Chronic Polyphenon-60 or Catechin Treatments Increase Brain Monoamines Syntheses and Hippocampal SIRT1 LEVELS Improving Cognition in Aged Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Drugs, Reagents, and Treatments
2.2. Behavioral Tests
2.2.1. Radial Maze Test
2.2.2. Novel Object Recognition Test
2.2.3. Motor Coordination in Rotarod Test
2.3. TPH Activity (Synthesis of 5-HT) and TH Activity (Synthesis of DA and NA)
2.4. Western Blot Analysis
2.5. Statistics
3. Results
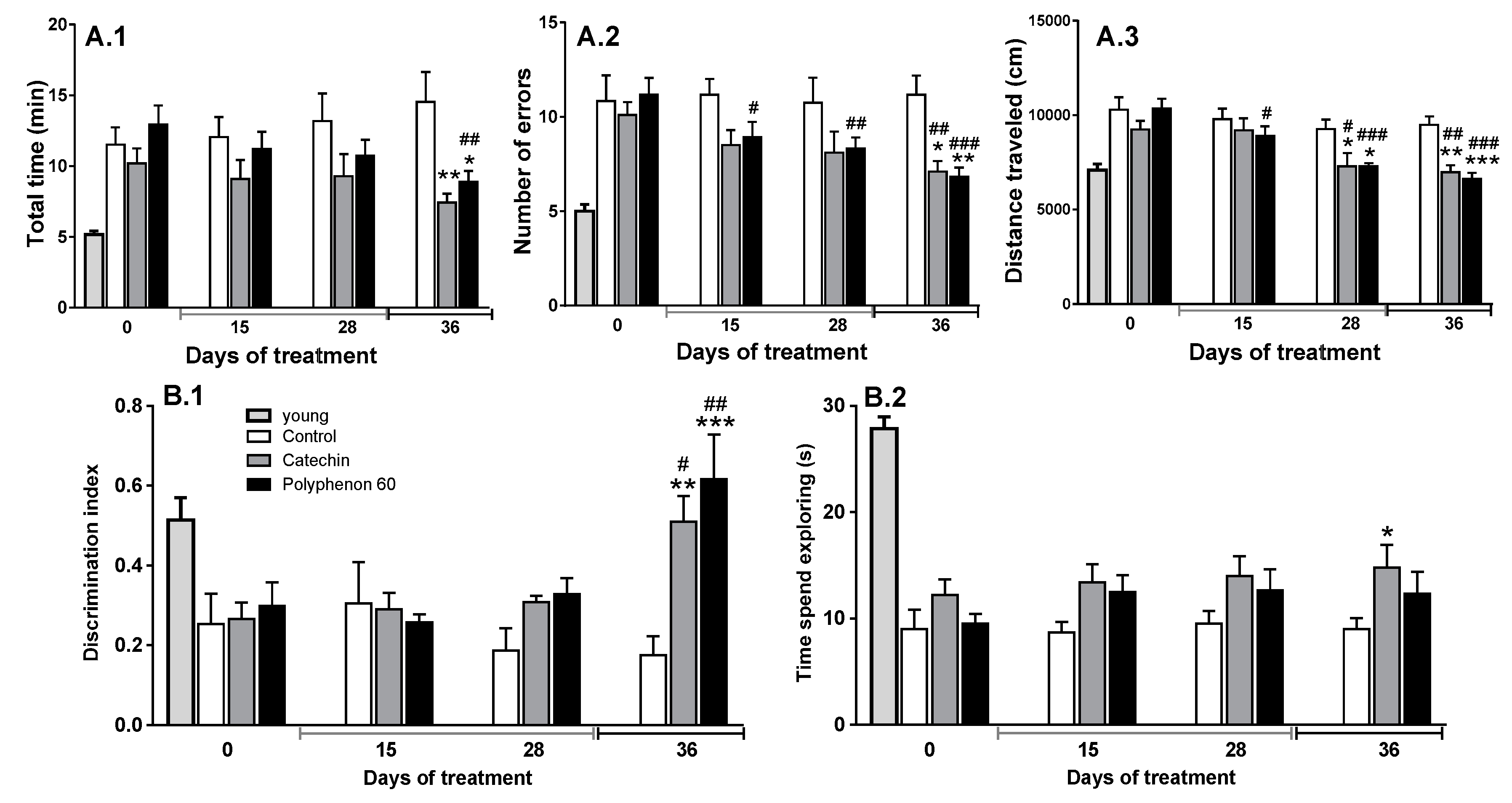
3.1. Old Rats’ Cognitive Abilities Evolution during Chronic Treatment with Cathechin and Polyphenon-60
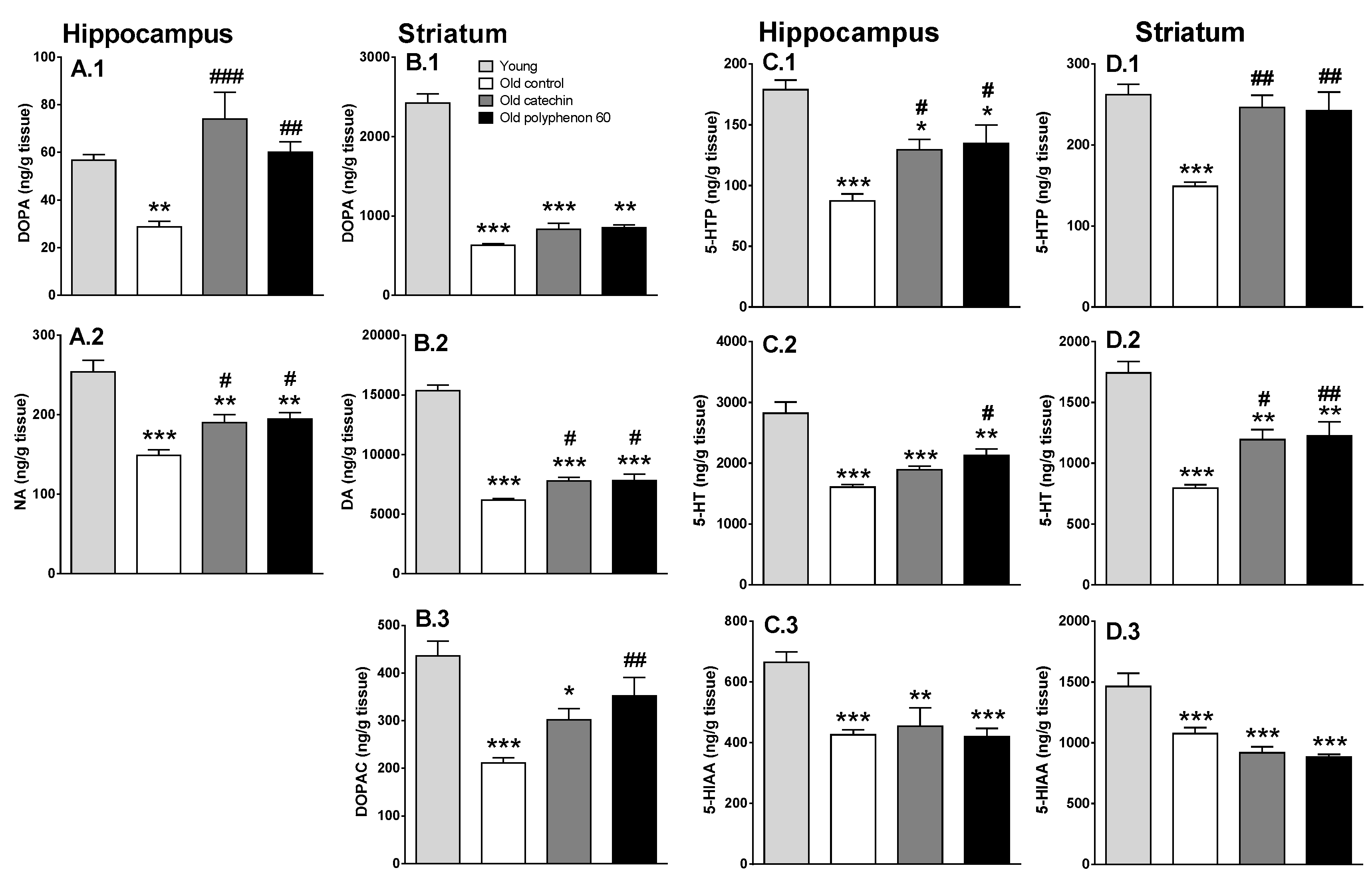
3.2. Effect of Chronic Catechin and Polyphenol-60 Treatments in Old Rats on Monoamine Synthesis and Metabolism in Hippocampus and Striatum
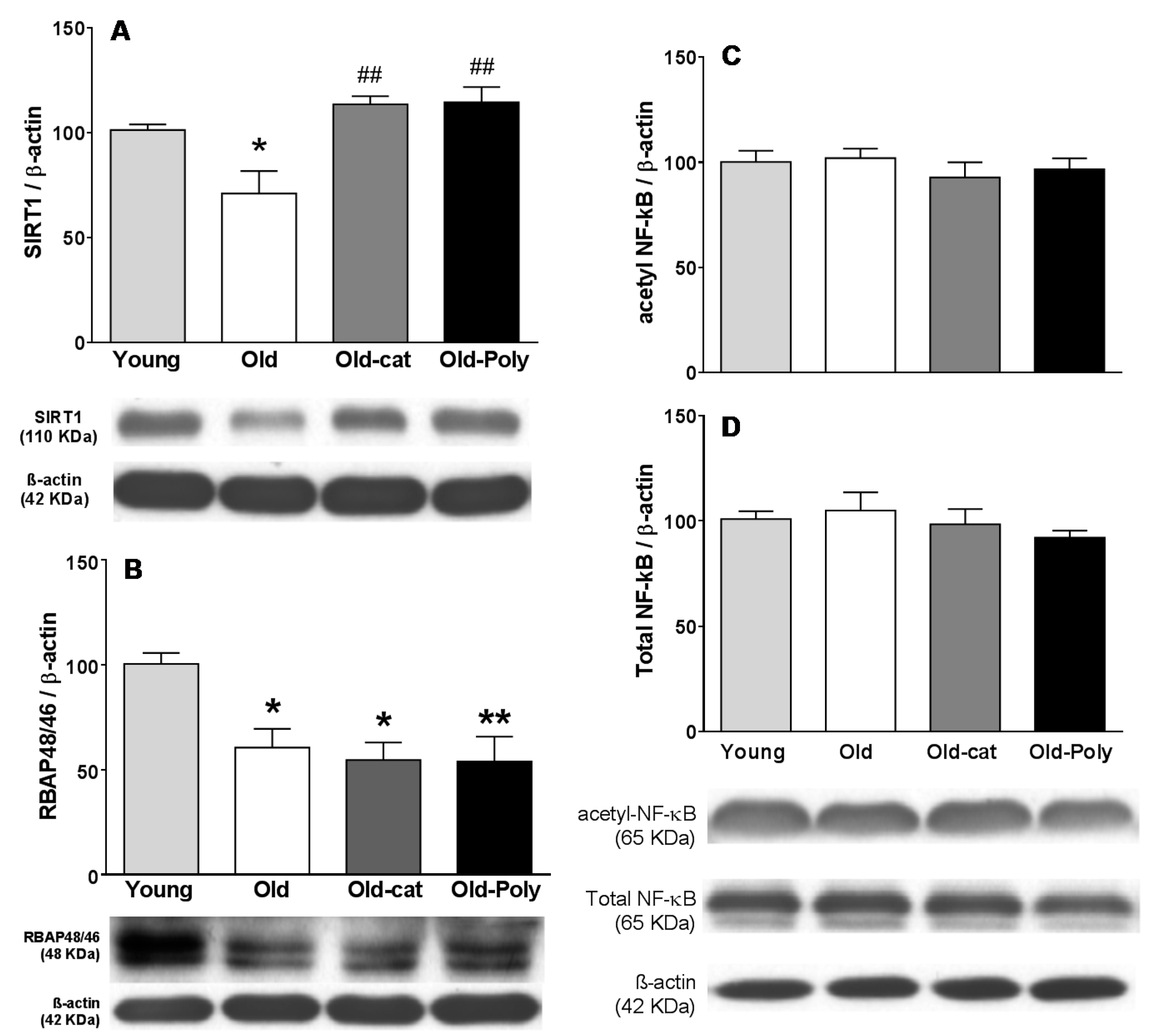
3.3. Effect of Chronic Catechin and Polyphenol 60 Treatments in Old Rats on SIRT1, RBAP46/48, and NF-κB Immunoreactivity in Hippocampus
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, H.T.A. Population ageing in a globalized world: Risks and dilemmas? J. Eval. Clin. Pract. 2018, 25, 754–760. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [Green Version]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging--matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [Green Version]
- Sarubbo, F.; Ramis, M.R.; Aparicio, S.; Ruiz, L.; Esteban, S.; Miralles, A.; Moranta, D. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age (Omaha) 2015, 37, 9777. [Google Scholar] [CrossRef] [Green Version]
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Milacic, V.; Chen, M.S.; Wan, S.B.; Lam, W.H.; Huo, C.; Landis-Piwowar, K.R.; Cui, Q.C.; Wali, A.; Chan, T.H.; et al. Tea polyphenols, their biological effects and potential molecular targets. Histol. Histopathol. 2008, 23, 487. [Google Scholar] [PubMed]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.K.; Ha, S.; Son, J.; Song, J.H.; Houh, Y.; Cho, E.; Chun, J.H.; Yoon, S.R.; Yang, Y.; Bang, S.I.; et al. Polyphenon-60 displays a therapeutic effect on acne by suppression of TLR2 and IL-8 expression via down-regulating the ERK1/2 pathway. Arch. Dermatol. Res. 2012, 304, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Q.; Wang, Z.