Occurrence and Risk of Metal(loid)s in Thelesperma megapotamicum Tea Plant
Abstract
:1. Introduction
2. Materials and Methods
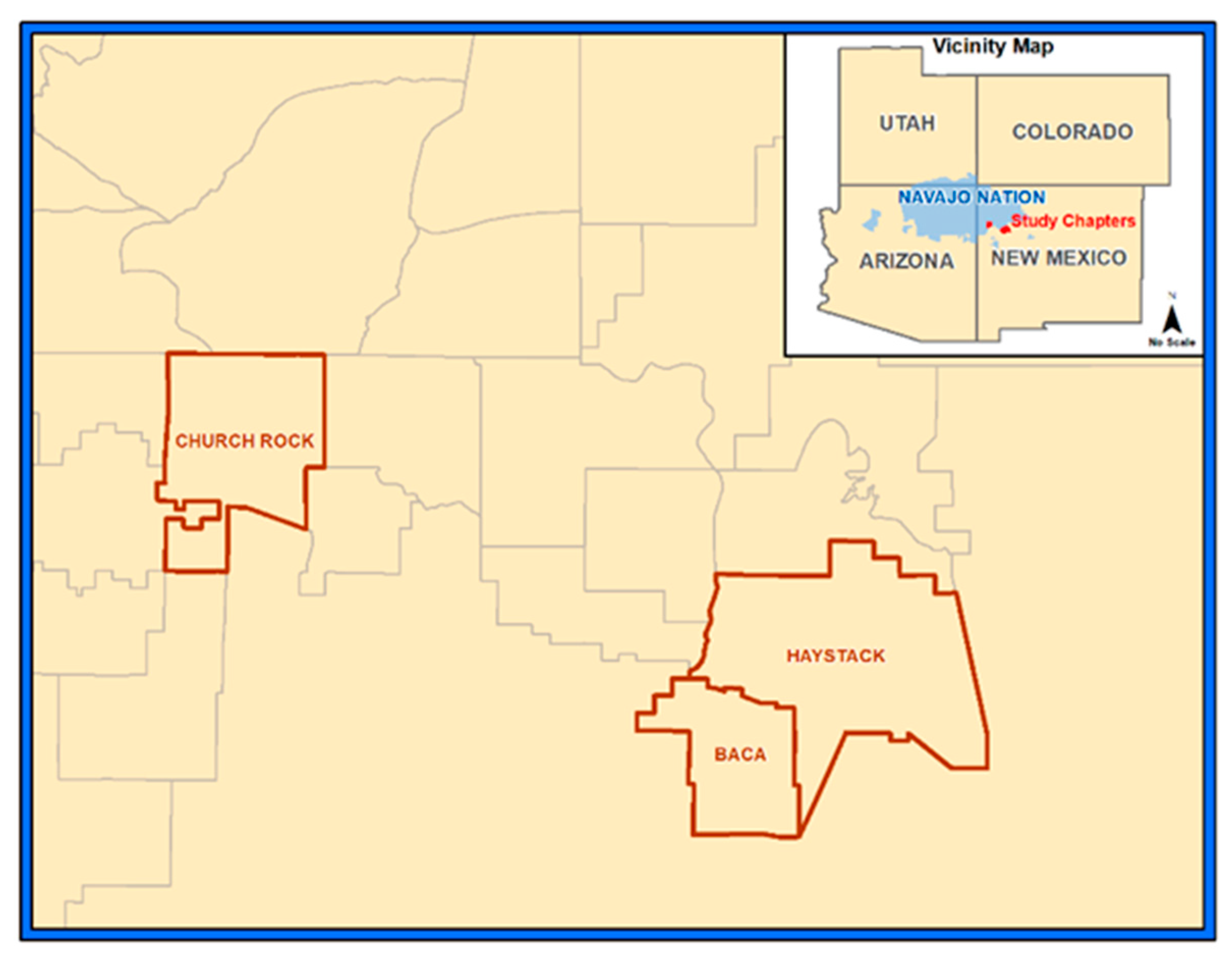
2.1. Setting
2.2. Herbal Tea Samples
2.3. HTA and LTA Classifications
2.4. Soil Samples
2.5. Human PTWI Calculation Equations
2.6. Plant Identification and Nomenclature
2.7. Sample Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Human Tea Harvester Data
3.2. Metal(loid) Concentration Levels in Tea Plant Parts and Soil
3.3. Metal(loid) Concentration Levels in HTAs and LTAs
3.4. Human PTWI Calculations
3.5. Human Intake Calculations for RDI or RDA and UL
3.6. Human PTWI Calculations for Samples from HTAs and LTAs
3.7. Human Intake Calculation Estimates for RDI or RDA and UL for Samples from HTAs and LTAs
3.8. Differences between the WHO RMPPL and the PTWI Guidelines
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Korkmaz Görür, F.; Keser, R.; Akçay, N.; Dizman, S.; Okumuşoğlu, N.T. Radionuclides and heavy metals concentrations in Turkish market tea. Food Control 2011, 22, 2065–2070. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes. (Lond.) 2009, 33, 956–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxley, R.; Lee, C.M.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Han, G.; Hu, Y.; Bi, Y.; Li, L.; Zhu, D. Tea consumption and risk of type 2 diabetes: A meta-analysis of cohort studies. J. Gen. Intern. Med. 2009, 24, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Chiu, A.; Barone, M.K.; Avino, D.; Wang, F.; Coleman, C.I.; Phung, O.J. Green tea catechins decrease total and low-density lipoprotein cholesterol: A systematic review and meta-analysis. J. Am. Diet. Assoc. 2011, 111, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Liu, X.X.; Hui, R.; Huang, X.H. Green tea intake lowers fasting serum total and LDL cholesterol in adults: A meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 2011, 94, 601–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpinel, C.; Ruiz, G.; Palacios, S. Screening of native plants of central Argentina for antifungal activity. Allelopath. J. 2010, 25, 423–432. [Google Scholar]
- Figueroa, A.C.; Soria, E.A.; Cantero, J.J.; Sanchez, M.S.; Goleniowski, M.E. Cytotoxic Activity of Thelesperma megapotamicum Organic Fractions against MCF-7 Human Breast Cancer Cell Line. J. Cancer Ther. 2012, 3, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Leong, H.; Mathur, P.S.; Greene, G.L. Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res. Treat. 2008, 107, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Shemluck, M. Medicinal and other uses of the Compositae by Indians in the United States and Canada. J. Ethnopharmacol. 1982, 5, 303–358. [Google Scholar] [CrossRef]
