Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal
Abstract
:1. Introduction
2. Results and Discussion
2.1. Possible Toxic Effects of the Extracts
2.2. Chemical Composition of the Extracts: Qualitative Profiling
2.3. Chemical Composition: Quantitative Profiling of Phenolic Compounds
2.4. Enzyme Inhibitory Activities
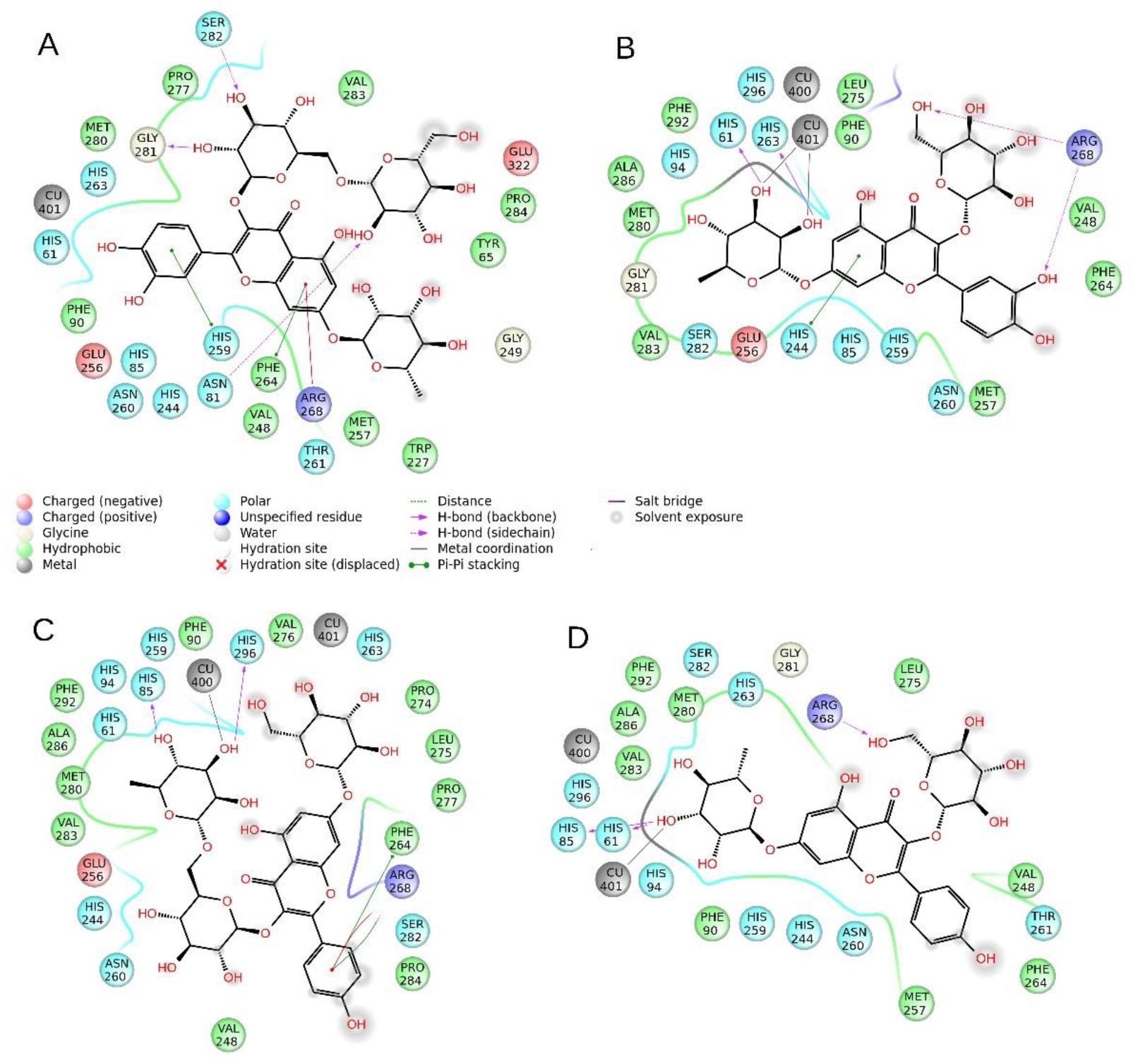
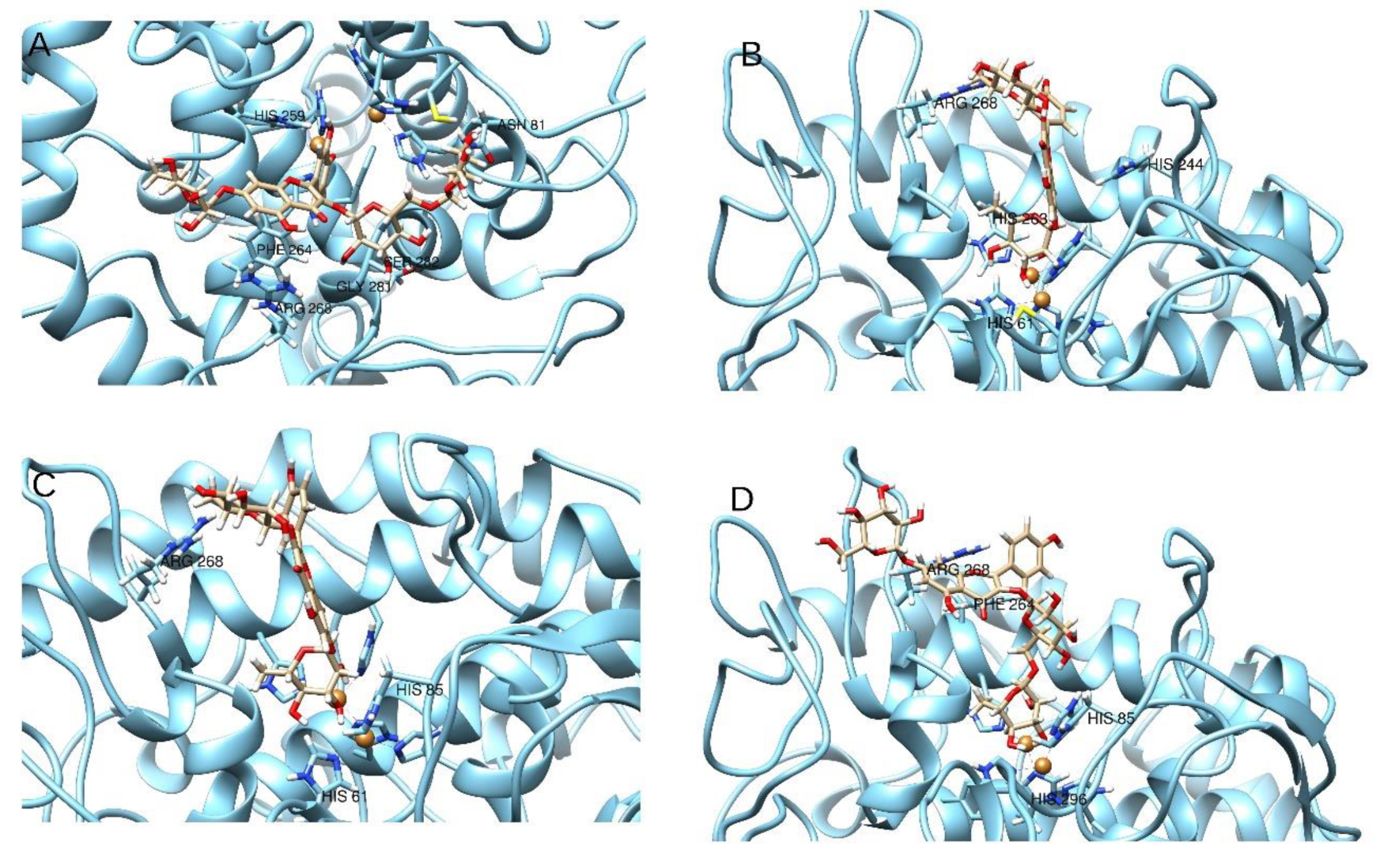
2.5. Molecular Modeling
2.6. In Silico Absorption, Distribution, Metabolism, and Excretion (ADME) Evaluation
2.7. Antioxidant Activity
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Preparation of the Extracts
3.4. Toxicological Evaluation
3.4.1. Cell Culture
3.4.2. Citotoxicity
3.5. Identification and Quantification of Phenolic Compounds by HPLC-MS
3.6. Enzyme Inhibitory Activities
3.6.1. Cholinesterase Inhibition
3.6.2. Tyrosinase Inhibition
3.6.3. Alpha-Amylase and α-Glucosidase Inhibition
3.7. Molecular Modeling
3.7.1. Receptor Preparation
3.7.2. Ligands Preparation
3.7.3. ADME Estimation
3.7.4. Self-Docking and Docking Method Validation
3.7.5. Molecular Docking
3.8. In Vitro Antioxidant Properties
3.8.1. Targeting Free Radicals: Radical Scavenging Activity (RSA) on DPPH and ABTS Radicals
3.8.2. Targeting Metal Ions: Metal Chelating Activity on Copper (CCA) and Iron (ICA) and Iron Reducing Power (FRAP)
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magne, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem. Toxicol. 2018, 114, 155–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Al-Oudat, M.; Qadir, M. The halophytic flora of Syria. Int. Cent. Agric. Res. Dry Areas Aleppo Syria 2011, 8, 186. [Google Scholar]
- Qasim, M.; Gulzar, S.; Khan, M. Halophytes as Medicinal Plants. In Proceedings of the NAM Meeting in Denizli, Denizli, Turkey, 27–29 June 2011. [Google Scholar]
- Arbelet-Bonnin, D.; Ben-Hamed-Louati, I.; Laurenti, P.; Abdelly, C.; Ben-Hamed, K.; Bouteau, F. Chapter Two-Cakile maritima, a promising model for halophyte studies and a putative cash crop for saline agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 155, pp. 45–78. [Google Scholar]
- Clausing, G.; Vickers, K.; Kadereit, J.W. Historical biogeography in a linear system: Genetic variation of sea rocket (Cakile maritima) and sea holly (Eryngium maritimum) along European coasts. Mol. Ecol. 2000, 9, 1823–1833. [Google Scholar] [CrossRef]
- Davy, A.J.; Scott, R.; Cordazzo, C.V. Biological flora of the British Isles: Cakile maritima Scop. J. Ecol. 2006, 94, 695–711. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Giménez-Martínez, J.J.; Torija-Isasa, M.E. Nutritional Composition of Wild Edible Crucifer Species. J. Food Biochem. 1999, 23, 283–294. [Google Scholar] [CrossRef]
