitus with well-controlled LDL cholesterol levels. Korean J. Intern. Med. 2018, 33, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Gwag, B.J.; Lee, Y.A.; Ko, S.Y.; Lee, M.J.; Im, D.S.; Yun, B.S.; Lim, H.R.; Park, H.R.; Byun, H.R.; Son, S.J.; et al. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J. Cereb. Blood Flow Metab. 2007, 27, 1142–1151. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Bowman, B.A.; Ford, E.S.; Vinicor, F.; Marks, J.S.; Koplan, J.P. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001, 286, 1195–1200. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Roncari, D.A.; Lau, D.C.; Kindler, S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism 1981, 30, 425–427. [Google Scholar] [CrossRef]
- Weigle, D.S. Pharmacological therapy of obesity: past, present, and future. J. Clin. Endocrinol. Metab. 2003, 88, 2462–2469. [Google Scholar] [CrossRef]
- Yun, J.W. Possible anti-obesity therapeutics from nature-a review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Heo, B.G.; Park, Y.J.; Park, Y.S.; Hee, I.M.; Cho, J.Y. Distribution status, physicochemical composition, and physiological activity of Spergularia marina cultivated. Korean J. Community Living Sci. 2009, 20, 181–191. [Google Scholar]
- Kim, M.S. Isolation and structural elucidation of antioxidants from Spergularia marina Griseb. Master’s Thesis, Chonnam National University, Gwangju, Korea, 2013. [Google Scholar]
- Lee, J.J.; Jung, H.O. Changes in physicochemical properties of Spergularia marina Griseb by blanching. Korean J. Food Preserv. 2012, 19, 866–872. [Google Scholar] [CrossRef]
- Cho, J.Y.; Huang, Z.; Park, S.Y.; Park, K.H.; Pai, T.K.; Kim, S.Y.; Kim, H.R.; Ham, K.S. The effects of several halophytes on insulin resistance in Otsuka long-evans tokushima fatty rats. Korean. J. Food Sci. Technol. 2014, 46, 100–107. [Google Scholar] [CrossRef]
- Chen, C.L.; Brodie, A.E.; Hu, C.Y. CCAAT/enhancer-binding protein beta is not affected by tetrachlorodibenzo-p-dioxin (TCDD) inhibition of 3T3-L1 preadipocyte differentiation. Obes. Res. 1997, 5, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Bohan, A.E.; Purvis, K.N.; Bartosh, J.L.; Brandebourg, T.D. The proliferation and differentiation of primary pig preadipocytes is suppressed when cultures are incubated at 37 degrees Celsius compared to euthermic conditions in pigs. Adipocyte 2014, 3, 322–332. [Google Scholar] [CrossRef]
- Wise, L.S.; Green, H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem. 1979, 254, 273–275. [Google Scholar]
- Nilsson-Ehle, P.; Garfinkel, A.S.; Schotz, M.C. Intra- and extracellular forms of lipoprotein lipase in adipose tissue. Biochim. Biophys. Acta 1976, 431, 147–156. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Rosenfeld, L. Lipoprotein analysis. Early methods in the diagnosis of atherosclerosis. Arch. Pathol. Lab. Med. 1989, 113, 1101–1110. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Biggs, H.G.; Erikson, J.M.; Moorehead, W.R. A manual colormetric assay of triglycerides in serum. Clin. Chem. 1975, 21, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Zlatkis, A.; Zak, B. Study of a new cholesterol reagent. Anal Biochem. 1969, 29, 143–148. [Google Scholar] [CrossRef]