- Dunmire, W.W.; Tierney, G.D. Wild Plants and Native Peoples of the Four Corners; Museum of New Mexico Press: Santa Fe, NM, USA, 1997. [Google Scholar]
- Matthews, W. Navajo names for plants. Am. Nat. 1886, 20, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.M.; Bridgforth, E.B.; Dalton, E.; Darby, W.J.; Efner, J.A.; Houk, N.; Johnson, H.F.; McGanity, W.J.; McKinley, P.; Pollard, M.; et al. A study of the dietary background and nutriture of the Navajo Indian. J. Nutr. 1956, 60, 1–85. [Google Scholar] [CrossRef] [PubMed]
- Ballew, C.; White, L.L.; Strauss, K.F.; Benson, L.J.; Mendlein, J.M.; Mokdad, A.H. Intake of nutrients and food sources of nutrients among the Navajo: Findings from the Navajo Health and Nutrition Survey. J. Nutr. 1997, 127, 2085S–2093S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, S.M.; Maggi, M.E.; Bazan, C.M.; Carpinella, M.C.; Turco, M.; Munoz, A.; Alonso, R.A.; Nunez, C.; Cantero, J.J.; Defago, M.T.; et al. Screening of Argentinian plants for pesticide activity. Fitoterapia 2007, 78, 580–584. [Google Scholar] [CrossRef]
- Shaw, D.; Graeme, L.; Pierre, D.; Elizabeth, W.; Kelvin, C. Pharmacovigilance of herbal medicine. J. Ethnopharmacol. 2012, 140, 513–518. [Google Scholar] [CrossRef]
- Da Silva Pinto, M. Tea: A new perspective on health benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Sarma, H.; Deka, S.; Deka, H.; Saikia, R.R. Accumulation of heavy metals in selected medicinal plants. Rev. Environ. Contam. Toxicol. 2011, 214, 63–86. [Google Scholar] [CrossRef]
- Lewis, J.; Hoover, J.; MacKenzie, D. Mining and Environmental Health Disparities in Native American Communities. Curr. Environ. Health Rep. 2017, 4, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Samuel-Nakamura, C.; Robbins, W.A.; Hodge, F.S. Uranium and Associated Heavy Metals in Ovis aries in a Mining Impacted Area in Northwestern New Mexico. Int. J. Environ. Res. Public Health 2017, 14, 848. [Google Scholar] [CrossRef] [PubMed]
- Samuel-Nakamura, C.; Hodge, F.S.; Valentine, J.L.; Robbins, W.A. Heavy metal contamination in Thelesperma megapotamicum. J. Toxicol. Environ. Health Sci. 2017, 9, 4–22. [Google Scholar] [CrossRef]
- Samuel-Nakamura, C.; Hodge, F.S.; Sokolow, S.; Ali, A.S.; Robbins, W.A. Metal(loid)s in Cucurbita pepo in a Uranium Mining Impacted Area in Northwestern New Mexico, USA. Int. J. Environ. Res. Public Health 2019, 16, 2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karak, T.; Bhagat, R.M. Trace elements in tea leaves, made tea and tea infusion: A review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- DeLemos, J.L.; Bostick, B.C.; Quicksall, A.N.; Landis, J.D.; George, C.C.; Slagowski, N.L.; Rock, T.; Brugge, D.; Lewis, J.; Durant, J.L. Rapid dissolution of soluble uranyl phases in arid, mine-impacted catchments near Church Rock, NM. Environ. Sci. Technol. 2008, 42, 3951–3957. [Google Scholar] [CrossRef] [Green Version]