- Fuochi, V.; Barbagallo, I.; Distefano, A.; Puglisi, F.; Palmeri, R.; Rosa, M.D.I.; Giallongo, C.; Longhitano, L.; Fontana, P.; Sferrazzo, G.; et al. Biological properties of Cakile maritima Scop. (Brassicaceae) extracts. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2280–2292. [Google Scholar]
- Azim, W.M.; Yaecob, H.S. Effect of transplanting date on chemical constituents of Cakile Maritima scop. under two different habitats conditions. J. Appl. Sci. Res. 2009, 5, 384–391. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Mansour, R.; Dakhlaouia, S.; Msahli, W.; Megdiche, W. Differential Responses of Cakile maritima at Two Development Stages to Salinity: Changes on Phenolic Metabolites and Related Enzymes and Antioxidant Activity. Med. Chem. 2018, 8, 100–108. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magne, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, H.; Shams, K.; Tawfik, W.; Soliman, A. Investigation of the Glucosinolates and Lipids Constituents of Cakile maritima (Scope) growing in Egypt and Their Biological Activity. Res. J. Med. Med. Sci. 2008, 3, 182–187. [Google Scholar]
- Shams, K.; Radwan, H.; Tawfik, W.; Habib, A.; Soliman, A. Flavonoid Constituents of Cakile maritima (Scope) Growing in Egypt and Their Biological Activity. Asian J. Chem. 2010, 22, 3981–3988. [Google Scholar]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Parra, A.L.; Yhebra, R.S.; Sardiñas, I.G.; Buela, L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef]
- Yan, S.X.; Li, X.; Sun, C.D.; Chen, K.S. Hypoglycemic and hypolipidemic effects of quercetin and its glycosides. China J. Chin. Mater. Med. 2015, 40, 4560–4567. [Google Scholar]
- Adhikari-Devkota, A.; Dirar, A.I.; Kurizaki, A.; Tsushiro, K.; Devkota, H.P. Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana. Separations 2019, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Dauguet, J.; Maume, D.; Dallemagne, P. The polyphenols of Cakile maritima. Plantes Médicinales Et Phytothérapie 1985, 19, 277–285. [Google Scholar]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; Garcia-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, H.; Yao, M.; Kamada, K.; Takeda, Y.; Alangionosides, G. Glucosides of megastegmane derivatives from the leaves of Alangium premnifolium. Chem. Pharm. Bull. 1995, 43, 754–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, E.Y.; Kim, T.Y.; Hong, G.U.; Kang, H.; Lee, J.-Y.; Park, J.Y.; Kim, S.-C.; Kim, Y.H.; Chung, M.-H.; Kwon, Y.