-S.; Ma, Y.-X.; Zhang, W.; Lu, J.-L.; Liang, Y.-R.; Zheng, X.-Q. Neuroprotective Effects and Mechanisms of Tea Bioactive Components in Neurodegenerative Diseases. Molecules 2018, 23, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assunção, M.; Santos-Marques, M.J.; Carvalho, F.; Lukoyanov, N.V.; Andrade, J.P. Chronic green tea consumption prevents age-related changes in rat hippocampal formation. Neurobiol. Aging 2011, 32, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Takabayashi, F.; Kishido, T.; Oku, N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp. Gerontol. 2004, 39, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Takabayashi, F.; Yoshida, H.; Choba, D.; Fukutomi, R.; Kikunaga, N.; Kishido, T.; Oku, N.; Hoshino, M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology 2007, 8, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gwee, X.; Kua, E.-H.; Ng, T.-P. Cognitive function and tea consumption in community dwelling older Chinese in Singapore. J. Nutr. Health Aging 2010, 14, 433–438. [Google Scholar] [CrossRef]
- Tomata, Y.; Kakizaki, M.; Nakaya, N.; Tsuboya, T.; Sone, T.; Kuriyama, S.; Hozawa, A.; Tsuji, I. Green tea consumption and the risk of incident functional disability in elderly Japanese: The Ohsaki Cohort 2006 Study. Am. J. Clin. Nutr. 2012, 95, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Haskell-Ramsay, C.F.; Schmitt, J.; Actis-Goretta, L. The Impact of Epicatechin on Human Cognition: The Role of Cerebral Blood Flow. Nutrients 2018, 10, 986. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.M.; DeKosky, S.T. Monoamine neurons in aging and Alzheimer’s disease. J. Neural Transm. 1993, 91, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsunemi, A.; Utsuyama, M.; Seidler, B.K.H.; Kobayashi, S.; Hirokawa, K. Age-related decline of brain monoamines in mice is reversed to young level by Japanese herbal medicine. Neurochem. Res. 2005, 30, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Sarubbo, F.; Terrasa, J.L.; Moranta, D.; Aparicio, S.; Miralles, A.; Esteban, S. Chronic α-Tocopherol Increases