- DeLemos, J.L.; Brugge, D.; Cajero, M.; Downs, M.; Durant, J.L.; George, C.M.; Henio-Adeky, S.; Nez, T.; Manning, T.; Rock, T.; et al. Development of risk maps to minimize uranium exposures in the Navajo Churchrock mining district. Environ. Health 2009, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Arpadjan, S.; Celik, G.; Taskesen, S.; Gucer, S. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem. Toxicol. 2008, 46, 2871–2875. [Google Scholar] [CrossRef]
- Acuna, U.M.; Atha, D.E.; Ma, J.; Nee, M.H.; Kennelly, E.J. Antioxidant capacities of ten edible North American plants. Phytother Res. 2002, 16, 63–65. [Google Scholar] [CrossRef]
- Palumbo, M.J.; Talcott, S.T.; Putz, F.E. Ilex Vomitoria Ait. (Yaupon): A Native North American Source of a Caffeinated and Antioxidant-Rich Tea. Econ. Bot. 2009, 63, 130–137. [Google Scholar] [CrossRef]
- Kurttio, P.; Harmoinen, A.; Saha, H.; Salonen, L.; Karpas, Z.; Komulainen, H.; Auvinen, A. Kidney toxicity of ingested uranium from drinking water. Am. J. Kidney Dis. 2006, 47, 972–982. [Google Scholar] [CrossRef]
- Kirkham, M.B. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Caldas, E.D.; Machado, L.L. Cadmium, mercury and lead in medicinal herbs in Brazil. Food Chem. Toxicol. 2004, 42, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Eisler, R. Arsenic Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; Report 12; Biological Report 85(1.12): Laurel, MD, USA, 1988. [Google Scholar]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant. Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Grignard, E.; Gueguen, Y.; Grison, S.; Lobaccaro, J.M.; Gourmelon, P.; Souidi, M. In vivo effects of chronic contamination with 137 cesium on testicular and adrenal steroidogenesis. Arch. Toxicol. 2008, 82, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Cadmium, lead, and other metals in relation to semen quality: Human evidence for molybdenum as a male reproductive toxicant. Environ. Health Perspect. 2008, 116, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Padmanahban, V.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Environmental exposure to metals and male reproductive hormones: Circulating testosterone is inversely associated with blood molybdenum. Fertil. Steril. 2010, 93, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Thorium. U.S. Department of Health and Human Services; Public Health Service, Ed.; CDC: Atlanta, GA, USA, 1990.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Vanadium. U.S. Department of Health and Human Services; Public Health Service, Ed.; CDC: Atlanta, GA, USA, 2012.
