Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Preparation of the Ginger Water
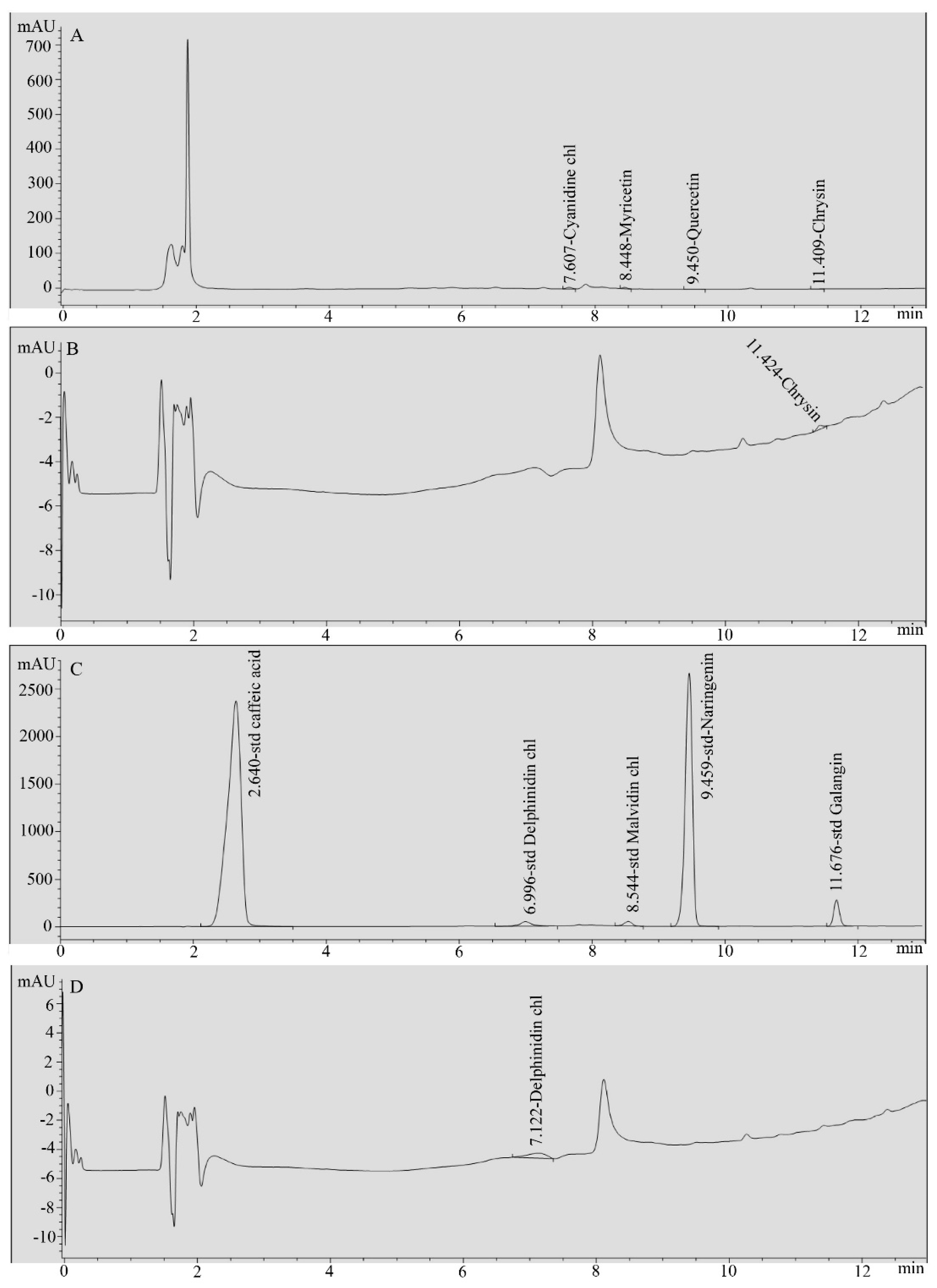
2.3. HPLC Analysis of Ginger Water
2.4. Animal Treatment
2.5. Sampling
2.6. Biochemical Assays
2.7. Gene Expression Analysis
2.7.1. RNA Extraction and cDNA Synthesis
2.7.2. Semi-Quantitative-PCR
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Ginger Water
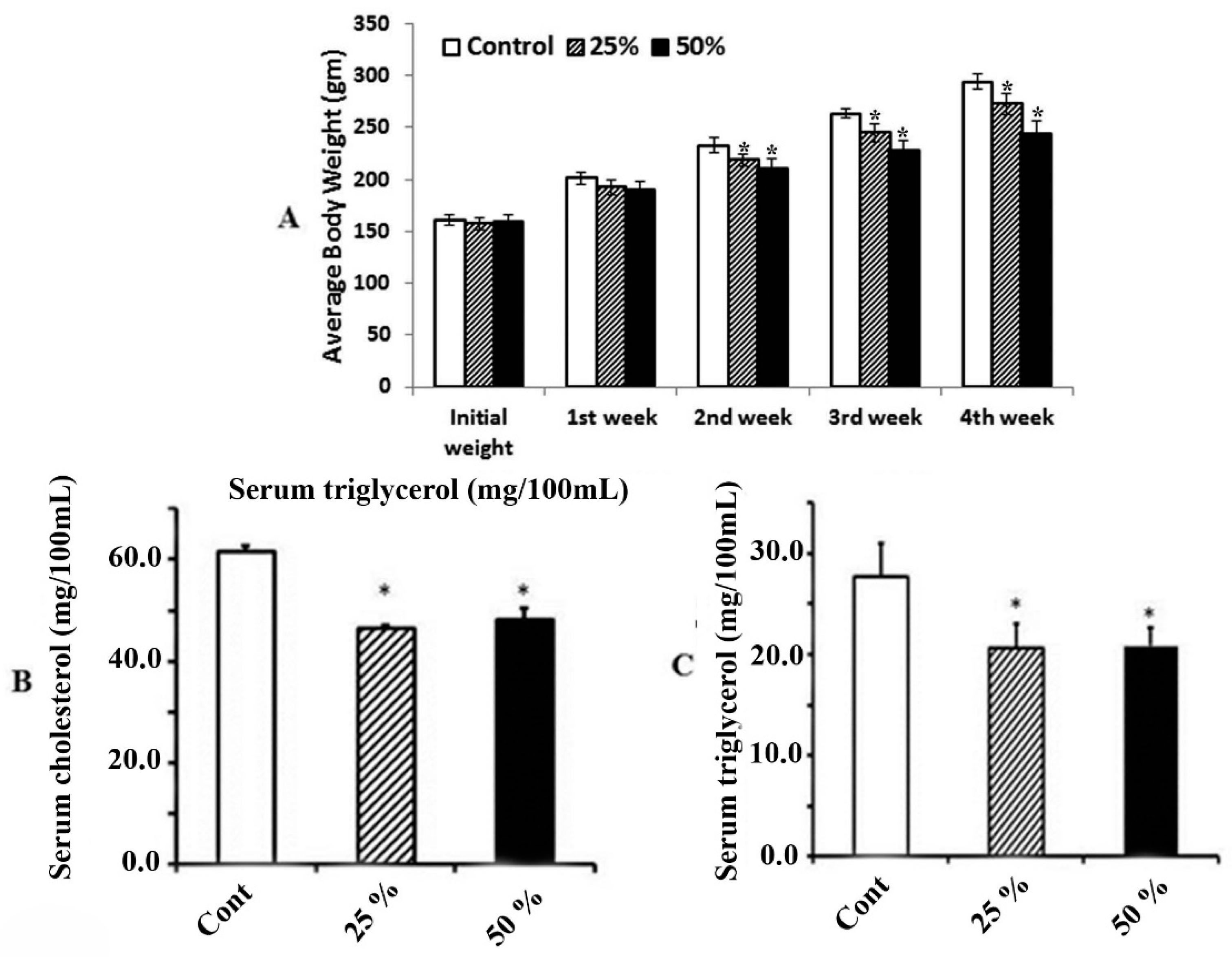
3.2. Effect of Ginger Water on Food Consumption and Average Change of Body Weight
