Ammonium Sulphate from a Bio-Refinery System as a Fertilizer—Agronomic and Economic Effectiveness on the Farm Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Ammonium Sulphate (Bio-AS)
2.2. Pot Experiment
2.3. Analytical Procedures
2.4. Indicator Calculation
2.5. Statistical Analysis
2.6. Analysis of Economic Performance
3. Results
3.1. Characteristic of Bio-AS from the Bio-Refinery
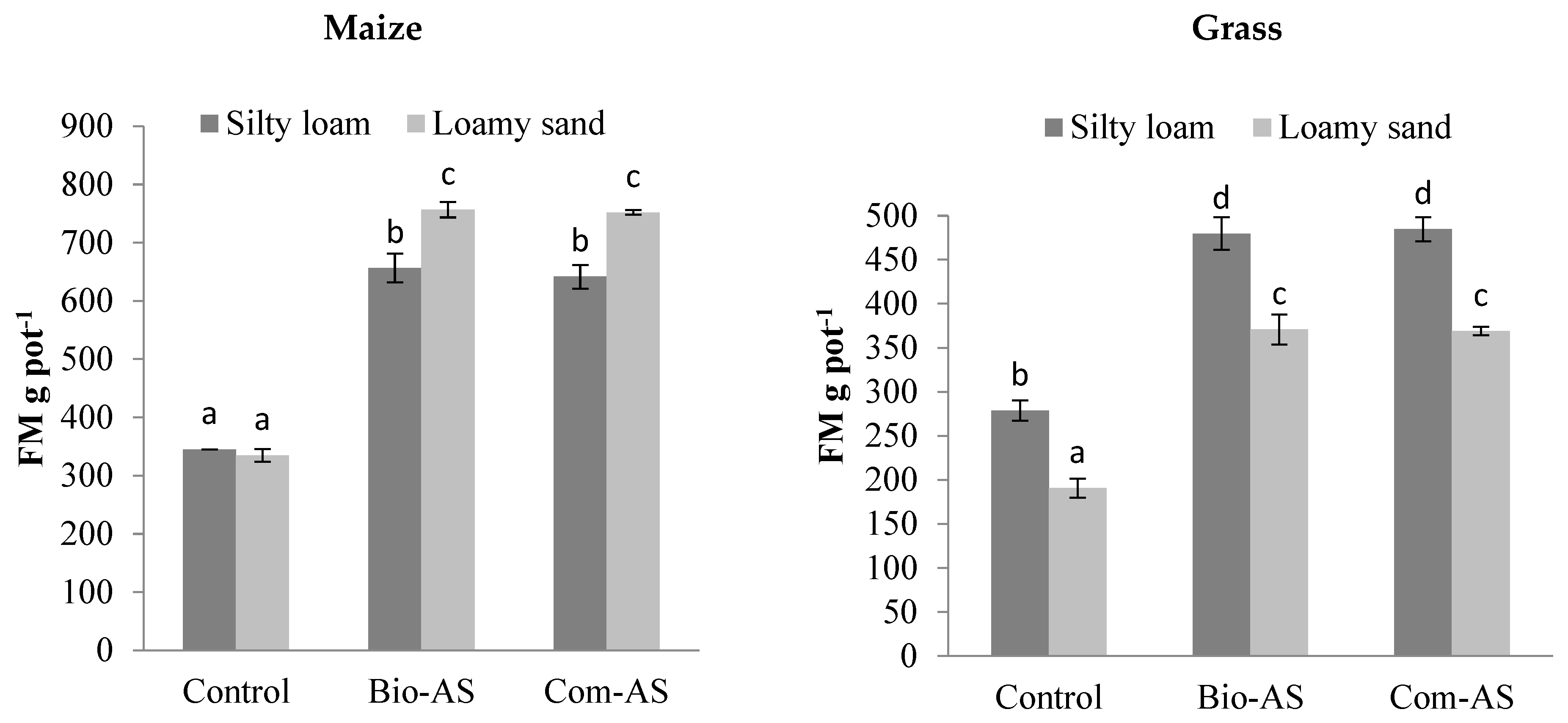
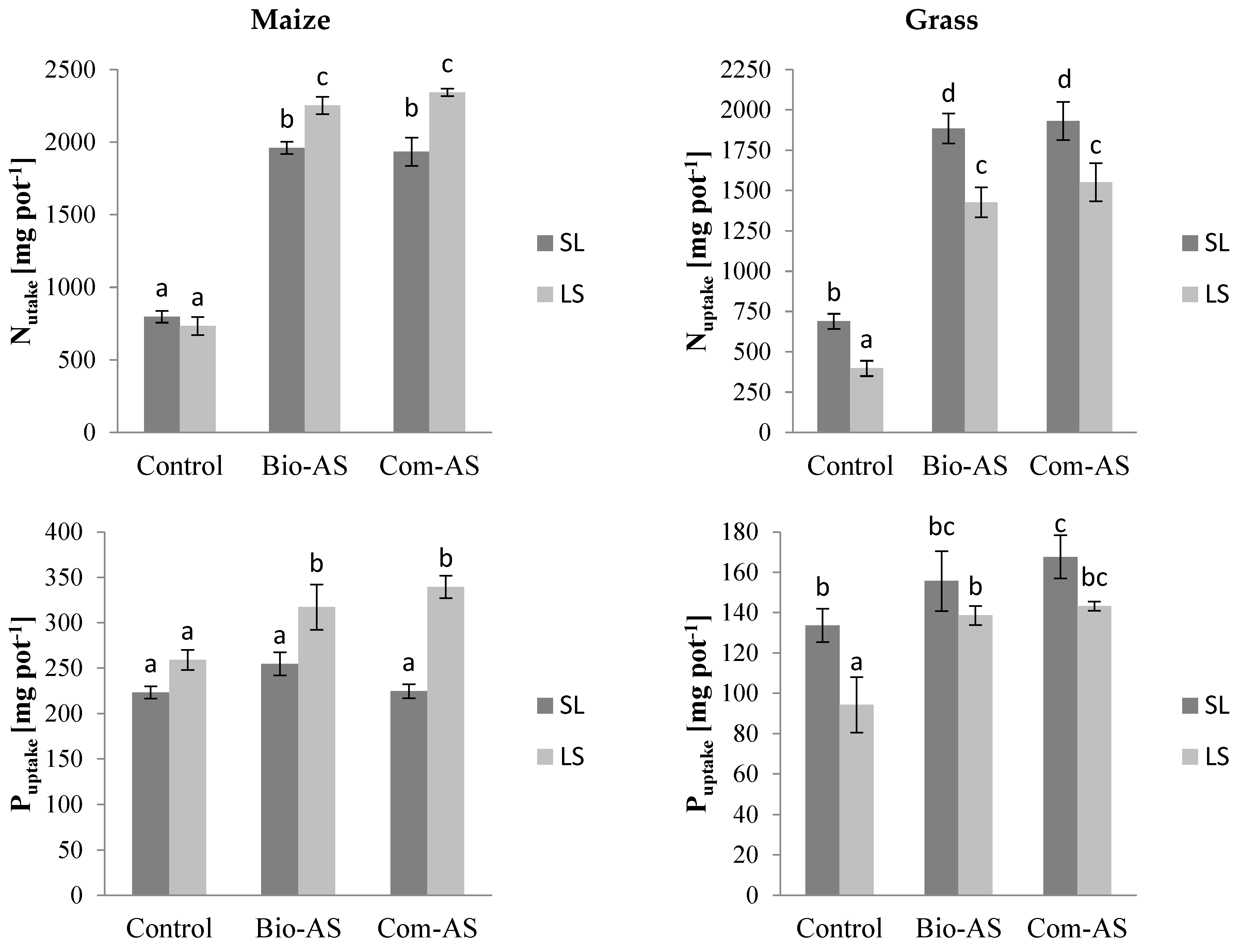
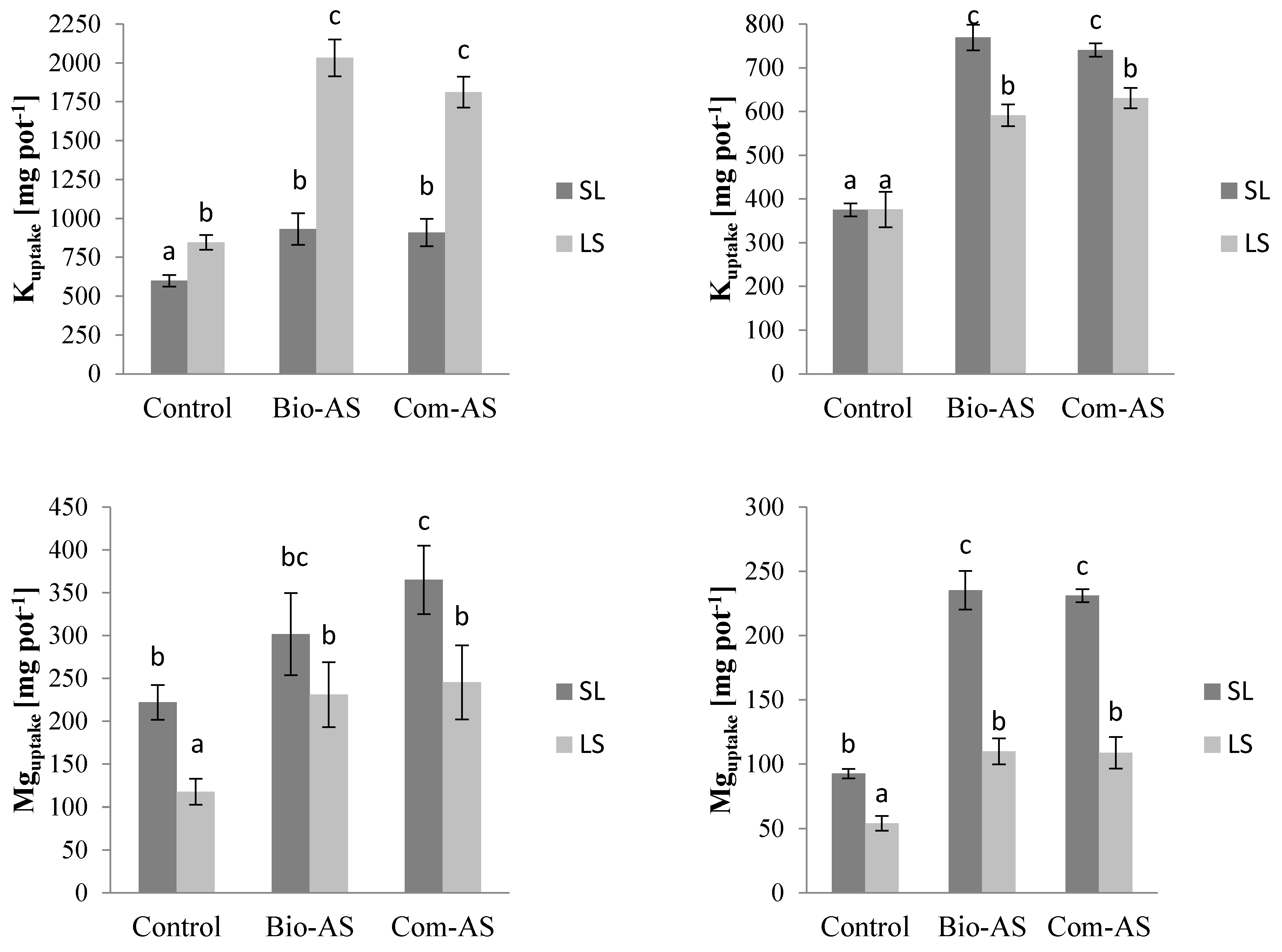
3.2. Agronomic Value of Bio-AS from the Bio-Refinery—Crop Characteristics
3.3. Agronomic Value of Bio-AS from the Bio-Refinery—Soil Characteristics
3.4. Economic Performance of Installation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Dry matter weight |
| FM | Fresh matter weight |
| Bio-AS | Ammonium sulphate from bio-refinery |
| Com-AS | Ammonium sulphate – commercial mineral fertilizer |
| LS | Soil—loamy sand |
| SL | Soil—silty loam |
| ANR | The apparent fertilizer N recovery |
| REF | The related fertilizer efficiency |
| NUE | The nitrogen use efficiency |
| PCaCl2, KCaCl2, Mg CaCl2 | Active forms of phosphorus, potassium, and magnesium in soil |
| PM3, KM3, MgM3 | Available forms of phosphorus, potassium, and magnesium in soil |
| UAN | Urea ammonium nitrate solution |
| NPV | Net present value |
| IRR | Internal return rate |
References
- Sonnenberg, A.; Baars, J.; Hendrickx, P. IEA Bioenergy Task 42 Biorefinery; Avantium, Biomass Research and Wageningen University and Research Centre: Wageningen, The Netherlands, 2007. [Google Scholar]
- Hagman, L.; Blumenthal, A.; Eklund, M.; Svensson, N. The role of biogas solutions in sustainable biorefineries. J. Clean Prod. 2018, 172, 3982–3989. [Google Scholar] [CrossRef]
- Moncada, J.; Aristizábal, V.; Cardona, C.A. Design strategies for sustainable biorefineries. Biochem. Eng. J. 2016, 116, 122–134. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, A.S.; Waqas, M.; Naqvi, M.; Ouda, M.; Shahzad, O.K.M.; Miandad, K.; Khan, R.; Syamsiro, M.Z.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Sauvee, L.; Viaggi, D. Biorefineries in the bio-based economy: Opportunities and challenges for economic research. Bio-Based Appl. Econ. 2016, 5, 1–4. [Google Scholar]