Central Monoamines Synthesis and Improves Cognitive and Motor Abilities in Old Rats. Rejuvenation Res. 2016, 19, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Faggi, L.; Porrini, V.; Lanzillotta, A.; Benarese, M.; Mota, M.; Tsoukalas, D.; Parrella, E.; Pizzi, M.; Faggi, L.; Porrini, V.; et al. A Polyphenol-Enriched Supplement Exerts Potent Epigenetic-Protective Activity in a Cell-Based Model of Brain Ischemia. Nutrients 2019, 11, 345. [Google Scholar] [CrossRef] [Green Version]
- Paulo Andrade, J.; Assuncao, M. Protective Effects of Chronic Green Tea Consumption on Age-related Neurodegeneration. Curr. Pharm. Des. 2012, 18, 4–14. [Google Scholar] [CrossRef]
- Cao, W.; Dou, Y.; Li, A. Resveratrol Boosts Cognitive Function by Targeting SIRT1. Neurochem. Res. 2018, 43, 1705–1713. [Google Scholar] [CrossRef]
- Bae, U.J.; Park, J.; Park, I.W.; Chae, B.M.; Oh, M.R.; Jung, S.J.; Ryu, G.S.; Chae, S.W.; Park, B.H. Epigallocatechin-3-gallate-rich green tea extract ameliorates fatty liver and weight gain in mice fed a high fat diet by activating the Sirtuin 1 and AMP activating protein kinase pathway. Am. J. Chin. Med. 2018, 46, 617–632. [Google Scholar] [CrossRef]
- Cheng, A.W.; Tan, X.; Sun, J.Y.; Gu, C.M.; Liu, C.; Guo, X. Catechin attenuates TNF-α induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS One 2019, 14, e0217090. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, W.Y.; Mao, Y.W.; Gräff, J.; Guan, J.S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Bi, Y.J.; Xue, L.X.; Wang, J.; Lu, Y.; Zhang, Z.G.; Chen, X.; Chu, Y.; Yang, R.; Wang, R.; et al. Multifaceted modulation of SIRT1 in cancer and inflammation. Crit. Rev. Oncog. 2015, 20, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulos, E.; Jones, S.; Kosmidis, S.; Close, M.; Kim, C.; Kovalerchik, O.; Small, S.A.; Kandel, E.R. Molecular Mechanism for Age-Related Memory Loss: The Histone-Binding Protein RbAp48. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [Green Version]
- Kosmidis, S.; Polyzos, A.; Harvey, L.; Youssef, M.; Denny, C.A.; Dranovsky, A.; Kandel, E.R. RbAp48 Protein Is a Critical Component of GPR158/OCN Signaling and Ameliorates Age-Related Memory Loss. Cell Rep. 2018, 25, 959–973. [Google Scholar] [CrossRef] [Green Version]