- Sottile, V.; Seuwen, K. A high-capacity screen for adipogenic differentiation. Anal Biochem. 2001, 293, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef] [PubMed]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef] [PubMed]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef]
- Di Renzo, D.; Owens, G.K.; Leeper, N.J. “Attack of the Clones”: Commonalities Between Cancer and Atherosclerosis. Circ. Res. 2017, 120, 624–626. [Google Scholar] [CrossRef]
- Maclean, P.S.; Bergouignan, A.; Cornier, M.A.; Jackman, M.R. Biology’s response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Phys. 2011, 301, R581–R600. [Google Scholar] [CrossRef]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Park, J.C.; Choi, J.W. Screening of marine natural products on inhibitory effect ofb the formation of lipid peroxidation. Kor. J. Pharmacogn. 1996, 27, 117–122. [Google Scholar]
- Cheong, J.H. Characteristics of seed germination in halophyte influenced by temperature. Master’s Thesis, Mokpo National University, Mokpo, Korea, 2011. [Google Scholar]
- Lee, H.J.; Kim, Y.A.; Ahn, J.W.; Lee, B.J.; Moon, S.G.; Seo, Y. Screening of peroxynitrite and DPPH radical scavenging activities from salt marsh plants. Korean J. Biotechnol. Bioeng. 2004, 19, 57–61. [Google Scholar]
- Kim, J.B.; Choe, S.N.; Choe, K.H.; Lim, S.H.; Chai, S.J. Functional components of halophyte—Antioxidant substances in Salicornia herbacea L. J. Fish. Mar. Sci. Educ. 2007, 19, 197–205. [Google Scholar]
- Kim, H.S.; Kim, J.A.; Karadeniz, F.; Ahn, B.N.; Kong, C.S. Radical scavenging and anti-inflammatory effects of the halophyte Spergularia marina Griseb. J. Biosci. 2014, 69, 425–433. [Google Scholar]
- Kim, K.; Lee, Y.M.; Rhyu, M.R.; Kim, H.Y. Spergularia marina induces glucagon-like peptide-1 secretion in NCI-H716 cells through bile acid receptor activation. J. Med. Food. 2014, 17, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H. Effects of Spergularia marina Griseb. powder on adipocytes and high-fat diet-induced obese rats. Ph.D. Thesis, Chosun University, Gwangju, Korea, 2016. [Google Scholar]
- Pang, W.; Wang, Y.; Wei, N.; Xu, R.; Xiong, Y.; Wang, P.; Shen, Q.; Yang, G. Sirt1 inhibits akt2-mediated porcine adipogenesis potentially by direct protein-protein interaction. PLoS ONE 2013, 8, e71576. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y. Effect of resveratrol and peanut sprout extracts on cell proliferation and lipid accumulation in mouse fibroblast 3T3-L1 preadipocytes. Master’s Thesis, Dankook University, Cheonan, Korea, 2011. [Google Scholar]
- Boone, C.; Gregoire, F.; Remacle, C. Culture of porcine stromal-vascular cells in serum-free medium: Differential action of various hormonal agents on adipose conversion. J. Anim. Sci. 2000, 78, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S. Studies on the bioactivities of Salsola komarovi extract. Master’s Thesis, Wonkwang University, Iksan, Korea, 2015. [Google Scholar]
- Kim, I.H.; Nam, T.J. The effects of polymannuronates on leptin in 3T3-L1 adipocytes. J. Kor. Fih. Soc. 2004, 37, 372–379. [Google Scholar] [CrossRef]
- Cha, S.Y.; Jang, J.Y.; Lee, Y.H.; Lee, G.Y.; Lee, H.J.; Hwang, K.T.; Kim, Y.J.; Jun, Y.J.; Lee, J.M. Lipolytic effect of methanol extracts from Luffa cylindrica in mature 3T3-L1 adipocytes. J. Korean Soc. Food Sci. Nutr. 2010, 39, 813–819. [Google Scholar] [CrossRef]