3.3. Effect of Ginger Water on Serum Total Cholesterol and Triacylglycerol
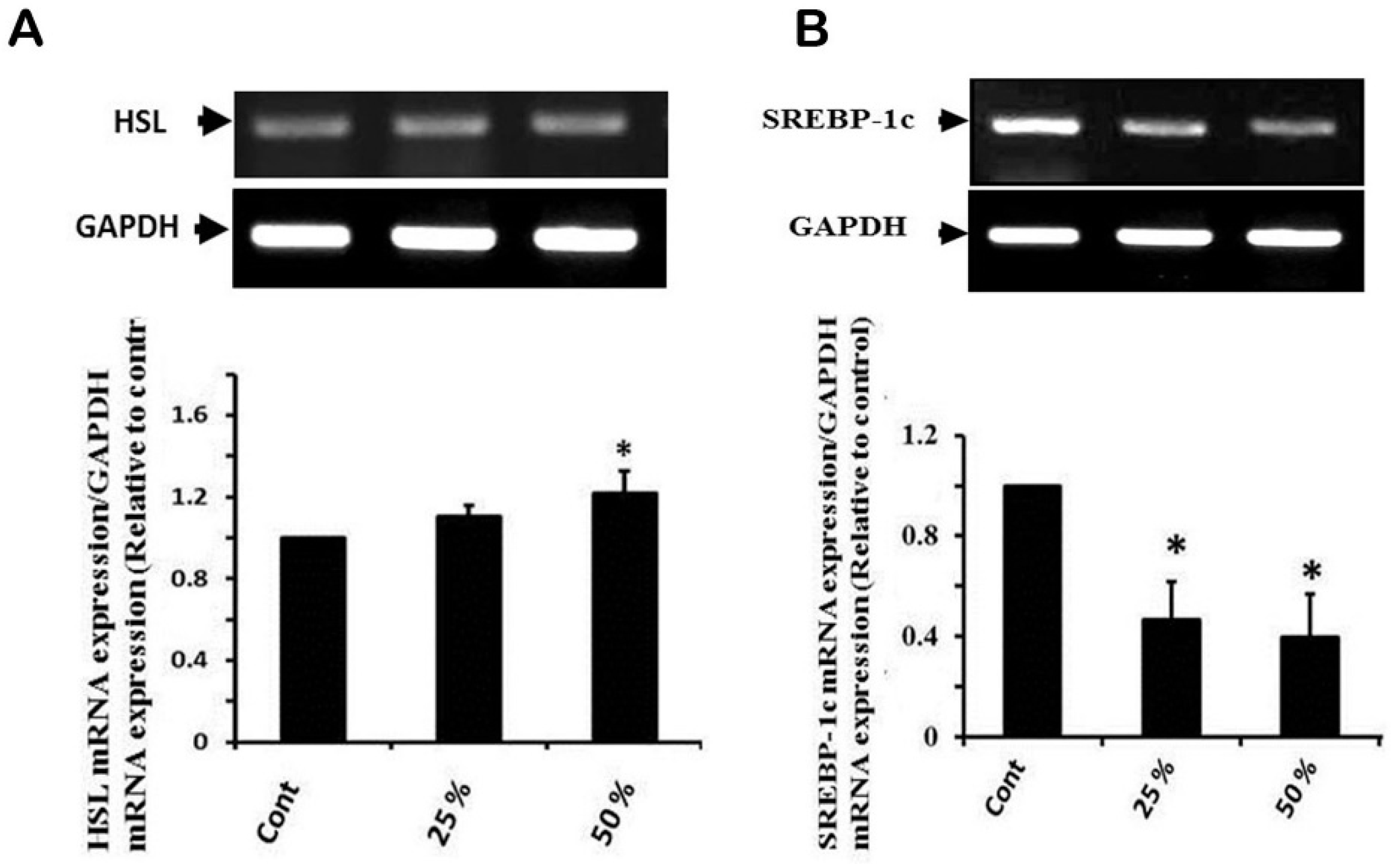
3.4. Effect of Ginger Water Treatment on HSL and SREBP-1c mRNA Expression
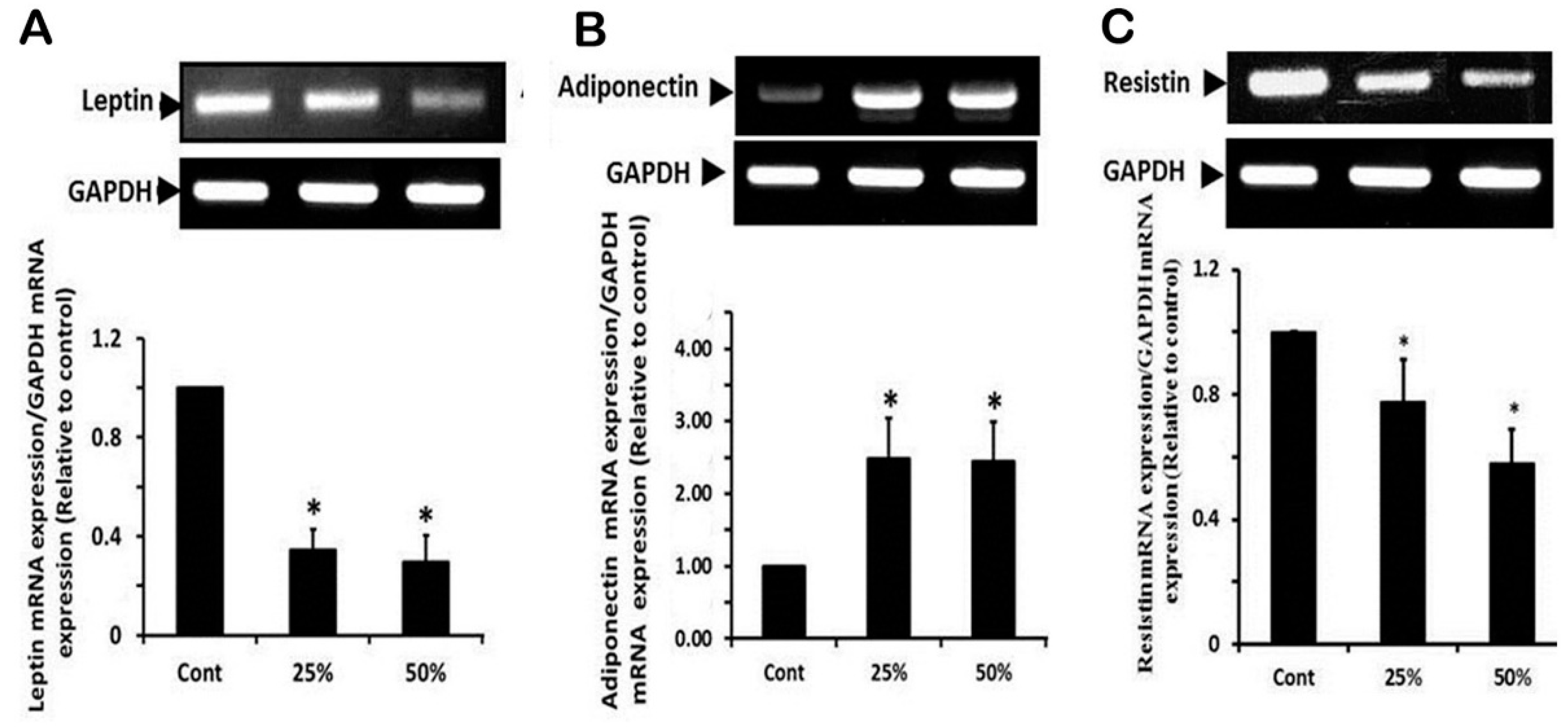
3.5. Effect of Ginger Water Treatment on the Leptin, Adiponectine, and Resistin mRNA Expression in White Adipose Tissue
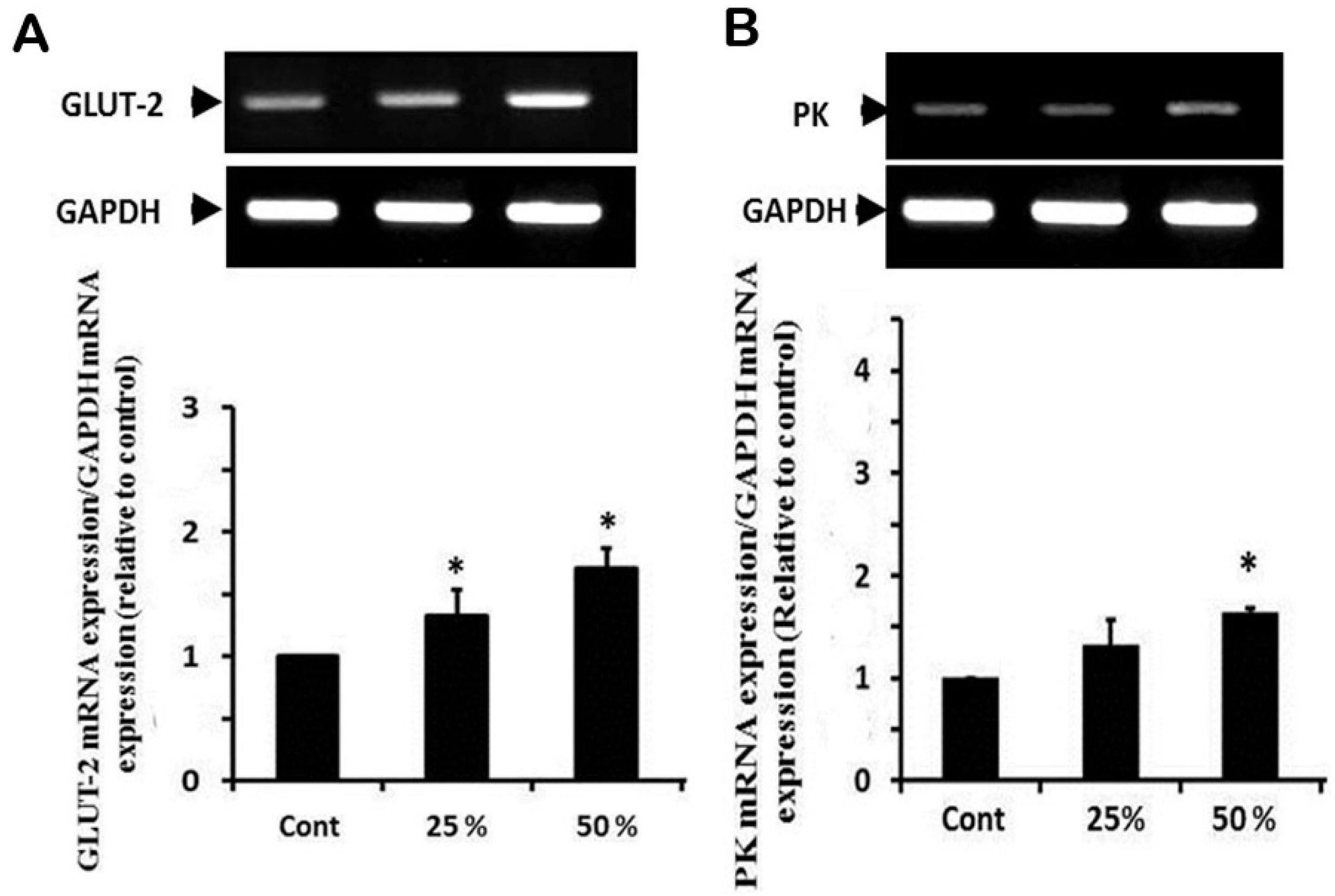
3.6. Effect of Ginger Water Treatment on the GLUT-2 and PK mRNA Expression
