Influence of Saccharomyces and non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making
Abstract
1. Color and Wine Freshness
2. Anthocyanins and Pyranoanthocyanins: Vitisins and Vinylphenolics Adducts
3. Vitisins
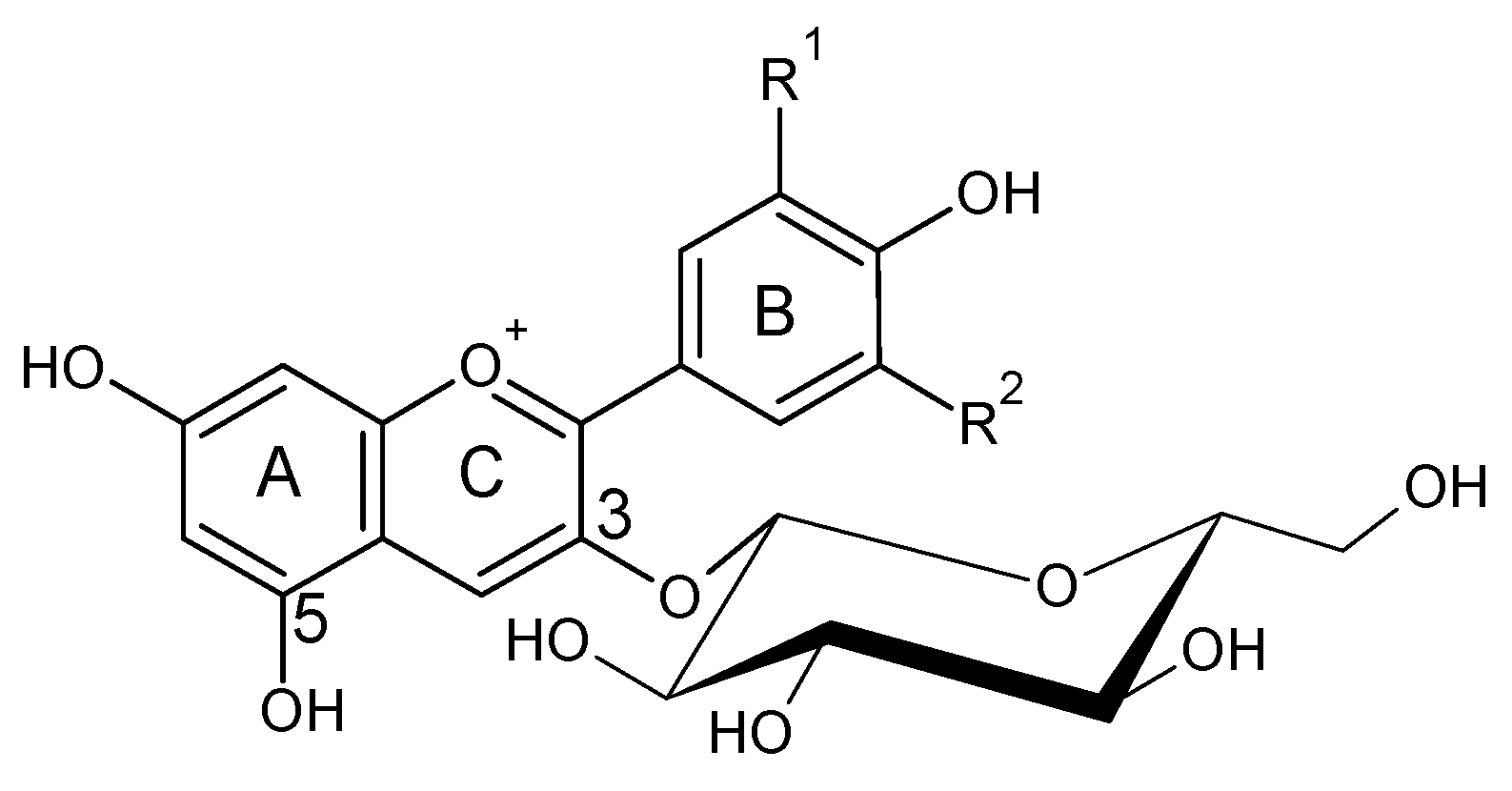
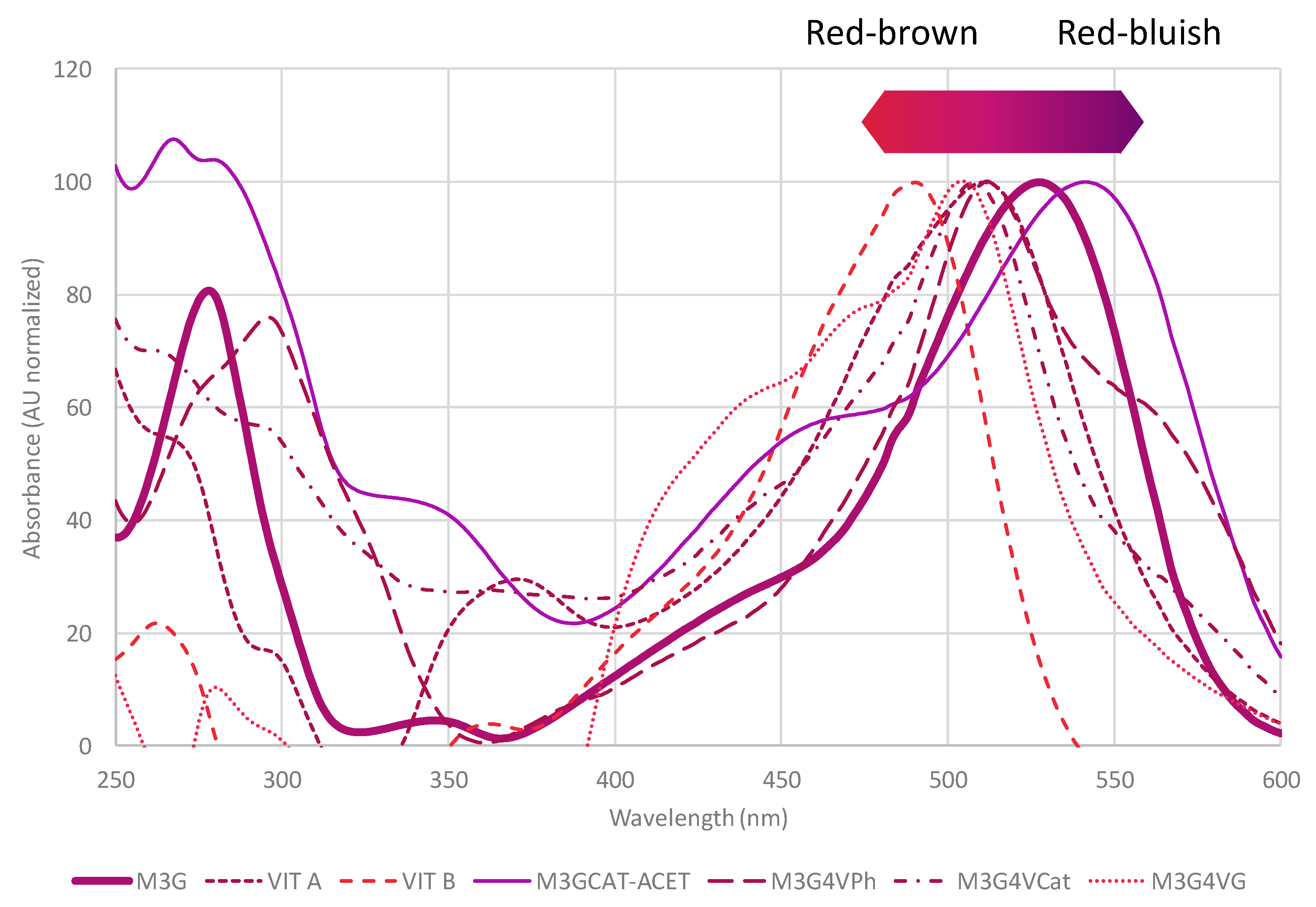
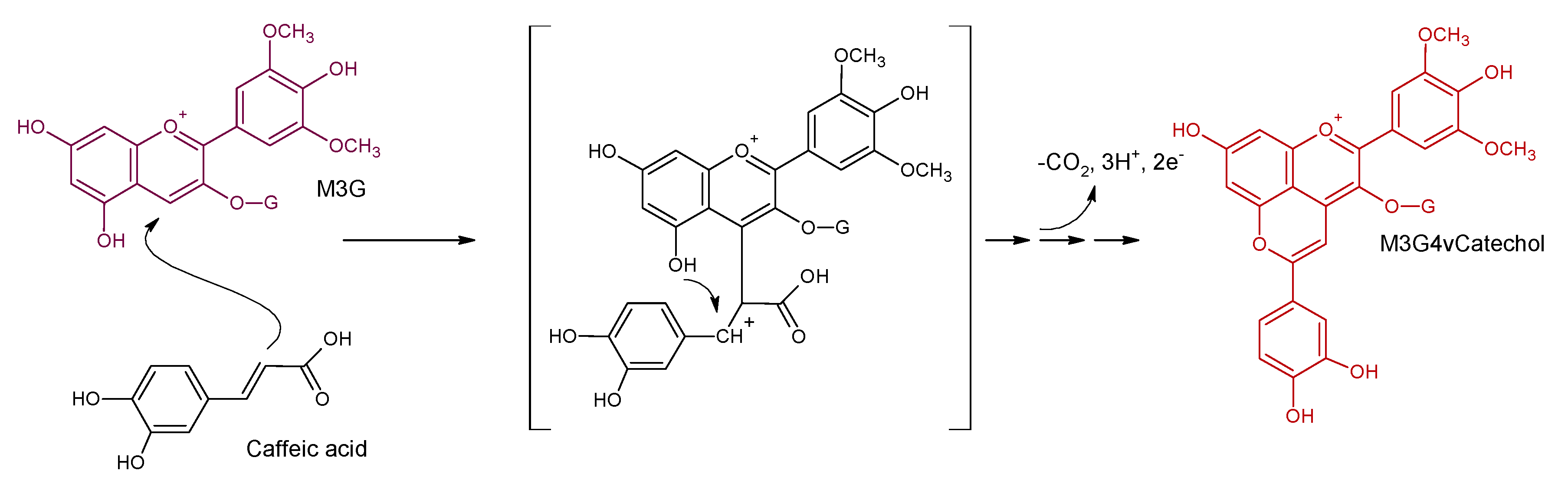
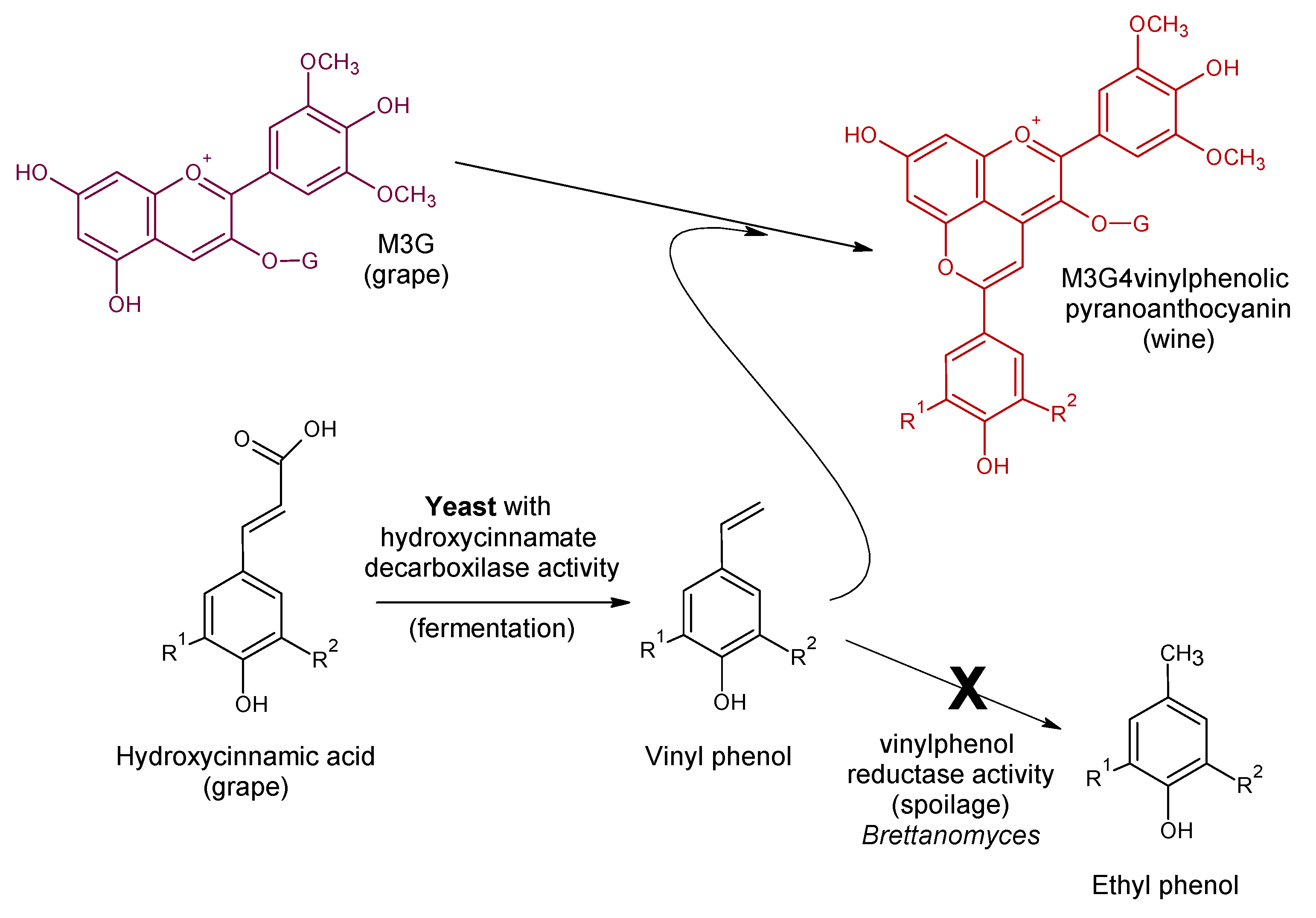
4. Vinylphenolic Pyranoanthocyanins
5. Polymeric Pigments: Catechin Derivatives, Derivates by Acetaldehyde Bridge and Procyanidin Derivatives
6. Anthocyanin Adsorption in Yeast Cell Walls
7. Stable Pyranoanthocyanins and Ageing on Lees
8. Emerging Techniques to Better Implant Non-Saccharomyces Yeasts during Fermentation
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Morata, A.; Loira, I.; Manuel del Fresno, J.; Escott, C.; Antonia Bañuelos, M.; Tesfaye, W.; González, C.; Palomero, F.; Antonio Suárez Lepe, J. Strategies to improve the freshness in wines from warm areas. In Advances in Grape and Wine Biotechnology; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar]
- Morrot, G.; Brochet, F.; Dubourdieu, D. The color of odors. Brain Lang. 2001, 79, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Suárez Lepe, J.A. Influence of yeasts in wine colour. Grape Wine Biotechnol. 2016, 4, 53. [Google Scholar]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.; González, C.; Suárez Lepe, J. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea thermotolerans as a tool to improve pH in red wines from warm regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; Freitas, V. de A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric. Food Chem. 2003, 51, 1919–1923. [Google Scholar] [CrossRef]
- Mateus, N.; Oliveira, J.; Haettich-Motta, M.; de Freitas, V. New family of bluish pyranoanthocyanins. J. Biomed. Biotechnol. 2004, 2004, 299–305. [Google Scholar] [CrossRef]
- Escott, C.; Morata, A.; Loira, I.; Tesfaye, W.; Suarez-Lepe, J.A. Characterization of polymeric pigments and pyranoanthocyanins formed in microfermentations of non- Saccharomyces yeasts. J. Appl. Microbiol. 2016, 121, 1346–1356. [Google Scholar] [CrossRef]
- Vasserot, Y.; Caillet, S.; Maujean, A. Study of anthocyanin adsorption by yeast lees. Effect of some physicochemical parameters. Am. J. Enol. Vitic. 1997, 48, 433–437. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Salmon, J.-M. Interactions between yeast, oxygen and polyphenols during alcoholic fermentations: Practical implications. LWT - Food Sci. Technol. 2006, 39, 959–965. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Morata, A.; Zamora, F.; Loira, I.; del Fresno, J.M.; Suárez-Lepe, J.A. Study of the interaction of anthocyanins with phenolic aldehydes in a model wine solution. ACS Omega 2018, 3, 15575–15581. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef] [PubMed]