- Western Regional Climate Center. Climatic Historic Summary January 2011–September 2012; Western Regional Climate Center: Reno, NV, USA, 2011. [Google Scholar]
- Navajo Nation Division of Natural Resources (NNDNR). Navajo Endangered Species List; Navajo Nation Division of Natural Resources (NNDNR): Window Rock, AZ, USA, 2008.
- New Mexico Energy, Minerals, and Natural Resources Department (NMEMNRD). New Mexico Rare Plant. Conservation Strategy; Forestry Division, Ed.; New Mexico Energy, Minerals, and Natural Resources Department (NMEMNRD): Santa Fe, NM, USA, 2017.
- New Mexico Department of Transportation. Functional Classification Guidance Manual; New Mexico Department of Transportation: Santa Fe, NM, USA, 2014.
- Joint Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Thirty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO: Geneva, Switzerland, 1989; pp. 9–10. [Google Scholar]
- Joint Expert Committee on Food Additives. Joint FAO/WHO Expert Committee on food additives. In Proceedings of the Sixty-First Meeting Summary and Conclusions, Rome, Italy, 10–19 June 2003; WHO: Geneva, Switzerland. [Google Scholar]
- Salahinejad, M.; Aflaki, F. Toxic and essential mineral elements content of black tea leaves and their tea infusions consumed in Iran. Biol. Trace Elem. Res. 2010, 134, 109–117. [Google Scholar] [CrossRef]
- Tsuji, L.J.; Manson, H.; Wainman, B.C.; Vanspronsen, E.P.; Shecapio-Blacksmith, J.; Rabbitskin, T. Identifying potential receptors and routes of contaminant exposure in the traditional territory of the Ouje-Bougoumou Cree: Land use and a geographical information system. Environ. Monit. Assess. 2007, 127, 293–306. [Google Scholar] [CrossRef]
- Han, W.Y.; Zhao, F.J.; Shi, Y.Z.; Ma, L.F.; Ruan, J.Y. Scale and causes of lead contamination in Chinese tea. Environ. Pollut. 2006, 139, 125–132. [Google Scholar] [CrossRef]
- Shi, Y.-Z.; Ruan, J.-Y.; Ma, L.-F.; Han, W.-Y.; Wang, F. Accumulation and distribution of arsenic and cadmium by tea plants. J. Zhejiang Univ. Sci. B 2008, 9, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.R.; Myhr, K. Cadmium uptake by barley as affected by Cd sources and pH levels. Geoderma 1998, 84, 185–194. [Google Scholar] [CrossRef]
- Wu, J.; Zou, Y.; Zhan, X.; Chen, S.; Lu, G.; Lai, F. Survey of heavy metal pollution in four chinese crude drugs and their cultivated soils. Bull. Environ. Contam. Toxicol. 2008, 81, 571–573. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Barthwal, J.; Nair, S.; Kakkar, P. Heavy metal accumulation in medicinal plants collected from environmentally different sites. Biomed. Environ. Sci. 2008, 21, 319–324. [Google Scholar] [CrossRef]