- Sosulski, T.; Szara, E.; Stępień, W.; Szymańska, M.; Borowska-Komenda, M. Carbon and nitrogen leaching in long-term experiments and DOC/N-NO3—Ratio in drainage water as an indicator of denitrification potential in different fertilization and crop rotation system. Fresenius Environ. Bull. 2016, 25, 2813–2824. [Google Scholar]
- Sosulski, T.; Szara, E.; Szymańska, M.; Stępień, W. N2O emission and nitrogen and carbon leaching from the soil in relation to long-term and current mineral and organic fertilization—A laboratory study. Plant Soil. Environ. 2017, 63, 97–104. [Google Scholar]
- Parker, D.B.; Gillyy, J.E.; Woodbury, B.; Kim, K.H.; Bartelt-Hunt, S.; Li, X.; Snow, D.; Galvin, G. Odours VOC emission following land application of swine manure slurry. Atmos. Environ. 2013, 66, 91–100. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Stępień, W.; Szymańska, M. Impact of liming management on N2O emissions from arable soils in three long-term fertilization experiments in Central Poland. Fresenius Environ. Bull. 2016, 25, 6111–6119. [Google Scholar]
- Council Directive of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). Available online: http://data.europa.eu/eli/dir/1991/676/2008-12-11 (accessed on 15 October 2019).
- Smith, H. Dutch Manure Policy; Ministry of Economic Affairs: Hague, The Netherlands, 2013. [Google Scholar]
- Einarsson, R.; Cederberg, C.; Kallus, J. Nitrogen flows on organic and conventional dairy farms: A comparison of three indicators. Nutr. Cycl. Agroecosyst. 2018, 110, 25–38. [Google Scholar] [CrossRef]
- Szymańska, M.; Nowaczewska, D.; Świerżewska, E.; Wrzosek-Jakubowska, J.; Gworek, B. An attempt to assess physicochemical properties of soil fertilized with fresh and treated digestate from biogas plant. Przem. Chem. 2016, 95, 572–576. [Google Scholar]