- Bridle, P.; Timberlake, C.F. Anthocyanins as natural food colours—selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; García-Estévez, I. Wine color evolution and stability. In Red Wine Technology; Academic Press: Cambridge, MA, USA, 2019; pp. 195–205. ISBN 9780128143995. [Google Scholar]
- Morata, A.; López, C.; Tesfaye, W.; González, C.; Escott, C. Anthocyanins as natural pigments in beverages. In Value-Added Ingredients and Enrichments of Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 383–428. ISBN 9780128166871. [Google Scholar]
- Santos-Buelga, C.; González-Paramás, A.M. Anthocyanins. In Encyclopedia of Food Chemistry; Academic Press: Cambridge, MA, USA, 2019; pp. 10–21. ISBN 9780128140451. [Google Scholar]
- Mazza, G. Anthocyanins in fruits, vegetables, and grains; Mazza, G., Miniati, E., Eds.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351069700. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2001, 18, 310–333. [Google Scholar] [CrossRef]
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Int. 2004, 87, 129–145. [Google Scholar]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: a new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Heier, A.; Blaas, W.; Droß, A.; Wittkowski, R. Anthocyanin Analysis by HPLC/ESI-MS. Am. J. Enol. Vitic. 2002, 53. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef]
- Morata, A.; Benito, S.; Loira, I.; Palomero, F.; González, M.C.; Suárez-Lepe, J.A. Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. Int. J. Food Microbiol. 2012, 159, 47–53. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Asenstorfer, R.E. Screening for potential pigments derived from anthocyanins in red wine using nanoelectrospray tandem mass spectrometry. J. Agric. Food Chem. 2002, 50, 756–761. [Google Scholar] [CrossRef]
- Schwarz, M.; Wabnitz, T.C.; Winterhalter, P. Pathway leading to the formation of anthocyanin−vinylphenol adducts and related pigments in red wines. J. Agric. Food Chem. 2003, 51, 3682–3687. [Google Scholar] [CrossRef]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef]
- Cameira-dos-Santos, P.-J.; Brillouet, J.-M.; Cheynier, V.; Moutounet, M. Detection and partial characterisation of new anthocyanin-derived pigments in wine. J. Sci. Food Agric. 1996, 70, 204–208. [Google Scholar] [CrossRef]
- Fulcrand, H.; Cameira Dos Santos, P.J.; Sarni-Manchado, P.; Cheynier, V.; Favre-Bonvin, J. Structure of new anthocyanin-derived wine pigments. J. Chem. Soc.-Perkin Trans. 1 1996, 735–739. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Separation of pyranoanthocyanins from red wine by column chromatography. Proc. Anal. Chim. Acta 2004, 513, 305–318. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. In Proceedings of the Analytica Chimica Acta, Amsterdam, The Netherlands, 23 March 2006; Volume 563, pp. 238–254. [Google Scholar]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Francia-Aricha, E.; Rivas-Gonzalo, J.C. Formation of anthocyanin-derived pigments in experimental red wines. Food Sci. Technol. Int. 1999, 5, 347–352. [Google Scholar] [CrossRef]