- Chan, K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 2003, 52, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Qiao, L.; Zhang, H.; Chen, J. Exposure and risk assessment for aluminium and heavy metals in Puerh tea. Sci. Total Environ. 2010, 408, 2777–2784. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, M.; Xu, J.; Chen, X.; Shi, J. Differentiation of eight tea (Camellia sinensis) cultivars in China by elemental fingerprint of their leaves. J. Sci. Food Agric. 2009, 89, 2350–2355. [Google Scholar] [CrossRef]
- Alasalvar, C.; Pelvan, E.; Ozdemir, K.S.; Kocadagli, T.; Mogol, B.A.; Pasli, A.A.; Ozcan, N.; Ozcelik, B.; Gokmen, V. Compositional, nutritional, and functional characteristics of instant teas produced from low- and high-quality black teas. J. Agric. Food Chem. 2013, 61, 7529–7536. [Google Scholar] [CrossRef]
- Matsuura, H.; Hokura, A.; Katsuki, F.; Itoh, A.; Haraguchi, H. Multielement determination and speciation of major-to-trace elements in black tea leaves by ICP-AES and ICP-MS with the aid of size exclusion chromatography. Anal. Sci. 2001, 17, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Antal, D.; Dehelean, C.; Dobrea, C.; Manfred, A. Vanadium in medicinal plants: New data on the occurence of an element both essential and toxic to plants and man. Analele Universitatii Din Oradea Fascicola 2009, 16, 5–10. [Google Scholar]
- Tokalioglu, S.; Kartal, S. Bioavailability of Soil-Extractable Metals to Tea Plant by BCR Sequential Extraction Procedure. Instrum. Sci. Technol. 2004, 32, 387–400. [Google Scholar] [CrossRef]
- Ashraf, W.; Mian, A.A. Levels of selected heavy metals in black tea varieties consumed in Saudi Arabia. Bull. Environ. Contam. Toxicol. 2008, 81, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Han, W.Y.; Shi, Y.Z.; Ma, L.F.; Ruan, J.Y.; Zhao, F.J. Effect of liming and seasonal variation on lead concentration of tea plant (Camellia sinensis (L.) O. Kuntze). Chemosphere 2007, 66, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kolachi, N.F.; Kazi, T.G.; Afridi, H.I.; Khan, S.; Wadhwa, S.K.; Shah, A.Q.; Shah, F.; Baig, J.A.; Sirajuddin. Determination of selenium content in aqueous extract of medicinal plants used as herbal supplement for cancer patients. Food Chem. Toxicol. 2010, 48, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Nookabkaew, S.; Rangkadilok, N.; Satayavivad, J. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. J. Agric. Food Chem. 2006, 54, 6939–6944. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, V.; Grimmer, H.; Semano, M.; Gulumian, M. Antioxidant and genotoxic properties of South African herbal extracts. Mutat. Res. 2005, 581, 35–42. [Google Scholar] [CrossRef]
- Ahmed, F.; Daif, M.M.; El-Masry, N.M.; Abo-Elmagd, M. External and internal radiation exposure of herbal plants used in Egypt. Radiat. Eff. Defects Solids 2010, 165, 65–71. [Google Scholar] [CrossRef]