- Orzi, V.; Scaglia, B.; Lonati, S.; Riva, C.; Boccasile, G.; Alborali, G.L.; Adani, F. The role of biological processes in reducing both odour impact and pathogen content during mesophilic anaerobic digestion. Sci. Total Environ. 2015, 526, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.; Orzi, V.; Carozzi, M.; Acutis, M.; Boccasile, G.; Lonati, S.; Tambone, F.; D’Imporzano, G.; Adani, F. Short-term experiments in using digestate products as substitutes for mineral (N) fertilizer: Agronomic performance, odours, and ammonia emission impacts. Sci. Total Environ. 2016, 547, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Orzi, V.; Riva, C.; Scaglia, B.; D’Imporzano, G.; Tambone, F.; Adanbi, F. Anaerobic digestion coupled with digestate injection reduced odour emissions from soil during manure distribution. Sci. Total Environ. 2018, 621, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, M.; Szara, E.; Sosulski, T.; Stepień, W.; Pilarski, K.; Pilarska, A.A. Chemical properties and fertilizer value of ten different anaerobic digestates. Fresenius Environ. Bull. 2018, 27, 3425–3432. [Google Scholar]
- Deng, L.W.; Zheng, P.; Chen, Z.A. Anaerobic digestion and post-treatment of swine wastewater using IC–SBR process with bypass of raw wastewater. Process Biochem. 2006, 41, 965–969. [Google Scholar] [CrossRef]
- Jia, G.; Zhang, H.; Krampe, J.; Muster, T.; Gao, B.; Zhu, N. Applying a chemical equilibrium model for optimizing struvite precipitation for ammonium recovery from anaerobic digester effluent. J. Clean. Prod. 2017, 147, 297–305. [Google Scholar] [CrossRef]
- Mondor, M.; Masse, L.; Ippersiel, D.; Lamarche, F.; Masse, D.I. Use of electrodialysis and reverse osmosis for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2009, 99, 7363–7368. [Google Scholar] [CrossRef]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmati, A. Influence of pig slurry characteristics on ammonia stripping efficiencies and quality of the recovered ammonium-sulfate solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Wąs, A.; Sosulski, T.; van Pruissen, G.W.; Cornelissen, R.L. Struvite—An Innovative Fertilizer from Anaerobic Digestate Produced in a Bio-Refinery. Energies 2019, 12, 296. [Google Scholar] [CrossRef]
- Di Laconi, C.; Pagano, M.; Ramadori, R.; Lopes, A. Nitrogen recovery from a stabilized municipal landfill leachate. Bioresour. Technol. 2010, 101, 1732–1736. [Google Scholar] [CrossRef]