- Benabdeljalil, C.; Cheynier, V.; Fulcrand, H.; Hafiki, A.; Mosaddak, M.; Moutounet, M. Mise en évidence de nouveaux pigments formés par réaction des anthocyanes avec des métabolites de levure. Sci. Aliments 2000, 20, 203–220. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Vercauteren, J.; de Freitas, V. Occurrence of anthocyanin-derived pigments in red wines. J. Agric. Food Chem. 2001, 49, 4836–4840. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; de Freitas, V. Evolution and stability of anthocyanin-derived pigments during Port wine aging. J. Agric. Food Chem. 2001, 49, 5217–5222. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Calderón, F.; González, M.C.; Gómez-Cordovés, M.C.; Suárez, J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.G.; Jones, G.P. Formation of vitisin A during red wine fermentation and maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Chinnici, F.; Sonni, F.; Natali, N.; Galassi, S.; Riponi, C. Colour features and pigment composition of Italian carbonic macerated red wines. Food Chem. 2009, 113, 651–657. [Google Scholar] [CrossRef]
- Jackowetz, J.N.; Dierschke, S.; Mira de Orduña, R. Multifactorial analysis of acetaldehyde kinetics during alcoholic fermentation by Saccharomyces cerevisiae. Food Res. Int. 2011, 44, 310–316. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Morata, A. El atractivo visual del color del vino tinto: Implicaciones microbiológicas en nuevas formas estables y pérdidas de antocianos durante la fermentación. In Levaduras para vinificacioón en tinto (Yeasts for red winemaking); AMV Ediciones: Madrid, Spain, 2015; p. 156. ISBN 9788494345197. [Google Scholar]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J. Schizosaccharomyces pombe: A promising biotechnology to modulate wine composition. Fermentation 2018, 4, 70. [Google Scholar] [CrossRef]
- Ciani, M. Continuous deacidification of wine by immobilized Schizosaccharomyces pombe cells: Evaluation of malic acid degradation rate and analytical profiles. J. Appl. Bacteriol. 1995, 79, 631–634. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem. 2018, 266, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; González Chamorro, C.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- Escott, C.; Del Fresno, J.M.; Loira, I.; Morata, A.; Tesfaye, W.; del Carmen González, M.; Suárez-Lepe, J.A. Formation of polymeric pigments in red wines through sequential fermentation of flavanol-enriched musts with non-Saccharomyces yeasts. Food Chem. 2018, 239, 975–983. [Google Scholar] [CrossRef]
- Oelofse, A.; Pretorius, I.S.; du Toit, M. Significance of Brettanomyces and Dekkera during winemaking: A synoptic review. South African J. Enol. Vitic. 2008, 29, 128–144. [Google Scholar] [CrossRef]