- Oprea, E.M.; Pintilie, V.; Bufnea, V.; Aprotosoaie, A.C.; Cioanca, O.; Trifan, A.; Hancianu, M. Radionuclides content in some medicinal plants commonly used in Romania. Farmacia 2014, 62, 658–663. [Google Scholar]
- Mitrović, B.M.; Grdović, S.N.; Vitorović, G.S.; Vitorović, D.P.; Pantelić, G.K.; Grubić, G.A. 137Cs and 40K in some traditional herbal teas collected in the mountain regions of Serbia. Isot. Environ. Heal. Stud. 2014, 50, 538–545. [Google Scholar] [CrossRef]
- Stanojkovic-Sebic, A.; Pivic, R.; Josic, D.; Dinić, Z.; Stanojković, A. Heavy metals content in selected medicinal plants commonly used as components for herbal formulations. Tarim Bilim. Derg. 2015, 21, 317–325. [Google Scholar] [CrossRef]
- World Health Organization. Quality Control. Methods for Medicinal Plant. Materials; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Blagojevic, N.; Vratnica, B.; Vukasinovic-Pesic, V.; Djurovic, D. Heavy Metals Content in Leaves and Extracts of Wild-Growing Salvia Officinalis from Montenegro. Pol. J. Environ. Stud. 2009, 18, 167–173. [Google Scholar]
- Apeagyei, E.; Bank, M.S.; Spengler, J.D. Distribution of heavy metals in road dust along an urban-rural gradient in Massachusetts. Atmos. Environ. 2011, 45, 2310–2323. [Google Scholar] [CrossRef]
- Naithani, V.; Kakkar, P. Effect of ecological variation on heavy metal content of some medicinal plants used as herbal tea ingredients in India. Bull. Environ. Contam. Toxicol. 2006, 76, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Naithani, V.; Kakkar, P. Evaluation of Heavy Metals in Indian Herbal Teas. Bull. Environ. Contam. Toxicol. 2005, 75, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gao, E.; He, B.; Jiang, G. Arsenic species and leaching characters in tea (Camellia sinensis). Food Chem. Toxicol. 2007, 45, 2381–2389. [Google Scholar] [CrossRef]
- Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Gil, F.; Hernández, A.F.; Martín-Domingo, M.C. Chapter 58—Toxic Contamination of Nutraceuticals and Food Ingredients. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 825–837. [Google Scholar] [CrossRef]
- Kosalec, I.; Cvek, J.; Tomic, S. Contaminants of medicinal herbs and herbal products. Arhiv Hig. Rada Toksikol. 2009, 60, 485–501. [Google Scholar] [CrossRef] [Green Version]
| Metal(loid)s | Herb (n = 14) | Root (n = 14) | Root Soil (n = 2) | Topsoil (n = 12) | WHO RMPPL mg/kg |
|---|---|---|---|---|---|
| As | 0.423 ± 0.103 1,3 | 0.758 ± 0.238 1 | 2.315 ± 1.168 | 1.844 ± 0.496 3 | 1 |
| Cd | 0.346 ± 0.311 1,3 | 0.634 ± 0.658 1 | 0.050 ± 0.029 | 0.512 ± 0.228 3 | 0.3 |
| Cs | 0.063 ± 0.074 1,4 | 0.213 ± 0.169 1 | 0.488 ± 0.264 | 1.082 ± 0.357 4 | --5 |