- Huang, H.; Xu, C.; Zhang, W. Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour. Technol. 2011, 102, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Lahav, O.; Telzhensky, M.; Zewuhn, A.; Gendel, Y.; Gerth, J.; Calmano, W.; Birnhack, L. Struvite recovery from municipal-wastewater sludge centrifuge supernatant using seawater NF concentrate as a cheap Mg(II) source. Sep. Purif. Technol. 2013, 108, 10–110. [Google Scholar] [CrossRef]
- Lee, S.I.; Weon, S.Y.; Lee, C.W.; Koopman, B. Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Gunay, A.; Karadag, D.; Tosun, I.; Ozturk, M. Use of magnesit as a magnesium source for ammonium removal from leachate. J. Hazard. Mater. 2008, 156, 619–623. [Google Scholar] [CrossRef]
- Escudero, A.; Blanco, F.; Lacalle, A.; Pinto, M. Struvite precipitation for ammonium removal from anaerobically treated effluents. J. Environ. Chem. Eng. 2015, 3, 413–419. [Google Scholar] [CrossRef]
- Ryu, H.D.; Kim, D.; Lee, S.I. Application of struvite precipitation in treating ammonium nitrogen from semiconductor wastewater. J. Hazard. Mater. 2008, 156, 163–169. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from Raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Szulc, W.; Rutkowska, B.; Sosulski, T.; Szara, E.; Stępień, W. Assessment of sulphur demand of crops under permanent fertilization experiment. Plant Soil Environ. 2014, 60, 135–140. [Google Scholar] [CrossRef]
- Jamal, A.; Moon, Y.S.; Abdin, M.Z. Sulphur—A general overview and interaction with nitrogen. Aust. J. Crop Sci. 2010, 4, 523–529. [Google Scholar]
- Törnwall, E.; Pettersson, H.; Thorin, E.; Schwede, S. Post-treatment of biogas digestate—An evaluation of ammonium recovery, energy use and sanitation. Energy Procedia. 2017, 142, 957–963. [Google Scholar] [CrossRef]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S.W. Sustainable Sewage Sludge Management: From Current Practices to Emerging Nutrient Recovery Technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef]