-I. Inhibitory Effects of Roseoside and Icariside E4 Isolated from a Natural Product Mixture (No-ap) on the Expression of Angiotensin II Receptor 1 and Oxidative Stress in Angiotensin II-Stimulated H9C2 Cells. Molecules 2019, 24, 414. [Google Scholar] [CrossRef] [Green Version]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Potapova, D.A.; Rendyuk, T.D. Identification of Phenol Compounds in Broccoli (Brassica Oleracea L. var. italica) by Uplc/Uv-Ms/Ms. Probl. Biol. Med. Pharm. Chem. 2019, 22. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerre-Tugaye, M.T.; Rosahl, S.; et al. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef] [Green Version]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, B.; Khan, H.; Bashir, S.; Nisar, M.; Hassan, M. Inhibition activities of Colchicum luteum baker on lipoxygenase and other enzymes. J. Enzym. Inhib. Med. Chem. 2006, 21, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Bhandari, U.; Jain, N.; Ansari, M.N.; Pillai, K.K. Beneficial effect of Embelia ribes ethanolic extract on blood pressure and glycosylated hemoglobin in streptozotocin-induced diabetes in rats. Fitoterapia 2008, 79, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.; Bailey, C. Type 2 Diabetes in Practice; RSM Press: London, UK, 2005. [Google Scholar]
- Zengin, G.; Llorent-Martínez, E.J.; Córdova, M.L.F.-d.; Bahadori, M.B.; Mocan, A.; Locatelli, M.; Aktumsek, A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crop. Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Chai, W.M.; Lin, M.Z.; Song, F.J.; Wang, Y.X.; Xu, K.L.; Huang, J.X.; Fu, J.P.; Peng, Y.Y. Rifampicin as a novel tyrosinase inhibitor: Inhibitory activity and mechanism. Int. J. Biol. Macromol. 2017, 102, 425–430. [Google Scholar] [CrossRef]
- Nesterov, A.; Zhao, J.; Minter, D.; Hertel, C.; Ma, W.; Abeysinghe, P.; Hong, M.; Jia, Q. 1-(2,4-dihydroxyphenyl)-3-(2,4-dimethoxy-3-methylphenyl)propane, a novel tyrosinase inhibitor with strong depigmenting effects. Chem. Pharm. Bull. 2008, 56, 1292–1296. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, M.; Mohammadi, H.T.; Mahdavi, A.; Shourian, M.; Ghafouri, H. Evaluation of thiazolidinone derivatives as a new class of mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2018, 108, 205–213. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoon, J.Y.; Yang, S.Y.; Choi, S.K.; Kwon, S.J.; Cho, I.S.; Jeong, M.H.; Ho Kim, Y.; Choi, G.S. Tyrosinase inhibitory components from Aloe vera and their antiviral activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.; Falqué, E.; Domínguez, H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custodio, L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorent-Martínez, E.J.; Zengin, G.; Lobine, D.; Molina-García, L.; Mollica, A.; Mahomoodally, M.F. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem. 2018, 42, 5204–5214. [Google Scholar] [CrossRef]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crop. Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger. Schrödinger Release 2012-1: Maestro; Schrödinger, LLC: New York, NY, USA, 2012. [Google Scholar]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Schrödinger. Schrödinger Release 2015-2, QikProp; Schrödinger, LLC: New York, NY, USA, 2019. [Google Scholar]
- Fakhari, S.; Stefanucci, A.; Mollica, A.; Nikkhoo, B.; Tafsiri, E.; Jalili, A.; Mirzaie, S. Designing new generation of potent inhibitors against membrane-type matrix metalloproteinase-2: A computational effort against multiple myeloma. J. Biomol. Struct. Dyn. 2019, 1–13. [Google Scholar] [CrossRef]
- Stefanucci, A.; Lei, W.; Pieretti, S.; Novellino, E.; Dimmito, M.P.; Marzoli, F.; Streicher, J.M.; Mollica, A. On resin click-chemistry-mediated synthesis of novel enkephalin analogues with potent anti-nociceptive activity. Sci. Rep. 2019, 9, 5771. [Google Scholar] [CrossRef]
- Poli, G.; Dimmito, M.P.; Mollica, A.; Zengin, G.; Benyhe, S.; Zador, F.; Stefanucci, A. Discovery of Novel µ-Opioid Receptor Inverse Agonist from a Combinatorial Library of Tetrapeptides through Structure-Based Virtual Screening. Molecules 2019, 24, 3872. [Google Scholar] [CrossRef] [Green Version]
- Mollica, A.; Zengin, G.; Durdagi, S.; Ekhteiari Salmas, R.; Macedonio, G.; Stefanucci, A.; Dimmito, M.P.; Novellino, E. Combinatorial peptide library screening for discovery of diverse alpha-glucosidase inhibitors using molecular dynamics simulations and binary QSAR models. J. Biomol. Struct. Dyn. 2019, 37, 726–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [PubMed]
- Nokinsee, D.; Shank, L.; Lee, V.; Nimmanpipug, P. Estimation of inhibitory effect against tyrosinase activity through homology modeling and molecular docking. Enzyme Res. 2015, 2015, 262364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorent-Martínez, E.J.; Zengin, G.; Fernández-de Córdova, M.L.; Bender, O.; Atalay, A.; Ceylan, R.; Mollica, A.; Mocan, A.; Uysal, S.; Guler, G.O. Traditionally used Lathyrus species: Phytochemical composition, antioxidant activity, enzyme inhibitory properties, cytotoxic effects, and in silico studies of L. czeczottianus and L. nissolia. Front. Pharmacol. 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, S.; Aktumsek, A.; Picot, C.M.; Sahan, A.; Mollica, A.; Zengin, G.; Mahomoodally, M.F. A comparative in vitro and in silico study of the biological potential and chemical fingerprints of Dorcycinum pentapyllum subsp. haussknechtii using three extraction procedures. New J. Chem. 2017, 41, 13952–13960. [Google Scholar] [CrossRef]