- Chatonnet, P.; Boidron, J.N.; Pons, M. Elevage des vins rouges en fûts de chêne: Évolution de certains composés volatils et de leur impact arômatique. Sci. des Aliment. 1990, 10, 587–656. [Google Scholar]
- Rodrigues, N.; Goncalves, G.; Pereira-da-Silva, S.; Malfeito-Ferreira, M.; Loureiro, V. Development and use of a new medium to detect yeasts of the genera Dekkera/Brettanomyces. J. Appl. Microbiol. 2001, 90, 588–599. [Google Scholar] [CrossRef]
- Morata, A.; Vejarano, R.; Ridolfi, G.; Benito, S.; Palomero, F.; Uthurry, C.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Reduction of 4-ethylphenol production in red wines using HCDC+ yeasts and cinnamyl esterases. Enzyme Microb. Technol. 2013, 52, 99–104. [Google Scholar] [CrossRef]
- Laitila, J.E.; Suvanto, J.; Salminen, J.-P. Liquid chromatography–tandem mass spectrometry reveals detailed chromatographic fingerprints of anthocyanins and anthocyanin adducts in red wine. Food Chem. 2019, 294, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Eglinton, J.; Griesser, M.; Henschke, P.; Kwiatkowski, M.; Parker, M.; Herderich, M. Yeast-mediated formation of pigmented polymers in red wine. In Red wine color: exploring the mysteries; Waterhouse, A.L., Kennedy, J.A., Eds.; American Chemical Society: Washington, DC, USA, 2004; pp. 7–21. ISBN 9780841239005. [Google Scholar]
- Dallas, C.; Ricardo-da-Silva, J.M.; Laureano, O. Products formed in model wine solutions involving anthocyanins, procyanidin B2, and acetaldehyde. J. Agric. Food Chem. 1996, 44, 2402–2407. [Google Scholar] [CrossRef]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Álvarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-O -glucoside and (+)-catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Cerreti, M.; Liburdi, K.; Nardi, T.; Vagnoli, P.; Ortiz-Julien, A.; Esti, M. Pre-fermentative cold maceration in presence of non-Saccharomyces strains: Evolution of chromatic characteristics of Sangiovese red wine elaborated by sequential inoculation. Food Res. Int. 2018, 107, 257–266. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Birse, M.; Eglinton, J.; Herderich, M. The effect of Saccharomyces cerevisiae and Saccharomyces bayanus yeast on colour properties and pigment profiles of a Cabernet Sauvignon red wine. Aust. J. Grape Wine Res. 2007, 13, 176–185. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Domizio, P.; Fatichenti, F. Secondary products formation as a tool for discriminating non-Saccharomyces wine strains. Antonie Van Leeuwenhoek 1997, 71, 239–242. [Google Scholar] [CrossRef]
- Rivas-Gonzalo, J.C.; Bravo-Haro, S.; Santos-Buelga, C. Detection of Compounds Formed through the Reaction of Malvidin 3-Monoglucoside and Catechin in the Presence of Acetaldehyde. J. Agric. Food Chem. 1995, 43, 1444–1449. [Google Scholar] [CrossRef]