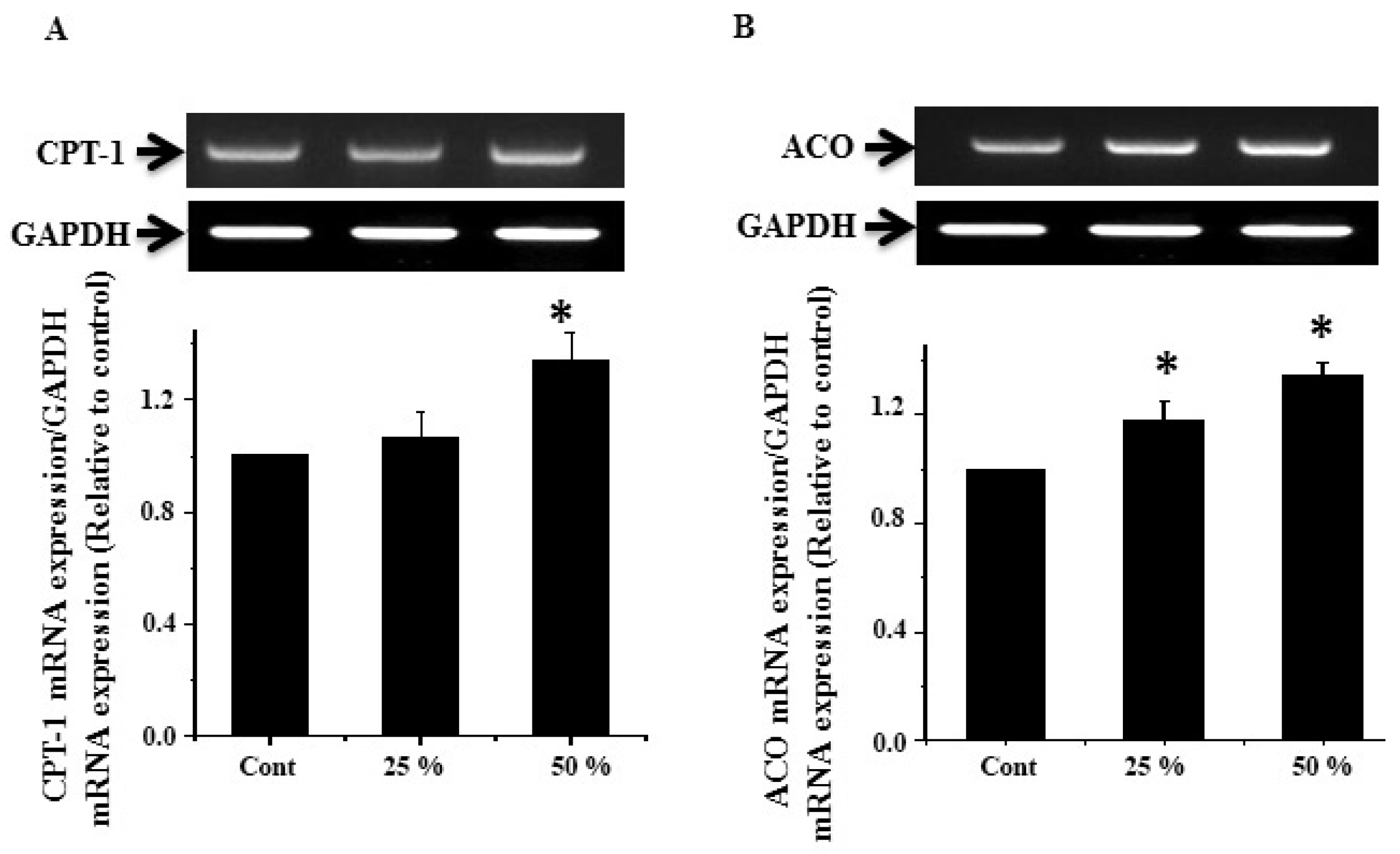
3.7. Effect of Ginger Water Treatment on the CPT-1 and ACO mRNA Expression
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- WHO. 2000 Obesity: Preventing and Managing the Global Epidemic Report of a WHO Consultation; WHO Technical Report Series No. 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- WHO 2013 Factson Obesity. Available online: http://www.who.int/features/factfiles/obesity/en/ (accessed on 24 February 2014).
- Choquet, H.; Meyre, D. Molecular basis of obesity: Current status and future prospects. Curr. Genom. 2011, 12, 154–168. [Google Scholar] [CrossRef]
- Nawrocki, A.R.; Scherer, P.E. Keynote review: The adipocyte as a drug discovery target. Drug Discov. Today 2005, 10, 1219–1230. [Google Scholar] [CrossRef]
- Abdollahi, M.; Afshar-Imani, B. A review on obesity and weight loss measures. Middle East Pharm. 2003, 11, 6–10. [Google Scholar]
- Hardy, G. Nutraceuticals and functional foods: Introduction and meaning. Nutrition 2000, 16, 688–689. [Google Scholar] [CrossRef]
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. Off. J. Eur. Communities 2002, L183/51–L183/57. Available online: http%3A%2F% 2Feur-lex.europa.eu%2Flegal-content%2FEN%2FTXT%2FPDF% 2F%3Furi%3DCELEX%3A32002L0046%26amp%3Bfrom% 3DENhttp%3A%2F%2Feur-lex.europa.eu%2Flegal-content% 2FEN%2FTXT%2FPDF%2F%3Furi%3DCELEX% 3A32002L0046%26amp%3Bfrom%3DEN (accessed on 31 December 2019).