- Schröder, J.J.; Uenk, D.; Hilhorst, G.J. Long-term nitrogen fertilizer replacement value of cattle manures applied to cut grassland. Plant Soil. 2007, 299, 83–99. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Sosulski, T.; Wąs, A.; van Pruissen, G.W.P.; Cornelissen, R.L.; Borowik, M.; Konkol, M. A Bio-Refinery Concept for N and P Recovery—A Chance for Biogas Plant Development. Energies 2019, 12, 155. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Mangi, C.A. Investment decisions, net present value and bounded rationality. Quant. Financ. 2009, 9, 967–979. [Google Scholar]
- Thomas, D.S. Investment Analysis Methods. A practitioner’s guide to understanding the basic principles for investment decisions in manufacturing. In NIST Advanced Manufacturing Series 200-5; National Institute of Standards and Technology US: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Cramer, M.D.; Lewis, O.A.M. The influence of Nitrate and Ammonium Nutrition on the Growth of Wheat (Triticum aestivum) and Maize (Zea mays) Plants. Ann. Bot. 1993, 72, 359–365. [Google Scholar] [CrossRef]
- Sogn, T.A.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.H. Recycling of biogas digestates in plant production: NPK fertilizer value and risk of leaching. Int. J. Recycl. Org. Waste. Agric. 2018, 7, 49–59. [Google Scholar] [CrossRef]
- Kandil, A.-H.T.; Cheira, M.F.; Gado, H.S.; Soliman, M.H.; Akl, H.M. Ammonium sulfate preparation from phosphogypsum waste. J. Radiat. Res. Appl. Sci. 2017, 10, 24–33. [Google Scholar] [CrossRef]
- Szara, E.; Sosulski, T.; Szymańska, M.; Szyszkowska, K. Usefulness of Mehlich-3 test in the monitoring of phosphorus dispersion from Polish arable soils. Environ. Monit. Assess. 2018, 190, 298. [Google Scholar] [CrossRef]
- Wąs, A.; Sulewski, P.; Szymańska, M. Biorafinerie Rolnicze Jako Element Trwałej Biogospodarki; SGGW: Warsaw, Poland, 2019; p. 154. [Google Scholar]
- Ferreira, A. Biorafinery concept. In Biorafineries: Targeting Energy, High Value Products and Waste Valorisation (Red.); Rabaçal, M., Ferreira, A.F., Silva, C.A.M., Cost, M., Eds.; Springer Publishing AG: Basel, Switzerland, 2017. [Google Scholar]
- Peters, J.F.; Petrakopoulou, F.; Dufour, J. Exergy analysis of synthetic biofuel production via fast pyrolysis and hydroupgrading. Energy 2015, 79, 325–336. [Google Scholar] [CrossRef]
| Soil | pH | N total (g·kg−1) | Active Forms of Nutrients (mg·kg−1) | Available Forms of Nutrients (mg·kg−1) | ||||