- Lea, A.G.H.; Bridle, P.; Timberlake, C.F.; Singleton, V.L. The procyanidins of white grapes and wines. Am. J. Enol. Vitic. 1979, 30, 289–300. [Google Scholar]
- Ricardo da Silva, J.M.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin dimers and trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Francia-Aricha, E.M.; Escribano-Bailón, M.T. Comparative flavan-3-ol composition of seeds from different grape varieties. Food Chem. 1995, 53, 197–201. [Google Scholar] [CrossRef]
- Escribano-Bailon, T.; Gutierrez-Fernandez, Y.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Characterization of procyanidins of Vitis vinifera variety Tinta del Pais grape seeds. J. Agric. Food Chem. 1992, 40, 1794–1799. [Google Scholar] [CrossRef]
- Fulcrand, H.; Remy, S.; Souquet, J.-M.; Cheynier, V.; Moutounet, M. Study of wine tannin oligomers by on-line liquid chromatography electrospray ionization mass spectrometry. J. Agric. Food Chem. 1999, 47, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Saucier, C.; Bourgeois, G.; Vitry, C.; Roux, D.; Glories, Y. Characterization of (+)-catechin−acetaldehyde polymers: A model for colloidal state of wine polyphenols. J. Agric. Food Chem. 1997, 45, 1045–1049. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Scariot, F.J.; Menegotto, M.; Delamare, A.P.L. Anthocyanin adsorption by Saccharomyces cerevisiae during wine fermentation is associated to the loss of yeast cell wall/membrane integrity. Int. J. Food Microbiol. 2020, 314. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Poncet-Legrand, C.; Sieczkowski, N.; Vernhet, A. Interactions of grape tannins and wine polyphenols with a yeast protein extract, mannoproteins and β-glucan. Food Chem. 2016, 210, 671–682. [Google Scholar] [CrossRef]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Caridi, A. Enological functions of parietal yeast mannoproteins. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 2006, 89, 417–422. [Google Scholar] [CrossRef]
- Caridi, A. New perspectives in safety and quality enhancement of wine through selection of yeasts based on the parietal adsorption activity. Int. J. Food Microbiol. 2007, 120, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Navajas, M.P.; Echavarri, F.; Ferreira, V.; Fernández-Zurbano, P. Pigment composition and color parameters of commercial Spanish red wine samples: Linkage to quality perception. Eur. Food Res. Technol. 2011, 232, 877–887. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Fornairon-Bonnefond, C.; Camarasa, C.; Moutounet, M.; Salmon, J.M. New trends on yeast autolysis and wine ageing on lees: A bibliographic review. J. Int. des Sci. la Vigne du Vin 2002, 36, 49–69. [Google Scholar] [CrossRef]
- Kulkarni, P.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Use of non-Saccharomyces yeast strains coupled with ultrasound treatment as a novel technique to accelerate ageing on lees of red wines and its repercussion in sensorial parameters. LWT - Food Sci. Technol. 2015, 64, 1255–1262. [Google Scholar] [CrossRef]