- Esteban, S.; Garau, C.; Aparicio, S.; Moranta, D.; Barceló, P.; Ramis, M.R.; Tresguerres, J.A.F.; Rial, R.V. Improving effects of long-term growth hormone treatment on monoaminergic neurotransmission and related behavioral tests in aged rats. Rejuvenation Res. 2010, 13, 707–716. [Google Scholar] [CrossRef]
- Sharma, S.; Rakoczy, S.; Brown-Borg, H. Assessment of spatial memory in mice. Life Sci. 2010, 87, 521–536. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Ramis, M.; Sarubbo, F.; Sola, J.; Aparicio, S.; Garau, C.; Miralles, A.; Esteban, S. Cognitive improvement by acute growth hormone is mediated by NMDA and AMPA receptors and MEK pathway. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2013, 45, 11–20. [Google Scholar] [CrossRef]
- Zhang, X.; Beaulieu, J.-M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan Hydroxylase-2 Controls Brain Serotonin Synthesis. Science (80-) 2004, 305, 217. [Google Scholar] [CrossRef]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Yasunaga, K.; Matsuo, N.; Katsuragi, Y.; Komikado, M.; Tokimitsu, I.; Wilder, D.; Jones, F.; et al. Green Tea Catechin Consumption Enhances Exercise-Induced Abdominal Fat Loss in Overweight and Obese Adults. J. Nutr. 2009, 139, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Tanaka, Y.; Kamimaki, I.; Nagao, T.; Tokimitsu, I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity 2008, 16, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Abbasabadi, Z.; Braidy, N.; Nabavi, S.M. Role of green tea catechins in prevention of age-related cognitive decline: Pharmacological targets and clinical perspective. J. Cell. Physiol. 2019, 234, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, H.F.; Zhang, Z.F.; Liu, Z.G.; Pei, X.R.; Wang, J.B.; Cai, M.Y.; Li, Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience 2009, 159, 1208–1215. [Google Scholar] [CrossRef]
- Esteban, S.; Moranta, D.; Asensio, V.J.V.J.; Miralles, A.; Sarubbo, F.; Moranta, D.; Asensio, V.J.V.J.; Miralles, A.; Esteban, S. Effects of Resveratrol and Other Polyphenols on the Most Common Brain Age-Related Diseases. Curr. Med. Chem. 2017, 24, 4245–4266. [Google Scholar]