- Sung, N.J.; Lee, S.J.; Shin, J.H.; Chung, M.J.; Lim, S.S. Effects of Houttuynia cordata Thunb powder and juice on lipid composition of liver, brain and kidney in dietary hypercholesterolemic rats. J. Korean Soc. Food Sci. Nutr. 1998, 27, 1230–1235. [Google Scholar]
- Frizell, E.; Liu, S.L.; Abraham, V.; Ozaki, I.; Eghbali, M.; Sage, E.H.; Zern, M.A. Expression of SPARC in normal and fibrotic livers. Hepatology 1995, 21, 847–854. [Google Scholar]
- Kim, H.S.; Kim, T.W.; Kim, D.J.; Hwang, H.J.; Lee, H.J.; Cheo, M. Effects of natural plants supplementation on adipocyte size of the epididymal fat pads in rat. J. Korean Soc. Food Sci. Nutr. 2007, 36, 419–423. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Zhao, Y.; Li, W.; Yang, X. Effects of Dietary Fiber Supplementation on Fatty Acid Metabolism and Intestinal Microbiota Diversity in C57BL/6J Mice Fed with a High-Fat Diet. J. Agric. Food Chem. 2018, 66, 12706–12718. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, F.; Liu, J.; Liao, S.; Zou, Y. Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites. Nutrients 2019, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Plaza, G.L.; Charbonneau, M. Detection and evaluation of chemically induced liver injury. In Hayes’ Principles and Methods of Toxicology, 5th ed.; CRC Prress: Boca Raton, FL, USA, 1994; Chapter 29; pp. 1465–1507. [Google Scholar]
- Lee, S.H.; Park, Y.B.; Choi, M.S. The effect of dietary citrus flavonoid supplementation on cholesterol biosynthesis control in rats. In The Autumnal Symposium of Korean Nutrition Association; Korean Nutrition Association: Seoul, Korea, 1998; Volume 79. [Google Scholar]
- Moreno-Fernandez, S.; Garces-Rimon, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Davignon, J.; Cohn, J.S. Triglycerides: a risk factor for coronary heart disease. Atherosclerosis. 1996, 124, S57–S64. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, M.R.; Kim, A.R.; Lee, M.Y. Effects of ramie leaves on improvement of lipid metabolism and anti-obesity effect in rats fed a high fat/high cholesterol diet. Korean J. Food Sci. Technol. 2011, 43, 83–90. [Google Scholar] [CrossRef]
- Kim, M.J.; Jun, H.Y.; Kim, J.H. Antiadipigenic effect of korean glasswort (Salicomia herbacea L.) water extract on 3T3-L1 adipocytes. J. Korean Soc. Food Sci. Nutr. 2014, 43, 814–821. [Google Scholar] [CrossRef][Green Version]
- Kim, M.J.; Jun, H.Y.; Kim, J.H. Anti-obesity effect of korean hamcho (Salicomia herbacea L.) powder on high-fat diet-induced obese rats. J. Nutr. Health. 2015, 48, 123–132. [Google Scholar] [CrossRef]
- Seo, H.B.; Kwak, Y.Y.; Nam, J.O.; Son, Y.J.; Kim, B.O.; Ryu, S.P. Glasswort powder diet activates lipid metabolism in rat. J. Life Sci. 2012, 22, 478–485. [Google Scholar] [CrossRef][Green Version]
- Lee, H.S.; Choi, J.H.; Kim, Y.E.; Lee, C.H. Effect of dietary intake of Salicornia herbacea L. hot water extract on anti-obesity in diet-induced obese rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 950–956. [Google Scholar] [CrossRef]
- Kim, N.H.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Anti-obesity Effect of Halophyte Crop, Limonium tetragonum in High-Fat Diet-Induced Obese Mice and 3T3-L1 Adipocytes. Biol. Pharm. Bull. 2017, 40, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y. Effect of Oenanthe javanica extracts on fat accumulation of the liver in rats. Master’s Thesis, Yeungnam University, Gyeongsan City, Korea.