|---|---|---|---|---|---|---|---|---|
| PCaCl2 | K CaCl2 | Mg CaCl2 | P M3 | K M3 | Mg M3 | |||
| Silty loam (SL) | 6.3 | 2.6 | 14.6 | 27.7 | 187.9 | 87.2 | 87.3 | 306.2 |
| Loamy sand (LS) | 6.3 | 0.8 | 19.1 | 45.9 | 20.0 | 95.7 | 97.5 | 65.0 |
| Fertilizer | Total N Content (TKN; %) | ST (%) | Pure (NH4)2SO4 (%) | Free H2SO4 (%) | pH | KT (%) |
|---|---|---|---|---|---|---|
| Bio-AS | 1.41 | 1.68 | 6.6 | 0.22 | 2.1 | - |
| Com-AS a | 20.8 | 24.0 | 98.1 | max 0.1 | - | - |
| KCl a | - | - | - | - | - | 50.0 |
| Soil | Fertilization | ANR (%) | NUE (g DM g N−1) | REF (%) | |||
|---|---|---|---|---|---|---|---|
| Maize | Grass | Maize | Grass | Maize | Grass | ||
| SL | Bio-AS | 58.2 | 59.8 | 17.9 | 10.4 | 102.4 | 96.3 |
| Com-AS | 56.8 | 62.1 | 10.5 | 11.5 | 100.0 | 100.0 | |
| LS | Bio-AS | 75.9 | 51.5 | 29.0 | 9.6 | 94.3 | 89.2 |
| Com-AS | 80.5 | 57.7 | 27.6 | 9.0 | 100.0 | 100.0 | |
| Soil | Fertilization | Maize | Grass | Maize | Grass | ||||
| I Cut | II Cut | III Cut | I Cut | II Cut | III Cut | ||||
| N·g·kg·DM−1 | P·g·kg·DM−1 | ||||||||
| SL | Control | 9.4 a | 24.1 b | 23.8 a | 17.3 bc | 2.6 c | 3.6 ab | 5.6 b | 3.6 ns |
| Bio-AS | 16.3 b | 39.6 c | 41.9 b | 19.2 cd | 2.1 a | 2.6 a | 3.6 a | 2.8 ns | |
| Com-AS | 18.3 c | 39.1 c | 40.3 b | 20.2 d | 2.1 a | 2.7 a | 4.1 a | 2.5 ns | |
| LS | Control | 8.5 a | 19.4 a | 20.7 a | 15.5 ab | 3.0 d | 4.4 b | 5.8 b | 2.8 ns |
| Bio-AS | 15.6 b | 43.6 d | 39.6 b | 13.0 a | 2.2 ab | 3.6 ab | 3.7 a | 2.8 ns | |
| Com-AS | 16.6 b | 45.1 d | 47.4 c | 17.8 bcd | 2.4 b | 3.9 ab | 3.8 a | 3.1 ns | |
| Soil a | *** | ** | ** | *** | *** | ** | ns | ns | |
| Fertilizer | *** | *** | *** | *** | *** | * | *** | ns | |
| Soil × Fertilizer | ns | *** | *** | ** | * | ns | ns | ns | |
| K·g·kg·DM−1 | Mg·g·kg·DM−1 | ||||||||
| SL | Control | 7.0 a | 12.2 a | 12.5 bc | 12.1 a | 2.6 b | 2.6 b | 3.2 a | 3.7 d |
| Bio-AS | 7.7 a | 13.3 ab | 14.0 c | 21.5 c | 2.5 b | 4.1 c | 6.0 b | 3.1 c | |
| Com-AS | 8.6 ab | 14.0 b | 10.2 ab | 20.1 bc | 3.4 c | 3.7 c | 5.6 b | 3.5 d | |
| LS | Control | 9.8 b | 16.7 c | 11.4 abc | 28.4 d | 1.4 a | 2.0 a | 2.9 a | 3.0 bc |
| Bio-AS | 14.1 c | 15.8 c | 9.2 a | 20.0 bc | 1.6 a | 2.2 ab | 3.7 a | 2.3 a | |