| Pb | 0.304 ± 0.731 1,3 | 0.809 ± 0.286 1 | 5.783 ± 3.704 2 | 5.207 ± 1.266 2,3 | 10 |
| Mo | 7.916 ± 9.291 1,4 | 18.304 ± 21.563 1 | nd | 10.562 ± 5.232 4 | --5 |
| Se | 0.738 ± 0.393 3 | 1.242 ± 0.557 | nd | 1.122 ± 0.113 3 | --5 |
| Th | 0.200 ± 0.248 | 0.268 ± 0.163 | 2.642 ± 1.946 | 2.940 ± 0.815 | --5 |
| U | 0.019 ± 0.012 1,3 | 0.114 ± 0.0411 | 0.829 ± 0.420 | 1.290 ± 0.224 3 | --5 |
| V | 0.244 ± 0.096 1,4 | 2.343 ± 1.589 1 | 9.203 ± 4.008 | 16.297 ± 5.799 4 | --5 |
| Metal(loid)s | High Traffic Areas (HTAs) (n = 6) | Low Traffic Areas (LTAs) (n = 8) |
|---|---|---|
| As | 0.360 ± 0.093 | 0.470 ± 0.086 |
| Cd | 0.681 ± 0.106 2 | 0.095 ± 0.062 2 |
| Cs | 0.021 ± 0.008 1 | 0.095 ± 0.085 1 |
| Pb | 0.320 ± 0.030 | 0.292 ± 0.094 |
| Mo | 18.042 ± 3.011 2 | 0.321 ± 0.171 2 |
| Se | 0.996 ± 0.246 | 0.544 ± 0.379 |
| Th | 0.501 ± 0.264 | 0.087 ± 0.121 |
| U | 0.029 ± 0.013 1 | 0.013 ± 0.005 1 |
| V | 0.306 ± 0.119 1 | 0.198 ± 0.035 1 |
| Metal(loid)s * | Weekly Intake (μg/kg BW) | PTWI (μg/kg BW) | % below PTWI |
|---|---|---|---|
| As | 0.25 | 15 | 1.66 |
| Cd | 0.20 | 7 | 2.85 |
| Pb | 0.18 | 25 | 0.72 |
| Metal(loid)s * | Daily Intake (μg) | RDI or RDA and UL (μg/day) | % below RDI or RDA and UL |
|---|---|---|---|
| Mo | 8.71 | RDA: 45 | 19.35 |
| UL: 2000 | 0.44 | ||
| Se | 0.81 | RDI: 55 | 1.47 |
| UL: 400 | 0.20 | ||
| V | 0.27 | UL: 1800 | 0.02 |
| Metal(loid)s in HTAs and LTAs | Weekly Intake μg/kg BW | PTWI (μg/kg BW) | % Below PTWI |
|---|---|---|---|
| HTA As | 0.21 | 1.4 | |
| LTA As | 0.27 | 15 | 1.8 |
| HTA Cd | 0.40 | 5.71 | |
| LTA Cd | 0.06 | 7 | 0.86 |
| HTA Pb | 0.19 | 0.76 | |
| LTA Pb | 0.17 | 25 | 0.68 |
| Metal(loid)s in HTAs and LTAs | Daily Intake (μg) | RDI or RDA and UL (μg/day) | % below RDI or RDA and UL |
|---|---|---|---|
| HTA Mo | 19.85 | RDA: 45; UL: 2000 | 44.1; 0.99 |
| LTA Mo | 0.4 | 0.88; 0.02 | |
| HTA Se | 1.10 | RDI: 55; UL: 400 | 2.0; 0.3 |
| LTA Se | 0.61 | 1.11; 0.15 | |
| HTA V | 0.34 | UL: 1800 | 0.02 |
| LTA V | 0.22 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel-Nakamura, C.; Hodge, F.S. Occurrence and Risk of Metal(loid)s in Thelesperma megapotamicum Tea Plant. Plants 2020, 9, 21. https://doi.org/10.3390/plants9010021
Samuel-Nakamura C, Hodge FS. Occurrence and Risk of Metal(loid)s in Thelesperma megapotamicum Tea Plant. Plants. 2020; 9(1):21. https://doi.org/10.3390/plants9010021
Chicago/Turabian StyleSamuel-Nakamura, Christine, and Felicia S. Hodge. 2020. "Occurrence and Risk of Metal(loid)s in Thelesperma megapotamicum Tea Plant" Plants 9, no. 1: 21. https://doi.org/10.3390/plants9010021
APA StyleSamuel-Nakamura, C., & Hodge, F. S. (2020). Occurrence and Risk of Metal(loid)s in Thelesperma megapotamicum Tea Plant. Plants, 9(1), 21. https://doi.org/10.3390/plants9010021