- European Nutraceutical Association (ENA) (Ed.) Science Behind Nutraceuticals; European Nutraceutical Association: Basel, Switzerland, 2016; Volume 2016, p. 594. [Google Scholar]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Exp. Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef]
- Chung, M.J.; Kang, A.Y.; Park, K.W.; Jun, H.J.; Lee, S.J. The effect of essential oil if dietary wormwood (Artemisia princeps), with and without added vitamin E, on oxidative stress and some genes involved in cholesterol metabolism. Food Chem. Toxicol. 2007, 45, 1400–1409. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Larijani, B.; Abdollahi, M. A systematic review of Iranian medicinal plants useful in diabetes mellitus. Arch. Med. Sci. 2008, 4, 285–292. [Google Scholar]
- Moreno, D.A.; Ilic, N.; Poulev, A.; Raskin, I. Effects of Arachis hypogaea nutshell extract on lipid metabolic enzymes and obesity parameters. Life Sci. 2006, 78, 2797–2803. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Ibrahim, Z.S.; Alkafafy, M.; El-Shazly, S.A. L-Carnitine protects against testicular dysfunction caused by gamma irradiation in mice. Acta Histochem. 2014, 116, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Nammi, S.; Sreemantula, S.; Roufogalis, B.D. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin. Pharmacol. Toxicol. 2009, 104, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, Z.M.; Thomson, M.; Al-Qattan, K.K. Antidiabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Ponmurugan, P.; Deepa, M.A.; Senthilkumar, B. Anti-obesity action of gingerol: Effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J. Sci. Food Agric. 2014, 94, 2972–2977. [Google Scholar] [CrossRef]
- Gao, H.; Guan, T.; Li, C.; Zuo, G.; Yamahara, J.; Wang, J.; Li, Y. Treatment with ginger ameliorates fructose-induced fatty liver and hypertriglyceridemia in rats: Modulation of the hepatic carbohydrate response element binding protein-mediated pathway. Evid. Based Complement. Altern. Med. 2012, 2012, 570948. [Google Scholar] [CrossRef]
- Wang, J.; Gao, H.; Ke, D.; Zuo, G.; Yang, Y.; Yamahara, J.; Li, Y. Improvement of liquid fructose-induced adipose tissue insulin resistance by ginger treatment in rats is associated with suppression of adipose macrophage-related pro-inflammatory cytokines. Evid. Based Complement. Altern. Med. 2013, 2013, 590376. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Jiang, J.; Zuo, G.; Lin, X.; Yamahara, J.; Wang, J.; Li, Y. Ginger extract diminishes chronic fructose consumption-induced kidney injury through suppression of renal overexpression of proinflammatory cytokines in rats. BMC Complement. Altern. Med. 2014, 14, 174. [Google Scholar] [CrossRef]
- Latona, D.F.; Oyeleke, G.O.; Olayiwola, O.A. Chemical Analysis of Ginger Root. IOSR J. Appl. Chem. 2012, 1, 47–49. [Google Scholar]
- Boissonneault, G.A. Obesity: The current treatment protocols. JAAPA 2009, 22, 18–19. [Google Scholar]
- Thomson, M.; Al Qattan, K.K.; Al sawan, S.M. The use of ginger (Zingiber officinale rose) as a potential antiflammatory and antithrombotic agent. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 475–478. [Google Scholar] [CrossRef]
- Jung, H.A.; Kim, Y.S.; Choi, J.S. Quantitative HPLC analysis of two key flavonoids and inhibitory activities against aldose reductase from different parts of the Korean thistle, Cirsium maackii. Food Chem. Toxicol. 2009, 47, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M. Pineapple juice ameliorates the high fat diet-induced alterations in cardiac gene expression pattern in male rats. Int. J. Biochem. Res. Rev. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Surh, Y.J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 1999, 428, 305–327. [Google Scholar] [CrossRef]
- Brett, W. Ginger: An Overview. Complement. Altern. Med. 2007, 75, 1689–1691. [Google Scholar]
- Choi, Y.Y.; Kim, M.H.; Hong, J.; Kim, S.H.; Yang, W.M. Dried ginger (Zingiber officinalis) inhibits inflammation in a lipopolysaccharide-induced mouse model. Evid. Based Complement. Altern. 2013, 2013, 914563. [Google Scholar] [CrossRef]
- Misawa, K.; Hashizume, K.; Yamamoto, M.; Minegishi, Y.; Hase, T.; Shimotoyodome, A. Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor δ pathway. J. Nutr. Biochem. 2015, 26, 1058–1067. [Google Scholar] [CrossRef]
- Fuhrman, B.; Rosenblat, M.; Hayek, T.; Coleman, R.; Aviram, M. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J. Nutr. 2000, 130, 1124–1131. [Google Scholar] [CrossRef]
- Alizadeh-Navaei, R.; Roozbeh, F.; Saravi, M.; Pouramir, M.; Jalali, F.; Moghadamnia, A.A. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Saudi Med. J. 2008, 29, 1280–1284. [Google Scholar]