- Liu, L.; Loira, I.; Morata, A.; Suárez-Lepe, J.A.; González, M.C.; Rauhut, D. Shortening the ageing on lees process in wines by using ultrasound and microwave treatments both combined with stirring and abrasion techniques. Eur. Food Res. Technol. 2016, 242, 559–569. [Google Scholar] [CrossRef]
- del Fresno, J.M.; Loira, I.; Morata, A.; González, C.; Suárez-Lepe, J.A.; Cuerda, R. Application of ultrasound to improve lees ageing processes in red wines. Food Chem. 2018, 261, 157–163. [Google Scholar] [CrossRef]
- del Fresno, J.; Morata, A.; Escott, C.; Loira, I.; Cuerda, R.; Suárez-Lepe, J. Sonication of yeast biomasses to improve the ageing on lees technique in red wines. Molecules 2019, 24, 635. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S.; Iacumin, L.; Ginaldi, F.; Palacios Paz, A.E.; Zironi, R. Potential of high pressure homogenization to induce autolysis of wine yeasts. Food Chem. 2015, 185, 340–348. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S.; Iacumin, L.; Ginaldi, F.; Voce, S.; Zironi, R. Application of multi-pass high pressure homogenization under variable temperature regimes to induce autolysis of wine yeasts. Food Chem. 2017, 224, 105–113. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S. Potential applications of high pressure homogenization in winemaking: A review. Beverages 2019, 5, 56. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Ferreras-Charro, R.; Ferrer-Gallego, R.; Rivero, F.J.; Heredia, F.J.; Escribano-Bailón, M.T. Monitoring the effects and side-effects on wine colour and flavonoid composition of the combined post-fermentative additions of seeds and mannoproteins. Food Res. Int. 2019, 126, 108650. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, Z.; Palacios, A.; Ayestarán, B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and color extraction in red wines. J. Agric. Food Chem. 2007, 55, 4854–4862. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J.A. New genera of yeasts for over-lees aging of red wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; González, M.C.; Suárez-Lepe, J.A. Conventional and enzyme-assisted autolysis during ageing over lees in red wines: Influence on the release of polysaccharides from yeast cell walls and on wine monomeric anthocyanin content. Food Chem. 2007, 105, 838–846. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Palomero, F.; Benito, S.; Calderón, F.; Morata, A. Oenological versatility of Schizosaccharomyces spp. Eur. Food Res. Technol. 2012, 235, 375–383. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; Bañuelos, M.A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape processing by high hydrostatic pressure: Effect on microbial populations, phenol extraction and wine quality. Food Bioprocess Technol. 