- Yagi, S.; Mohammed, A.B.; Tzanova, T.; Schohn, H.; Abdelgadir, H.; Stefanucci, A.; Mollica, A.; Zengin, G. Chemical profile, antiproliferative, antioxidant, and enzyme inhibition activities and docking studies of Cymbopogon schoenanthus (L.) Spreng. and Cymbopogon nervatus (Hochst.) Chiov. from Sudan. J. Food Biochem. 2019, e13107. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]

| Organs | Extract | RAW 264.7 | HEK 293 | HepG2 |
|---|---|---|---|---|
| Aerial vegetative organs | Ethanol | 67.7 ± 4.50 b | 78.4 ± 6.01 b | 103 ± 9.12 a |
| Acetone | 83.1 ± 7.91 a | 72.2 ± 2.72 b | 78.3 ± 19.1 b | |
| Water | 72.3 ± 1.52 ab | 76.5 ± 15.0 b | 115 ± 13.5 a | |
| Fruits | Ethanol | 80.9 ± 4.80 a | 82.2 ± 5.33 b | 101 ± 16.7 a |
| Acetone | 73.1 ± 12.9 a | 79.4 ± 4.70 b | 96.2 ± 6.52 a | |
| Water | 90.1 ± 14.6 a | 140 ± 25.1 a | 110 ± 4.02 a |
| No. | tR (min) | [M-H]− m/z | m/z (% Base Peak) | Assigned Identification | Fruits | Aerial Organs | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water | Ethanol | Acetone | Water | Ethanol | Acetone | |||||
| 1 | 1.8 | 377 | MS2 [377]: 341 (100) MS3 [377→341]: 179 (64), 161 (59), 143 (100), 119 (5), 113 (7) | Disaccharide (HCl adduct) | √ | √ | √ | √ | ||
| 2 | 2.0 | 374 | MS2 [374]: 294 (16), 275 (68), 259 (100), 241 (14), 227 (16), 163 (17), 145 (28) | Dihydrogluconapin isomer-1 | √ | √ | √ | √ | √ | |
| 3 | 2.6 | 360 | MS2 [360]: 275 (100), 259 (86), 257 (25), 119 (71) | Unknown | √ | √ | √ | |||
| 4 | 2.7 | 191 | MS2 [191]: 173 (37). 111 (100) | Citric acid | √ | √ | ||||
| 5 | 3.1 | 374 | MS2 [374]: 294 (9), 275 (14), 259 (100), 241 (12), 163 (12), 145 (6) | Dihydrogluconapin isomer-2 | √ | √ | √ | √ | √ | |
| 6 | 3.7 | 315 | MS2 [315]: 153 (100) MS3 [315→153]: 109 (100) | Dihydroxybenzoic acid hexoside | √ | √ | ||||
| 7 | 7.0 | 323 | MS2 [323]: 179 (74), 161 (53), 119 (99), 113 (100), 101 (30) | Hexose derivative | √ | √ | ||||
| 8 | 10.7 | 431 | MS2 [431]: 385 (100) MS3 [431→385]: 223 (100), 205 (51), 161 (34), 153 (54) | Roseoside (formate adduct) | √ | √ | √ | |||
| 9 | 11.4 | 294 | MS2 [294]: 279 (100), 264 (10) | Unknown | √ | √ | √ | |||
| 10 | 13.1 | 609 | MS2 [609]: 447 (100), 301 (54) MS3 [609→447]: 301 (100) MS4 [609→447→301]: 271 (100), 255 (54), 179 (15) | Quercetin-O-hexoside-O-deoxyhexoside | √ | √ | ||||
| 11 | 15.0 | 771 | MS2 [771]: 609 (55), 463 (100), 301 (28) MS3 [771→463]: 343 (46), 301 (100), 271 (35), 179 (18), 151 (21) | Quercetin-O-dihexoside-O-deoxyhexoside | √ | √ | √ | √ | √ | √ |
| 12 | 15.9 | 609 | MS2 [609]: 463 (33), 447 (100), 301 (50) MS3 [609→447]: 301 (100) MS4 [609→447→301]: 301 (100), 179 (22), 151 (8) | Quercetin-O-hexoside-O-deoxyhexoside | √ | √ | √ | √ | √ | √ |