- Dias, M.C.; Spinardi-Barbisan, A.L.; Rodrigues, M.A.; de Camargo, J.L.; Teran, E.; Barbisan, L.F. Lack of chemopreventive effects of ginger on colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Food Chem. Toxicol. 2006, 44, 877–884. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially processed dry ginger (Zingiber officinale): Composition and effects on LPS-stimulated PGE2 production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; International Book Distributors Book Sellers and Publishers: Deheradun, India, 1999; Volume 3. [Google Scholar]
- Patel, D.K.; Patel, K.; Gadewar, M.; Tahilyani, V. Pharmacological and bioanalytical aspects of galangin—A concise report. Asian Pac. J. Trop. Biomed. 2012, 2, 5449–5455. [Google Scholar] [CrossRef]
- Campana, R.; Patrone, V.; Franzini, I.T.; Diamantini, G.; Vittoria, E.; Baffone, W. Antimicrobial activity of two propolis samples against human Campylobacter jejuni. J. Med. Food 2009, 12, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, Q.; Bi, J.; Ding, J.; Ge, S.; Chen, F. Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo. Chem. Biol. Interact. 2014, 224, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.A.; Jeong, S.; Lee, S.; Park, H.J.; Kim, N.J.; Lim, S. Anti-inflammatory, anti-nociceptive, and anti-psychiatric effects by the rhizomes of Alpinia officinarum on complete Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2009, 126, 258–264. [Google Scholar] [CrossRef]
- Heo, M.Y.; Sohn, S.J.; Au, W.W. Anti-genotoxicity of galangin as a cancer chemoprotective agent candidate. Mutat. Res. 2001, 488, 135–150. [Google Scholar] [CrossRef]
- Hamada, M.; Satsum, H.; Ashida, H.; Sugita-Konishi, Y.; Shimizu, M. Metabolites of Galangin by 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin-Inducible Cytochrome P450 1A1 in Human Intestinal Epithelial Caco-2 Cells and Their Antagonistic Activity toward Aryl Hydrocarbon Receptor. J. Agric. Food Chem. 2010, 58, 8111–8118. [Google Scholar] [CrossRef]
- Jung, C.H.; Jang, S.J.; Ahn, J.; Gwon, S.Y.; Jeon, T.I.; Kim, T.W.; Ha, T.Y. Alpinia officinarum inhibits adipocyte differentiation and high-fat diet-induced obesity in mice through regulation of adipogenesis and lipogenesis. J. Med. Food 2012, 15, 959–967. [Google Scholar] [CrossRef]
- Kumar, S.; Alagawadi, K.R. Anti-obesity effects of galangin, a pancreatic lipase inhibitor in cafeteria diet fed female rats. Pharm. Biol. 2013, 151, 607–613. [Google Scholar] [CrossRef]
- Tsai, M.S.; Chien, C.C.; Lin, T.H.; Liu, C.C.; Liu, R.H.; Su, H.L.; Chiu, Y.T.; Wang, S.H. Galangin prevents acute hepatorenal toxicity in novel propacetamol-induced acetaminophen-overdosed mice. J. Med. Food 2015, 18, 1187–1197. [Google Scholar] [CrossRef]
- Bae, Y.; Lee, S.; Kim, S.H. Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol. Appl. Pharmacol. 2011, 254, 56–64. [Google Scholar] [CrossRef]
- Bai, J.; Luo, Y.; Song, Z.; Fan, W.; Wang, Z.; Luan, T.; Jiang, J.; Zang, B. Effects and the mechanisms of chrysin on sepsis-associated acute lung injury of rats chrysin inhibits acute lung injury. Life Sci. J. 2013, 10, 1052–1058. [Google Scholar]
- Wadibhasme, P.G.; Ghaisas, M.M.; Thakurdesai, P.A. Anti-asthmatic potential of chrysin on ovalbumin-induced bronchoalveolar hyperresponsiveness in rats. Pharm. Biol. 2011, 49, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.N.; Huang, J.M.; Xiong, X.K.; Chen, M.F.; Ong, C.N.; Shen, H.M.; Yang, X.F. Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Toxicol. In Vitro 2011, 25, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Maruyama, T.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Yagita, H.; Ruchirawat, S. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol. Rep. 2013, 30, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Anandhi, R.; Annadurai, T.; Anitha, T.S.; Muralidharan, A.R.; Najmunnisha, K.; Nachiappan, V.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and antioxidative effects of an extract of the Oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in triton WR-1339-induced hypercholesterolemic rats. J. Physiol. Biochem. 2013, 69, 313–323. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013, 65, 750–756. [Google Scholar] [CrossRef]
- Van Heerden, F.R. Hoodia gordonii: A natural appetite suppressant. J. Ethnopharmacol. 2008, 119, 434–437. [Google Scholar] [CrossRef]
- Okuda, H.; Han, L.; Kimura, Y.; Saito, M.; Murata, T. Anti-Obesity Action of Herb Tea (Part 1). Effects or Various Herb Teas on Noradrenaline-Induced Lipolysis in Rat Fat Cells and Pancreatic Lipase Activity. Jpn. J. Const. Med. 2001, 63, 60–65. [Google Scholar]
- Geoffroy, P.; Ressault, B.; Marchioni, E.; Miesch, M. Synthesis of Hoodigogenin A, aglycone of a natural appetite suppressant glycosteroid extracted from Hoodia gordonii. Steroids 2011, 76, 702–708. [Google Scholar] [CrossRef]