2015, 8, 277–286. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Puig-Pujol, A.; Guamis, B.; González, C.; Suárez-Lepe, J.A. Use of Ultra-High Pressure Homogenization processing in winemaking: Control of microbial populations in grape musts and effects in sensory quality. Innov. Food Sci. Emerg. Technol. 2018, 50, 50–56. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Álvarez, I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Saldaña, G.; Álvarez, I.; Raso, J. Evaluation of phenolic extraction during fermentation of red grapes treated by a continuous pulsed electric fields process at pilot-plant scale. J. Food Eng. 2010, 98, 120–125. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Escott, C.; Vaquero, C.; del Fresno, J.M.; Bañuelos, M.A.; Loira, I.; Han, S.Y.; Bi, Y.; Morata, A.; Suárez-Lepe, J.A. Pulsed light effect in red grape quality and fermentation. Food Bioprocess Technol. 2017, 10, 1540–1547. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Tesfaye, W.; Loira, I.; Palomero, F.; Benito, S.; Callejo, M.J.; Villa, A.; González, M.C.; Suárez-Lepe, J.A. Electron beam irradiation of wine grapes: Effect on microbial populations, phenol extraction and wine quality. Food Bioprocess Technol. 2015, 8, 1845–1853. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Loira, I.; Escott, C.; Del Fresno, J.M.; Morata, A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape processing by high hydrostatic pressure: Effect on use of non-Saccharomyces in must fermentation. Food Bioprocess Technol. 2016, 9, 1769–1778. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Morata, A.; González, C.; Tesfaye, W.; Loira, I.; Suárez-Lepe, J.A. Maceration and Fermentation: New technologies to increase extraction. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–49. [Google Scholar]
- Liu, Y.; He, F.; Shi, Y.; Zhang, B.; Duan, C.Q. Effect of the high pressure treatments on the physicochemical properties of the young red wines supplemented with pyruvic acid. Innov. Food Sci. Emerg. Technol. 2018, 48, 56–65. [Google Scholar] [CrossRef]
- Sun, J.; Luo, H.; Li, X.; Li, X.; Lu, Y.; Bai, W. Effects of low power ultrasonic treatment on the transformation of cyanidin-3-O-glucoside to methylpyranocyanidin-3-O-glucoside and its stability evaluation. Food Chem. 2019, 276, 240–246. [Google Scholar] [CrossRef]


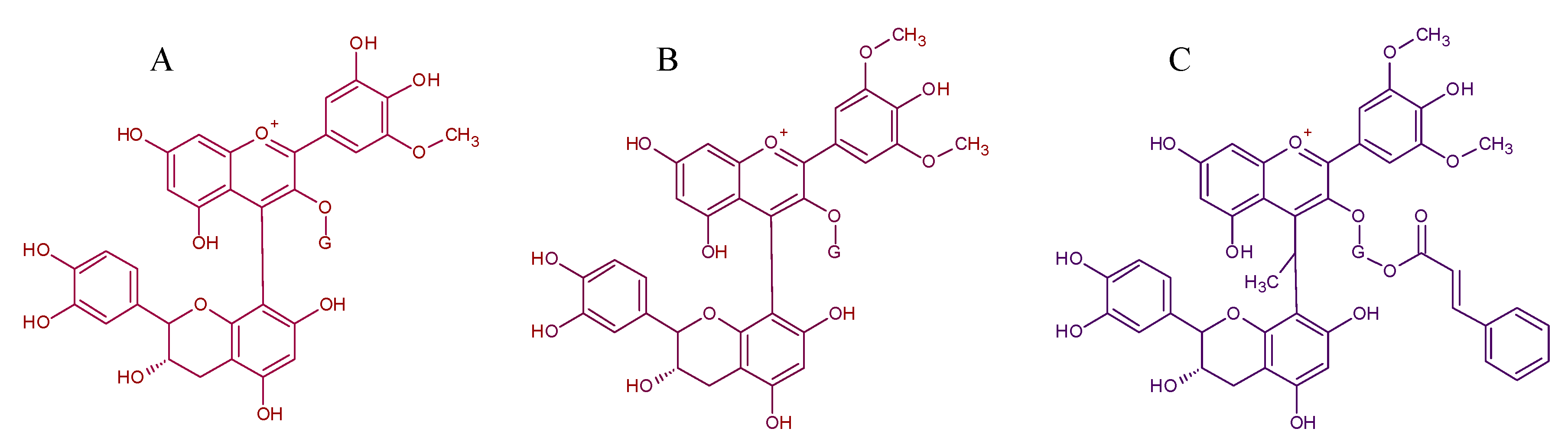
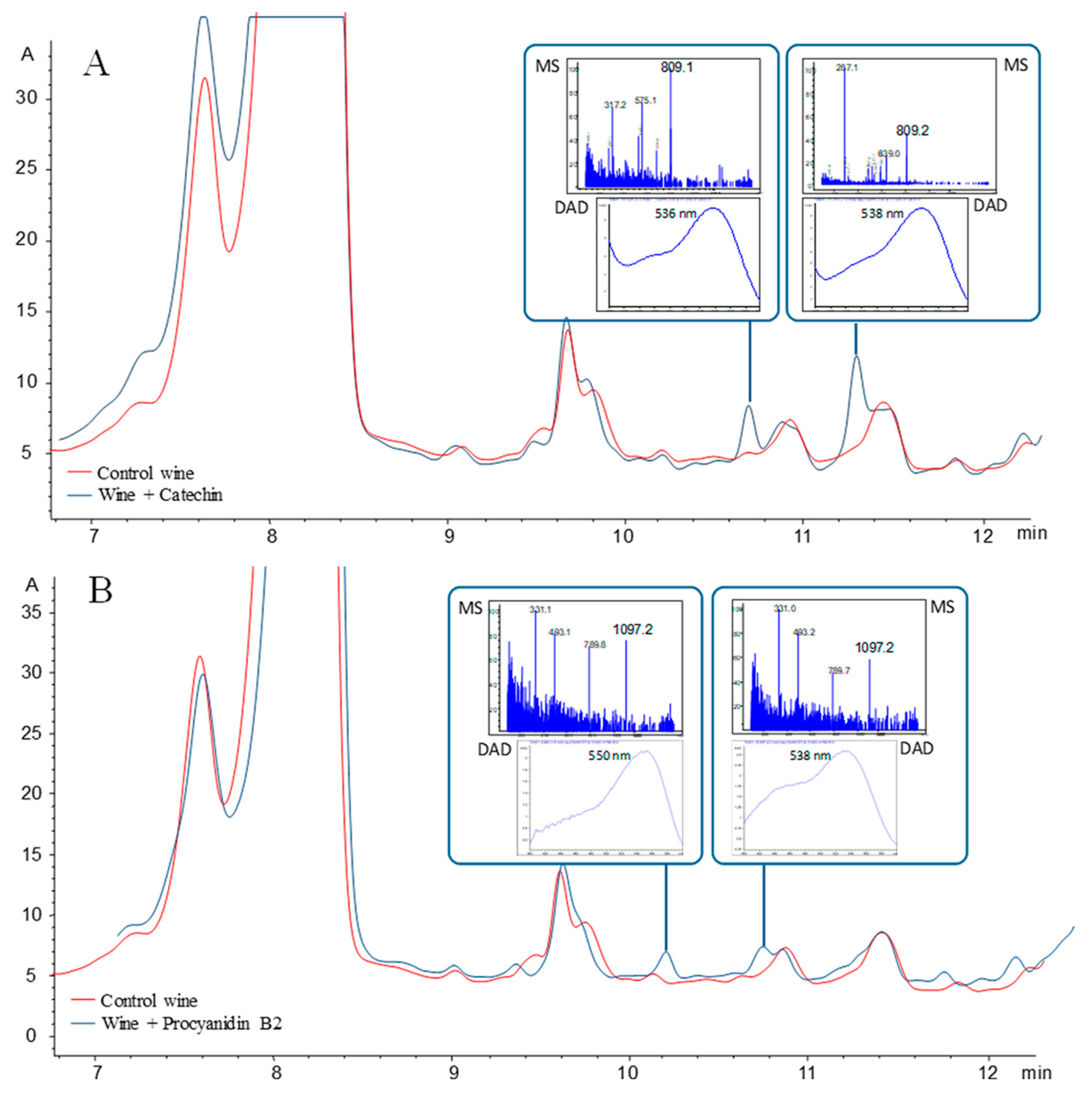
| Pigment | Type | Structure and Tentative Color | Visible λ max. (nm) | m/z [M − H]+ | m/z aglycone | Tentative RGB Color | Promoter Yeast Species | Reference |
|---|---|---|---|---|---|---|---|---|
| Monomeric | ||||||||
| Malvidin3-O-glucoside | Anthocyanin | 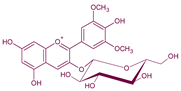 | 528 | 493 | 331 |  | From grape | - |