| 13 | 17.3 | 755 | MS2 [755]: 593 (100), 447 (76), 285 (43) MS3 [755→593]: 285 (100), 255 (42) | Kaempferol-O-hexoside-O-rutinoside | √ | √ | √ | √ | √ | √ |
| 14 | 18.6 | 593 | MS2 [593]: 447 (100), 431 (41), 285 (42) MS3 [593→447]: 285 (36), 284 (100), 255 (42), 151 (12) | Kaempferol-O-deoxyhexoside-O-hexoside | √ | √ | √ | √ | √ | √ |
| 15 | 21.0 | 463 | MS2 [463]: 301 (100) MS3 [463→301]: 271 (47), 179 (100), 151 (74) | Quercetin-O-hexoside | √ | √ | √ | √ | ||
| 16 | 23.9 | 447 | MS2 [447]: 285 (100), 255 (16) MS3 [447→285]: 255 (100), 227 (12) | Kaempferol-O-hexoside | √ | |||||
| 17 | 25.4 | 580 | MS2 [580]: 580 (100), 373 (23), 223 (15) | Unknown | √ | √ | √ | √ | √ | |
| 18 | 26.5 | 609 | MS2 [609]: 301 (100) MS3 [609→301]: 179 (14), 151 (100) | Quercetin-O-rutinoside | √ | √ | √ | √ | √ | √ |
| 19 | 26.5 | 753 | MS2 [753]: 529 (100) MS3 [753→529]: 511 (52), 247 (78), 223 (100) MS4 [753→529→223]: 208 (100) | Disinapoylgentiobioside | √ | √ | √ | √ | ||
| 20 | 27.3 | 539 | MS2 [539]: 377 (100), 307 (63), 275 (58) MS3 [539→377]: 307 (70), 275 (100) | Oleuropein | √ | √ | ||||
| 21 | 28.8 | 447 | MS2 [447]: 301 (100) MS3 [447→301]: 179 (18), 151 (100) | Quercetin-O-deoxyhexoside | √ | √ | √ | √ | √ | √ |
| 22 | 30.3 | 591 | MS2 [591]: 367 (86), 223 (100) MS3 [591→223]: 208 (69), 164 (100) | Disinapoyl-hexoside | √ | √ | √ | |||
| 23 | 31.2 | 959 | MS2 [959]: 735 (100), 529 (11) MS3 [959→735]: 717 (14), 529 (100), 511 (76), 497 (43), 457 (18) MS4 [959→735→529]: 245 (100), 223 (73) | Trisinapoylgentiobioside | √ | √ | ||||
| 24 | 31.5 | 593 | MS2 [593]: 285 (100) MS3 [593→285]: 241 (100), 151 (50) | Kaempferol-O-rutinoside | √ | √ | √ | √ | √ | √ |
| 25 | 35.6 | 431 | MS2 [431]: 285 (100) MS3 [431→285]: 257 (36), 151 (100) | Kaempferol-O-deoxyhexoside | √ | √ | √ | √ | √ | √ |
| 26 | 36.3 | 461 | MS2 [461]: 315 (100), 300 (19) | Isorhamnetin-O-deoxyhexoside | √ | √ | √ | |||
| 27 | 39.2 | 327 | MS2 [327]: 291 (35), 229 (100), 211 (84), 171 (62) | Oxo-dihydroxy-octadecenoic acid | √ | √ | √ | √ | √ | √ |
| 28 | 40.5 | 329 | MS2 [329]: 311 (33), 229 (100), 211 (66), 171 (77) | Trihydroxy-octadecenoic acid | √ | √ | √ | √ | √ | √ |
| Fruits | Aerial Organs | ||||||
|---|---|---|---|---|---|---|---|
| Compounds * | Assigned Identification | Water | Ethanol | Acetone | Water | Ethanol | Acetone |
| Flavonoids | |||||||
| 11 | Quercetin-di-Hex-dHex | 1.42 ± 0.06 a | 1.20 ± 0.06 b | 0.16 ± 0.01 d | 1.05 ± 0.05 c | 1.25 ± 0.07 b | 0.12 ± 0.008 d |
| 12 | Quercetin-Hex-dHex | 1.30 ± 0.07 b | 1.05 ± 0.06 c | 0.43 ± 0.03 e | 0.86 ± 0.04 d | 1.56 ± 0.08 a | 0.48 ± 0.03 e |