- Zar Kalai, F.; Han, J.; Ksouri, R.; Abdelly, C.; Isoda, H. Oral administration of Nitraria retusa ethanolic extract enhances hepatic lipid metabolism in db/db mice model ‘BKS.Cg-Dock7(m)+/+ Lepr(db/) J’ through the modulation of lipogenesis-lipolysis balance. Food Chem. Toxicol. 2014, 72, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Choi, J.H.; Lee, S.K.; Woo, M.H.; Choi, S.W. Effect of enzymatic hydrolysate of Hamcho (Salicornia herbacea) on antioxidative defense system in rats fed high cholesterol diet. J. Korean Soc. Food Sci. Nutr. 2006, 35, 1356–1362. [Google Scholar] [CrossRef]
- Kang, K.J.; Lim, S.J.; Jeong, J.G.; Han, H.K.; Choi, S.S.; Kim, M.H.; Kwon, S.Y. Effects of wax guard on weight, triglyceride, leptin and fat cell size in rats fed on a high fat diet. J. Nutr. Health. 2003, 36, 446–451. [Google Scholar]
- Oka, S.; Alcendor, R.; Zhai, P.; Park, J.Y.; Shao, D.; Cho, J.; Yamamoto, T.; Tian, B.; Sadoshima, J. PPARalpha-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab. 2011, 14, 598–611. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kim, J.A.; Ahn, B.N.; Kim, M.; Kong, C.S. Anti-adipogenic and pro-osteoblastogenic activities of Spergularia marina Griseb. Prev. Nutr. Food Sci. 2014, 19, 187–193. [Google Scholar] [CrossRef][Green Version]



| Diet Composition (g1) | ND 2 | HFD 3 | HFD-SL | HFD-SH |
|---|---|---|---|---|
| Casein | 200 | 200 | 200 | 200 |
| L-Cystine | 3 | 3 | 3 | 3 |
| Corn starch | 399.986 | 299.986 | 269.986 | 249.986 |
| Dextrose | 100 | 100 | 100 | 100 |
| Sucrose | 50 | 50 | 50 | 50 |
| Cellulose | 50 | 50 | 50 | 50 |
| Lard | 100 | 200 | 200 | 200 |
| Soybean oil | 50 | 50 | 50 | 50 |
| Mineral mix 4 | 35 | 35 | 35 | 35 |
| Vitamin mix 5 | 10 | 10 | 10 | 10 |
| Choline chloride | 2 | 2 | 2 | 2 |
| tert-Butylhydroquinone | 0.014 | 0.056 | 0.056 | 0.056 |
| Spergularia marina Griseb | 0.0 | 0.0 | 30 | 50 |
| Total (g) | 1000 | 1000.042 | 1000.042 | 1000.042 |
| Total energy (kcal) | 4401.94 | 4901.944 | 4781.944 | 4701.944 |
| Fat (kcal %) | 30.67 | 45.90 | 47.05 | 47.85 |
| Group | n | Body Weight Gain (g/day) | Food Intake (g/day) | FER (Food Efficiency Ratio) |
|---|---|---|---|---|
| ND | 8 | 6.16 ± 0.52 c | 21.80 ± 1.24 a | 0.28 ± 0.01 b |
| HFD | 8 | 8.22 ± 1.30 a | 20.17 ± 2.29 a | 0.41 ± 0.04 a |
| HFD-SL (3%) | 8 | 7.48 ± 0.69 b | 19.03 ± 1.39 b | 0.39 ± 0.03 a |
| HFD-SH (5%) | 8 | 6.81 ± 1.03 c | 18.55 ± 1.05 b | 0.37 ± 0.04 a |
| Group | n | Liver (g) | EAT (g) | MAT (g) | RAT (g) | PAT (g) | Total AT (g) |
|---|---|---|---|---|---|---|---|
| ND | 8 | 11.03 ± 1.04 | 4.50 ± 1.14 c | 3.45 ± 0.96 | 6.24 ± 1.46 | 1.66 ± 0.32 b | 15.84 ± 2.17 b |
| HFD | 8 | 12.34 ± 2.70 | 8.34 ± 2.70 a,b | 4.73 ± 1.25 | 8.59 ± 2.12 | 2.63 ± 1.08 a,b | 24.28 ± 6.40 a |