| Com-AS | 12.8 c | 17.1 c | 14.0 c | 18.7 b | 1.7 a | 2.3 ab | 3.2 a | 2.6 ab | |
| Soil a | *** | *** | ** | ** | *** | *** | *** | *** | |
| Fertilizer | *** | * | * | * | ** | *** | *** | *** | |
| Soil × Fertilizer | ** | * | *** | *** | ns | *** | *** | ns | |
| Soil | Fertilization | pHKCl | TKN(g·kg−1) | PCaCl2 | PM3 | KCaCl2 | KM3 | MgCaCl2 | MgM3 |
| (mg·kg−1) | |||||||||
| After Maize Harvesting | |||||||||
| Silty loam SL | Control | 6.1 b | 2.7 ns | 10.9 c | 86.7 b | 25.2 b | 78.6 b | 130.5 ns | 351.0 b |
| Bio-AS | 5.4 a | 2.8 ns | 2.4 a | 80.0 a | 17.8 a | 66.1 a | 128.4 ns | 246.4 a | |
| Com-AS | 5.3 a | 2.7 ns | 3.8 b | 82.3 a | 18.2 a | 64.4 a | 129.2 ns | 252.6 a | |
| Fertilizer | ** | ns | *** | ** | *** | *** | ns | *** | |
| Loamy sand LS | Control | 6.1 b | 0.7 ns | 14.7 c | 84.1 b | 50.5 b | 94.6 b | 20.3 b | 63.5 b |
| Bio-AS | 5.1 a | 0.8 ns | 1.7 a | 73.3 a | 26.0 a | 64.4 a | 15.7 a | 48.6 a | |
| Com-AS | 5.2 a | 0.8 ns | 3.2 b | 77.5 a | 25.7 a | 67.3 a | 15.3 a | 46.7 a | |
| Fertilizer | *** | ns | *** | *** | *** | *** | ** | *** | |
| After Grass Harvesting | |||||||||
| Silty loam SL | Control | 6.4 b | 2.6 ns | 11.8 b | 81.2 b | 26.0 b | 84.6 ns | 133.0 ns | 312.6 b |
| Bio-AS | 5.4 a | 2.7 ns | 3.3 a | 78.4 a | 21.7 a | 82.0 ns | 122.3 ns | 245.6 a | |
| Com-AS | 5.3 a | 2.7 ns | 3.5 a | 78.6 a | 23.0 ab | 80.1 ns | 127.5 ns | 254.3 a | |
| Fertilizer | ** | ns | *** | * | * | ns | ns | ** | |
| Loamy sand LS | Control | 6.1 b | 0.8 ns | 15.0 c | 90.0 b | 48.1 b | 96.4 b | 19.8 b | 57.8 b |
| Bio-AS | 5.1 a | 0.8 ns | 2.2 a | 74.0 a | 20.2 a | 76.8 a | 14.8 a | 35.3 a | |
| Com-AS | 5.2 a | 0.8 ns | 3.5 b | 76.1 a | 25.0 a | 72.7 a | 15.0 a | 36.3 a | |
| Fertilizer | *** | ns | *** | *** | ** | *** | *** | *** | |
| Specification | Country | |||
|---|---|---|---|---|
| Poland | The Netherlands | United Kingdom | ||
| Revenues (EUR farm−1) | Electricity | 30,715 | 30,341 | 41,348 |
| Sales of ammonium sulphate solution (1.4% N) | 6700 | 9200 | 7258 | |
| Avoided expenditures on utilization of manure excess | 33,995 | |||
| Savings due to using duckweed as a feed for animals (based on value of saved feed). | 11,314 | 1632 | 11,314 | |