| 13 | Kaempferol-Hex-Rut | 1.14 ± 0.06 c | 0.95 ± 0.04 cd | 0.26 ± 0.01 e | 2.80 ± 0.10 b | 4.20 ± 0.30 a | 0.70 ± 0.04 d |
| 14 | Kaempferol-dHex-Hex | 0.73 ± 0.04 d | 0.49 ± 0.03 de | 0.29 ± 0.02 e | 1.80 ± 0.10 b | 3.60 ± 0.20 a | 1.50 ± 0.10 c |
| 16 | Kaempferol-Hex | nd | nd | nd | 0.11 ± 0.01 | nd | nd |
| 18 | Quercetin-Rut | 0.18 ± 0.01 | 0.06 ± 0.00 | nd | nd | nd | nd |
| 21 | Quercetin-dHex | 0.15 ± 0.01 a | 0.11 ± 0.01 b | 0.05 ± 0.00 c | 0.04 ± 0.00 c | 0.11 ± 0.01 b | 0.02 ± 0.00 c |
| 24 | Kaempferol-Rut | 0.13 ± 0.01 d | 0.03 ± 0.00 e | 0.0029 ± 0.0002 e | 0.85 ± 0.04 a | 0.23 ± 0.01 c | 0.54 ± 0.03 b |
| 25 | Kaempferol-dHex | 0.091 ± 0.005 cd | 0.081 ± 0.003 d | 0.08 ± 0.00 d | 0.12 ± 0.01 c | 0.37 ± 0.02 a | 0.23 ± 0.01 b |
| 26 | Isorhamnetin-dHex | --- | --- | 0.04 ± 0.00 | --- | --- | --- |
| Total | 5.10 ± 0.10 c | 4.00 ± 0.10 d | 1.28 ± 0.04 e | 7.6 ± 0.2 b | 11.3 ± 0.4 a | 3.6 ± 0.1 d | |
| Others | |||||||
| 20 | Oleuropein | 0.26 ± 0.01 | 0.27 ± 0.01 | nd | nd | nd | nd |
| 22 | Disinapoyl-Hex | 0.12 ± 0.01 c | 0.15 ± 0.01 bc | nd | 0.26 ± 0.02 a | 0.17 ± 0.01 b | 0.17 ± 0.01 b |
| Total | 0.38 ± 0.01 b | 0.42 ± 0.01 a | nd | 0.26 ± 0.02 c | 0.17 ± 0.01 d | 0.17 ± 0.01 d | |
| TIPC | 5.48 ± 0.1 c | 4.42 ± 0.10 d | 1.28 ± 0.04 f | 7.92 ± 0.2 b | 11.5 ± 0.4 a | 3.94 ± 0.11 e | |
| Organs | Extract | AChE (mg GALAE/g) | BuChE (mg GALAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) | Tyrosinase (mg KAE/g) |
|---|---|---|---|---|---|---|
| Aerial organs | Ethanol | 1.33 ± 0.14 a | 0.58 ± 0.10 b | 0.18 ± 0.01 a | na | 25.9 ± 0.13 a |
| Acetone | 1.35 ± 0.01 a | 0.66 ± 0.19 b | 0.26 ± 0.01 a | na | 24.7 ± 0.13 a | |
| Water | 0.53 ± 0.03 b | 0.06 ± 0.01 d | 0.02 ± 0.01 b | 0.16 ± 0.01 b | 19.9 ± 0.12 b | |
| Fruits | Ethanol | 1.33 ± 0.11 a | 0.86 ± 0.09 a | 0.20 ± 0.01 a | 2.19 ± 0.02 a | 24.9 ± 0.25 a |
| Acetone | 1.29 ± 0.01 a | 0.92 ± 0.07 a | 0.21 ± 0.02 a | 0.16 ± 0.04 b | 24.0 ± 0.33 a | |
| Water | 1.26 ± 0.06 a | 0.26 ± 0.05 c | 0.04 ± 0.01 b | 0.24 ± 0.04 b | 6.16 ± 0.30 c |
| Compounds | Residues | Copper Atoms | ∆G | Chem Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| His61 | Asn81 | His85 | His244 | His259 | His263 | Phe264 | Arg268 | Gly281 | Ser282 | His296 | Cu400 | Cu401 | |||
| 11 | -- | H-bond | -- | -- | π–π | -- | π–π | Cat-π | H-bond | H-bond | -- | -- | -- | −8.87 | 1.29 |
| 12 | π–π | -- | -- | π–π | -- | H-bond | -- | 2× H-bonds | -- | -- | -- | Coordinative bond | −26.46 | 13.22 | |
| 13 | -- | -- | H-bond | -- | -- | -- | π–π | Cat-π | -- | -- | H-bond | Coordinative bond | -- | −24.33 | 5.16 |