- Sun, B.; Wang, W.; He, Z.; Zhang, M.; Kong, F.; Sain, M.; Ni, Y. Improvement of Stability of Tea Polyphenols: A Review. Curr. Pharm. Des. 2018, 24, 3410–3423. [Google Scholar] [CrossRef]
- Zokti, J.A.; Baharin, B.S.; Mohammed, A.S.; Abas, F. Green tea leaves extract: Microencapsulation, physicochemical and storage stability study. Molecules 2016, 21, 940. [Google Scholar] [CrossRef] [Green Version]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Moranta, D.; Barceló, P.; Aparicio, S.; Garau, C.; Sarubbo, F.; Ramis, M.R.; Nicolau, C.; Esteban, S.; Nicolau, M.C.; Esteban, S.; et al. Intake of melatonin increases tryptophan hydroxylase type 1 activity in aged rats: Preliminary study. Exp. Gerontol. 2014, 49, 1–4. [Google Scholar] [CrossRef]
- Haider, S.; Saleem, S.; Perveen, T.; Tabassum, S.; Batool, Z.; Sadir, S.; Liaquat, L.; Madiha, S. Age-related learning and memory deficits in rats: Role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age (Omaha) 2014, 36, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic control of the striatum for high-level cognition. Curr. Opin. Neurobiol. 2011, 21, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Moranta, D.; Pani, G. Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition. Neurosci. Biobehav. Rev. 2018, 90, 456–470. [Google Scholar] [CrossRef]
- Hussain, A.M.; Mitra, A.K. Effect of aging on tryptophan hydroxylase in rat brain: Implications on serotonin level. Drug Metab. Dispos. 2000, 28, 1038–1042. [Google Scholar] [PubMed]
- De La Cruz, C.P.; Revilla, E.; Venero, J.L.; Ayala, A.; Cano, J.; Machado, A. Oxidative inactivation of tyrosine hydroxylase in substantia nigra of aged rat. Free Radic. Biol. Med. 1996, 20, 53–61. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Wu, J.-C.; Ho, C.-T. Epigenetic and Disease Targets by Polyphenols. Curr. Pharm. Des. 2013, 19, 6156–6185. [Google Scholar] [CrossRef]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. (Lausanne) 2018, 9, 702. [Google Scholar] [CrossRef]