| Total (EUR farm−1) | 48,729 | 75,168 | 59,920 | |
| Operational costs (EUR farm−1) | Materials (active carbon, sulphur acid, natrium hydroxide, water) | 6618 | 6618 | 6618 |
| labour costs | 4550 | 9100 | 9100 | |
| insurances | 2215 | 2606 | 2606 | |
| Equipment maintenance cost (4% value—digester, CHP, stripping unit facilities) | 17,721 | 20,848 | 20,848 | |
| Financial costs (interest on loans) | 11,296 | 2870 | 4417 | |
| Total | 42,400 | 42,042 | 43,589 | |
| Annual operational income (EUR farm−1) | 6329 | 33,126 | 16,331 | |
| Specification | Country | ||
|---|---|---|---|
| Poland | The Netherlands | United Kingdom | |
| Total investments costs (EUR) | 443,023 | 521,203 | 521,203 |
| Public support (investment grant) (EUR) | 69,767 | 331,034 | 208,481 |
| NPV (EUR) | −291,238 | 230,862 | −100,865 |
| IRR (%) | −14% | 15.4% | −3% |
| Simple payback period (year) | 58.98 | 5.74 | 19.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, M.; Sosulski, T.; Szara, E.; Wąs, A.; Sulewski, P.; van Pruissen, G.W.P.; Cornelissen, R.L. Ammonium Sulphate from a Bio-Refinery System as a Fertilizer—Agronomic and Economic Effectiveness on the Farm Scale. Energies 2019, 12, 4721. https://doi.org/10.3390/en12244721
Szymańska M, Sosulski T, Szara E, Wąs A, Sulewski P, van Pruissen GWP, Cornelissen RL. Ammonium Sulphate from a Bio-Refinery System as a Fertilizer—Agronomic and Economic Effectiveness on the Farm Scale. Energies. 2019; 12(24):4721. https://doi.org/10.3390/en12244721
Chicago/Turabian StyleSzymańska, Magdalena, Tomasz Sosulski, Ewa Szara, Adam Wąs, Piotr Sulewski, Gijs W.P. van Pruissen, and René L. Cornelissen. 2019. "Ammonium Sulphate from a Bio-Refinery System as a Fertilizer—Agronomic and Economic Effectiveness on the Farm Scale" Energies 12, no. 24: 4721. https://doi.org/10.3390/en12244721
APA StyleSzymańska, M., Sosulski, T., Szara, E., Wąs, A., Sulewski, P., van Pruissen, G. W. P., & Cornelissen, R. L. (2019). Ammonium Sulphate from a Bio-Refinery System as a Fertilizer—Agronomic and Economic Effectiveness on the Farm Scale. Energies, 12(24), 4721. https://doi.org/10.3390/en12244721