| 14 | H-bond | -- | H-bond | -- | -- | -- | -- | H-bond | -- | -- | -- | -- | Coordinative bond | −25.98 | 15.44 |
| Tropolone | π–π | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | Coordinative bond | −28.62 | 26.60 |
| Kojic acid | H-bond | H-bond To Asn 260 | -- | -- | -- | -- | -- | -- | -- | -- | -- | Coordinative bond | Coordinative bond | −30.11 | 25.43 |
| Ligand | QPlogP O/W a | QPPCaco b | QPLogBB c | Qual. Model for Human Oral Absorption d | Lipinski Rule of 5 Violations e | Jorgensen Rule of 3 Violations f | CNS g | HERG K+ h |
|---|---|---|---|---|---|---|---|---|
| 11 | −1.944 | 1.49 | −4.788 | low | 3 | 2 | −− | −6.198 |
| 12 | −4.130 | 0.094 | −7.230 | low | 3 | 2 | −− | −6.858 |
| 13 | −2.448 | 0.665 | −5.412 | low | 3 | 2 | −− | −6.298 |
| 14 | −4.9 | 0.032 | −7.856 | low | 3 | 2 | −− | −6.590 |
| Organs | Extract | DPPH | ABTS | FRAP | CCA | ICA |
|---|---|---|---|---|---|---|
| Aerial organs | Ethanol | 0.59 ± 0.35 b | 5.14 ± 0.33 b | 0.99 ± 0.05 a * | 8.53 ± 0.31 c | nr |
| Acetone | nr | nr | 1.38 ± 0.09 a * | nr | nr | |
| Water | nr | 5.69 ± 2.30 c | 1.12 ± 0.08 a | 2.80 ± 0.30 b | nr | |
| Fruits | Ethanol | nr | 4.88 ± 0.79 b | 2.03 ± 0.15 b | 8.17 ± 0.65 c | nr |
| Acetone | nr | nr | 2.80 ± 0.48 b | nr | nr | |
| Water | nr | 6.74 ± 0.95 c | 1.31 ± 0.32 a | 2.53 ± 0.13 b | 5.23 ± 0.41 b | |
| BHT * | 0.1 ± 0.02 a | 0.06 ± 0.0 a | ||||
| EDTA * | 0.11 ± 0.00 a | 0.07 ± 0.00 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Placines, C.; Castañeda-Loaiza, V.; João Rodrigues, M.; G. Pereira, C.; Stefanucci, A.; Mollica, A.; Zengin, G.; Llorent-Martínez, E.J.; Castilho, P.C.; Custódio, L. Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal. Plants 2020, 9, 142. https://doi.org/10.3390/plants9020142
Placines C, Castañeda-Loaiza V, João Rodrigues M, G. Pereira C, Stefanucci A, Mollica A, Zengin G, Llorent-Martínez EJ, Castilho PC, Custódio L. Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal. Plants. 2020; 9(2):142. https://doi.org/10.3390/plants9020142
Chicago/Turabian StylePlacines, Chloé, Viana Castañeda-Loaiza, Maria João Rodrigues, Catarina G. Pereira, Azzurra Stefanucci, Adriano Mollica, Gokhan Zengin, Eulogio J. Llorent-Martínez, Paula C. Castilho, and Luísa Custódio. 2020. "Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal" Plants 9, no. 2: 142. https://doi.org/10.3390/plants9020142
APA StylePlacines, C., Castañeda-Loaiza, V., João Rodrigues, M., G. Pereira, C., Stefanucci, A., Mollica, A., Zengin, G., Llorent-Martínez, E. J., Castilho, P. C., & Custódio, L. (2020). Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal. Plants, 9(2), 142. https://doi.org/10.3390/plants9020142