- Haaz, S.; Fontaine, K.R.; Cutter, G.; Limdi, N.; Perumean-Chaney, S.; Allison, D.B. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: An update. Obes. Rev. 2006, 7, 79–88. [Google Scholar] [CrossRef]
- Stefan, N.; Bunt, J.C.; Salbe, A.D.; Funahashi, T.; Matsuzawa, Y.; Tataranni, P.A. Plasma adiponectin concentrations in children: Relationships with obesity and insulinemia. J. Clin. Endocrinol. Metab. 2001, 87, 4652–4656. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh Attari, V.; Ostadrahimi, A.; Asghari Jafarabadi, M.; Mehralizadeh, S.; Mahluji, S. Changes of serum adipocytokines and body weight following Zingiber officinale supplementation in obese women: A RCT. Eur. J. Nutr. 2016, 55, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. 2005, 6, 13–21. [Google Scholar] [CrossRef]
- Park, J.; Rho, H.K.; Kim, K.H.; Choe, S.S.; Lee, Y.S.; Kim, J.B. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol. Cell Biol. 2005, 25, 5146–5157. [Google Scholar] [CrossRef]
- Thamilvaani, M.; Manaharan, T.; Kanthimathi, M.S. Ginger oil-mediated down-regulation of adipocyte specific genes inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Biochem. Biotechnol. Res. 2016, 4, 38–47. [Google Scholar]
- Ojewole, J.A. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother. Res. 2006, 20, 764–772. [Google Scholar] [CrossRef]
- Krskova-Tybitanclova, K.; Macejova, D.; Brtko, J.; Ba-culikova, M.; Krizanova, O.; Zorad, S. Short term 13-cis-retinoic acid treatment at therapeutic doses elevates expression of leptin, GLUT4, PPAR gamma and aP2 in rat adipose tissue. J. Physiol. Pharmacol. 2008, 59, 731–743. [Google Scholar]
- Landrier, J.F.; Gouranton, E.; Yazidi, E.L.; Malezet, C.; Balaguer, P.; Borel, P.; Amiot, M.J. Adiponectin expression is induced by vitamin E via a peroxisome proliferator activated receptor gamma-dependent mechanism. Endocrinology 2009, 150, 5318–5325. [Google Scholar] [CrossRef]
- Soliman, M.; Ahmed, M.; El-Shazly, S.; Ismail, T.; Attia, H.; Elkirdasy, A. Effect of vitamin A and E on carbohydrate and lipid metabolism in diet-induced obese wistar rats. Adv. Biosci. Biotechnol. 2014, 5, 4–11. [Google Scholar] [CrossRef][Green Version]
- Peluso, G.; Nicolai, R.; Reda, E.; Benatti, P.; Barbarisi, A.; .Calvani, M. Cancer and anticancer therapy-induced modifications on metabolism mediated by carnitine system. J. Cell Physiol. 2000, 182, 339–350. [Google Scholar] [CrossRef]
- Soliman, M.M.; Ahmed, M.M.; El-Sawy, H.B.; Ibrahim, Z.S.; El-Shazly, S.A. Effect of ginger extract and L-carnitine on the expression of genes related to lipids and carbohydrates metabolism. Biosci. Res. 2018, 15, 4381–4389. [Google Scholar]
- Hoehn, K.L.; Hohnen-Behrens, C.; Cederberg, A.; Wu, L.E.; Turner, N.; Yuasa, T.; Ebina, Y.; James, D.E. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008, 7, 421–433. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primer Sequence (5′–3′) | Annealing | Cycles | Product Size |
|---|---|---|---|---|
| GAPDH | F-AGATCCACAACGGATACATT | 52 °C | 25 cycles | 309 bP |
| R-TCCCTCAAGATTGTCAGCA | ||||
| SREP1-c | F-GGAGCCATGGATTGCACATT | 58 °C | 28 cycles | 191 bP |
| R-AGGAAGGCTTCCAGAGAGGA | ||||
| HSL | F-TGCCCAGGAGTGTGTCTGAG | 61 °C | 33 cycles | 313 bP |
| R-AGGACACCTTGGCTTGAGCG | ||||
| Leptin | F-CCTGTGGCTTTGGTCCTATCTG | 59 °C | 30 cycles | 244 bP |
| R-TATGCTTTGCTGGGGTTTTC | ||||
| Adiponectin | F-CTCCACCCAAGGAAACTTGT | 52 °C | 28 cycles | 500 bP |
| R-CTGGTCCACATTTTTTTCCT | ||||
| PK | F-ATTGCTGTGACTGGATCTGC | 52 °C | 28 cycles | 229 bP |
| R-CCCGCATGATGTTGGTATAG | ||||
| GLUT-2 | F-AAGGATCAAAGCCATGTTGG | 55 °C | 28 cycles | 330 bP |
| R-GGAGACCTTCTGCTCAGTGG | ||||
| ACO | F-GCCCTCAGCTATGGTATTAC | 53 °C | 28 cycles | 633 bP |
| R-AGGAACTGCTCTCACAATGG | ||||
| CPT-1 | F-TATGTGAGGATGCTGCTTCC | 52 °C | 28 cycles | 628 bP |
| R-CTCGGAGAGCTAAGCTTGCT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, S.; Ahmed, M.; El-Shehawi, A.; Alkafafy, M.; Al-Otaibi, S.; El-Sawy, H.; Farouk, S.; El-Shazly, S. Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats. Foods 2020, 9, 38. https://doi.org/10.3390/foods9010038
Sayed S, Ahmed M, El-Shehawi A, Alkafafy M, Al-Otaibi S, El-Sawy H, Farouk S, El-Shazly S. Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats. Foods. 2020; 9(1):38. https://doi.org/10.3390/foods9010038
Chicago/Turabian StyleSayed, Samy, Mohamed Ahmed, Ahmed El-Shehawi, Mohamed Alkafafy, Saqer Al-Otaibi, Hanan El-Sawy, Samy Farouk, and Samir El-Shazly. 2020. "Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats" Foods 9, no. 1: 38. https://doi.org/10.3390/foods9010038
APA StyleSayed, S., Ahmed, M., El-Shehawi, A., Alkafafy, M., Al-Otaibi, S., El-Sawy, H., Farouk, S., & El-Shazly, S. (2020). Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats. Foods, 9(1), 38. https://doi.org/10.3390/foods9010038