| Vitisin B | Pyranoanthocyanin-acetaldehyde derivative | 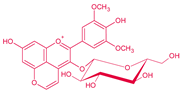 | 495 | 517 | 355 |  | S. cerevisiae | [26,28,29] |
| Vitisin A | Pyranoanthocyanin-pyruvate derivative |  | 515 | 561 | 399 |  | S. cerevisiae, S. pombe, | [26,28,29,30] |
| Malvidin-3-O-glucoside-4-vinylcatechol | Vinylphenolic pyranoanthocyanin-caffeic acid derivative |  | 504 | 625 | 463 |  | S. cerevisiae, S. pombe | [31,32,33] |
| Malvidin-3-O-glucoside-4-vinylphenol | Vinylphenolic pyranoanthocyanin -p-coumaric acid derivative | 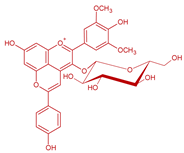 | 504 | 609 | 447 |  | S. cerevisiae, S. pombe | [30,31,33,34,35] |
| Malvidin-3-O-(6-acetyl)-glucoside-4-vinylphenol | Vinylphenolic pyranoanthocyanin -p-coumaric acid derivative | 508 | 651 | 447 | S. cerevisiae, S. pombe | [30,31,33] | ||
| Malvidin-3-O-(6-p-coumaroyl)-glucoside-4-vinylphenol | Vinylphenolic pyranoanthocyanin -p-coumaric acid derivative | 508 | 755 | 447 | S. cerevisiae, S. pombe | [30,31,33] | ||
| Malvidin-3-O-glucoside-4-vinylguaiacol | Vinylphenolic pyranoanthocyanin -ferulic acid derivative | 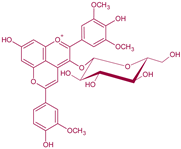 | 512 | 639 | 477 |  | S. cerevisiae, S. pombe | [30,31,33,36] |
| Malvidin-3-O-(6-acetyl)-glucoside-4-vinylguaiacol | Vinylphenolic pyranoanthocyanin -ferulic acid derivative | 520 | 681 | 477 | S. cerevisiae, S. pombe | [30,33,37,38] | ||
| Malvidin-3-O-(6-p-coumaroyl)-glucoside-4-vinylguaiacol | Vinylphenolic pyranoanthocyanin -ferulic acid derivative | 522 | 785 | 477 | S. cerevisiae, S. pombe | [30,33,37,38] | ||
| Polymeric | ||||||||
| Portisins | Vinylphenolic pyranoanthocyanin – phenol, catechin or procyanidin derivative in R2Glucose, acetylglucose or p-coumaroylglucose in R1 | 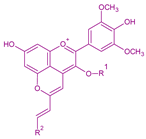 | Phenol:538Catechin:572Procyanidin:583 |  | [6,7] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morata, A.; Escott, C.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Influence of Saccharomyces and non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making. Molecules 2019, 24, 4490. https://doi.org/10.3390/molecules24244490
Morata A, Escott C, Loira I, Del Fresno JM, González C, Suárez-Lepe JA. Influence of Saccharomyces and non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making. Molecules. 2019; 24(24):4490. https://doi.org/10.3390/molecules24244490
Chicago/Turabian StyleMorata, Antonio, Carlos Escott, Iris Loira, Juan Manuel Del Fresno, Carmen González, and Jose Antonio Suárez-Lepe. 2019. "Influence of Saccharomyces and non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making" Molecules 24, no. 24: 4490. https://doi.org/10.3390/molecules24244490
APA StyleMorata, A., Escott, C., Loira, I., Del Fresno, J. M., González, C., & Suárez-Lepe, J. A. (2019). Influence of Saccharomyces and non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making. Molecules, 24(24), 4490. https://doi.org/10.3390/molecules24244490