- Corpas, R.; Revilla, S.; Ursulet, S.; Castro-Freire, M.; Kaliman, P.; Petegnief, V.; Giménez-Llort, L.; Sarkis, C.; Pallàs, M.; Sanfeliu, C. SIRT1 Overexpression in Mouse Hippocampus Induces Cognitive Enhancement Through Proteostatic and Neurotrophic Mechanisms. Mol. Neurobiol. 2017, 54, 5604–5619. [Google Scholar] [CrossRef]
- Shah, S.A.; Khan, M.; Jo, M.H.; Jo, M.G.; Amin, F.U.; Kim, M.O. Melatonin Stimulates the SIRT1/Nrf2 Signaling Pathway Counteracting Lipopolysaccharide (LPS)-Induced Oxidative Stress to Rescue Postnatal Rat Brain. CNS Neurosci. Ther. 2017, 23, 33–44. [Google Scholar] [CrossRef]
- Quintas, A.; de Solís, A.J.; Díez-Guerra, F.J.; Carrascosa, J.M.; Bogónez, E. Age-associated decrease of SIRT1 expression in rat hippocampus: Prevention by late onset caloric restriction. Exp. Gerontol. 2012, 47, 198–201. [Google Scholar] [CrossRef]
- Yao, H.; Rahman, I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem. Pharmacol. 2012, 84, 1332–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snow, W.M.; Albensi, B.C. Neuronal gene targets of NF-κB and their dysregulation in alzheimer’s disease. Front. Mol. Neurosci. 2016, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, E.C.; Meffert, M.K. Cellular specificity of NF-κB function in the nervous system. Front. Immunol. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Y.; Nan, Y.; Wang, X.; Chen, Y.; Wang, S. (-)-Epigallocatechin-3-gallate ameliorates memory impairment and rescues the abnormal synaptic protein levels in the frontal cortex and hippocampus in a mouse model of Alzheimer’s disease. Neuroreport 2017, 28, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Chiaverini, C.; Roger, C.; Fontas, E.; Bourrat, E.; Bourdon-Lanoy, E.; Labrèze, C.; Mazereeuw, J.; Vabres, P.; Bodemer, C.; Lacour, J.-P. Oral epigallocatechin-3-gallate for treatment of dystrophic epidermolysis bullosa: A multicentre, randomized, crossover, double-blind, placebo-controlled clinical trial. Orphanet J. Rare Dis. 2016, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhao, H.; Zhao, M.; Zhang, Z.; Li, Y. Chronic green tea catechins administration prevents oxidative stress-related brain aging in C57BL/6J mice. Brain Res. 2010, 1353, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Gill, M.; Kinra, M.; Shetty, R.; Krishnadas, N.; Rao, C.M.; Sumalatha, S.; Kumar, N. Catechin ameliorates depressive symptoms in sprague dawley rats subjected to chronic unpredictable mild stress by decreasing oxidative stress. Biomed. Reports 2019, 11, 79–84. [Google Scholar] [CrossRef] [Green Version]