| HFD-SL (3%) | 8 | 11.46 ± 1.11 | 5.73 ± 1.06 b,c | 4.19 ± 0.76 | 7.64 ± 2.35 | 2.13 ± 0.60 a,b | 19.68±4.36 a,b |
| HFD-SH (5%) | 8 | 11.59 ± 1.14 | 7.91 ± 2.0 a,b | 4.70 ± 1.0 | 8.20 ± 1.45 | 2.56 ± 0.66 a | 23.36 ± 4.09 a |
| Group | n | AST (U/L) | ALT (U/L) | ALP (U/L) | LDH (U/L) |
|---|---|---|---|---|---|
| ND | 8 | 120.50 ± 20.45 b | 30.75 ± 6.84 b | 650.3 ± 62.0 b | 687.1 ± 116.8 b |
| HFD | 8 | 189.38 ± 44.39 a | 61.75 ± 4.50 a | 905.8 ± 109.5 a | 888.4 ± 32.9 a |
| HFD-SL (3%) | 8 | 153.38 ± 31.50 b | 45.25± 6.65 a,b | 874.9 ± 126.2 a | 804.4 ± 156.8 a,b |
| HFD-SH (5%) | 8 | 136.25 ± 34.74 b | 38.75 ± 4.03 b | 809.0 ± 91.0 b | 736.1 ± 153.5 a,b |
| Group | n | HDL-C (mg/dL) | TC (mg/dL) | TG (mg/dL) | Glu (mg/dL) | LDL-C (mg/dL) | AI | CRF |
|---|---|---|---|---|---|---|---|---|
| ND | 8 | 65.13 ± 9.42a | 75.88 ± 8.66b | 64.75 ± 16.12b | 131.5 ± 16.6 c | 24.70 ± 7.08c | 0.17 ± 0.11b | 1.17 ± 0.11b |
| HFD | 8 | 47.63 ± 7.05b | 101.63 ± 12.92a | 111.75 ± 25.08a | 179.4 ± 12.5 a | 76.35 ± 12.45a | 1.15 ± 0.24a | 2.15 ± 0.24a |
| HFD-SL (3%) | 8 | 51.00 ± 5.71a,b | 85.75 ± 16.24a,b | 76.25 ± 8.41a,b | 159.3 ± 11.5a,b | 50.00 ± 20.02a,b | 0.71 ± 0.43b | 1.71 ± 0.43b |
| HFD-SH (5%) | 8 | 54.25 ± 13.13a,b | 77.88 ± 10.62a,b | 65.63 ± 13.79b | 148.9 ± 9.3b,c | 36.75 ± 18.81b,c | 0.51 ± 0.42b | 1.51 ± 0.42b |
| Group | n | Liver TG (mg/g) | Liver TC (mg/g) | EAT TG (mg/g) | EAT TC (mg/g) | MAT TG (mg/g) | MAT TC (mg/g) |
|---|---|---|---|---|---|---|---|
| ND | 8 | 25.8 ± 3.8c | 82.8 ± 10.0b | 88.2 ± 8.3c | 75.3 ± 12.9c | 35.5 ± 2.5c | 25.3 ± 3.9c |
| HFD | 8 | 60.0 ± 12.7a | 107.9 ± 18.4a | 107.3± 12.2a | 125.4 ± 15.5a | 62.7 ± 2.7a | 39.8 ± 4.5a |
| HFD-SL (3%) | 8 | 45.0 ± 12.7a,b | 98.0 ± 11.7b | 92.3 ± 4.7b,c | 103.7±16.2a,b | 44.3 ± 3.6a,b | 32.2 ± 4.5a,b |
| HFD-SH (5%) | 8 | 41.0 ± 7.4b,c | 97.6 ± 11.7b | 97.0± 13.5a,b | 93.5 ± 6.4b,c | 42.4 ± 4.5b,c | 29.5 ± 2.7b,c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-H.; Lee, J.-J.; Son, H.-K.; Kim, B.-H.; Byun, J.; Ha, J.-H. Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients 2020, 12, 336. https://doi.org/10.3390/nu12020336
Park Y-H, Lee J-J, Son H-K, Kim B-H, Byun J, Ha J-H. Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients. 2020; 12(2):336. https://doi.org/10.3390/nu12020336
Chicago/Turabian StylePark, Yong-Hyun, Jae-Joon Lee, Hee-Kyoung Son, Bok-Hee Kim, Jaemin Byun, and Jung-Heun Ha. 2020. "Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats" Nutrients 12, no. 2: 336. https://doi.org/10.3390/nu12020336
APA StylePark, Y.-H., Lee, J.-J., Son, H.-K., Kim, B.-H., Byun, J., & Ha, J.-H. (2020). Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients, 12(2), 336. https://doi.org/10.3390/nu12020336