| Radial Maze | Novel Object Recognition | |||
|---|---|---|---|---|
| Time | Errors | D.I. | Novel – Fam. | |
| Hippocampus | ||||
| NA | −0.67 ** | −0.8 *** | 0.39 | 0.64 ** |
| DOPA | −0.47* | −0.59 * | 0.44 | 0.48 * |
| 5-HT | −0.61** | −0.76 *** | 0.50 * | 0.6 * |
| 5-HTP | −0.48* | −0.48 * | 0.11 | 0.72 ** |
| 5-HIAA | −0.11 | −0.09 | 0.02 | −0.07 |
| Striatum | ||||
| DA | −0.55 * | −0.69 ** | 0.6 * | 0.7 ** |
| DOPA | −0.38 | −0.68 ** | 0.34 | 0.42 |
| DOPAC | −0.25 | −0.51 * | 0.53 * | 0.43 |
| 5-HT | −0.6 * | −0.72 ** | 0.22 | 0.75 ** |
| 5-HTP | −0.62 ** | −0.66 ** | 0.43 | 0.75 ** |
| 5-HIAA | 0.44 | 0.37 | −0.35 | −0.46 |
| Hippocampal Protein Level | ||||
| SIRT1 | −0.82 *** | −0.44 | 0.44 | 0.55 * |
| RBAP49/46 | 0.19 | 0.05 | −0.33 | −0.46 |
| Total NF-κB | 0.24 | 0.21 | −0.38 | −0.28 |
| Acetyl-NF-κB | −0.16 | −0.22 | −0.26 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramis, M.R.; Sarubbo, F.; Tejada, S.; Jiménez, M.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Polyphenon-60 or Catechin Treatments Increase Brain Monoamines Syntheses and Hippocampal SIRT1 LEVELS Improving Cognition in Aged Rats. Nutrients 2020, 12, 326. https://doi.org/10.3390/nu12020326
Ramis MR, Sarubbo F, Tejada S, Jiménez M, Esteban S, Miralles A, Moranta D. Chronic Polyphenon-60 or Catechin Treatments Increase Brain Monoamines Syntheses and Hippocampal SIRT1 LEVELS Improving Cognition in Aged Rats. Nutrients. 2020; 12(2):326. https://doi.org/10.3390/nu12020326
Chicago/Turabian StyleRamis, Margarita R., Fiorella Sarubbo, Silvia Tejada, Manuel Jiménez, Susana Esteban, Antoni Miralles, and David Moranta. 2020. "Chronic Polyphenon-60 or Catechin Treatments Increase Brain Monoamines Syntheses and Hippocampal SIRT1 LEVELS Improving Cognition in Aged Rats" Nutrients 12, no. 2: 326. https://doi.org/10.3390/nu12020326





