Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Konjac Glucomannan Oligosaccharides/Kojic Acid
2.3. Characterization
2.4. Antibacterial Activities
2.5. Moisture Absorption and Moisture Retention Properties
3. Results
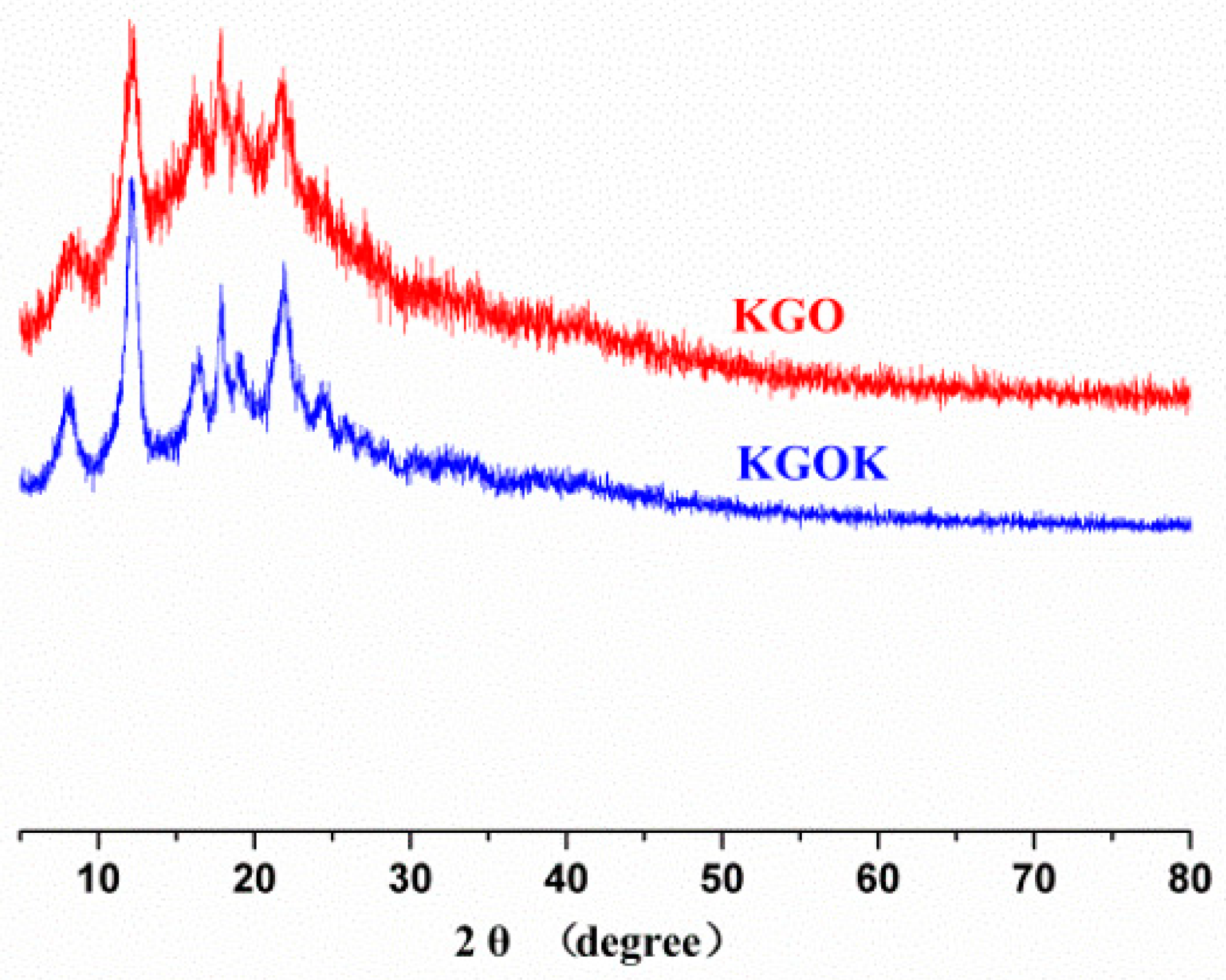
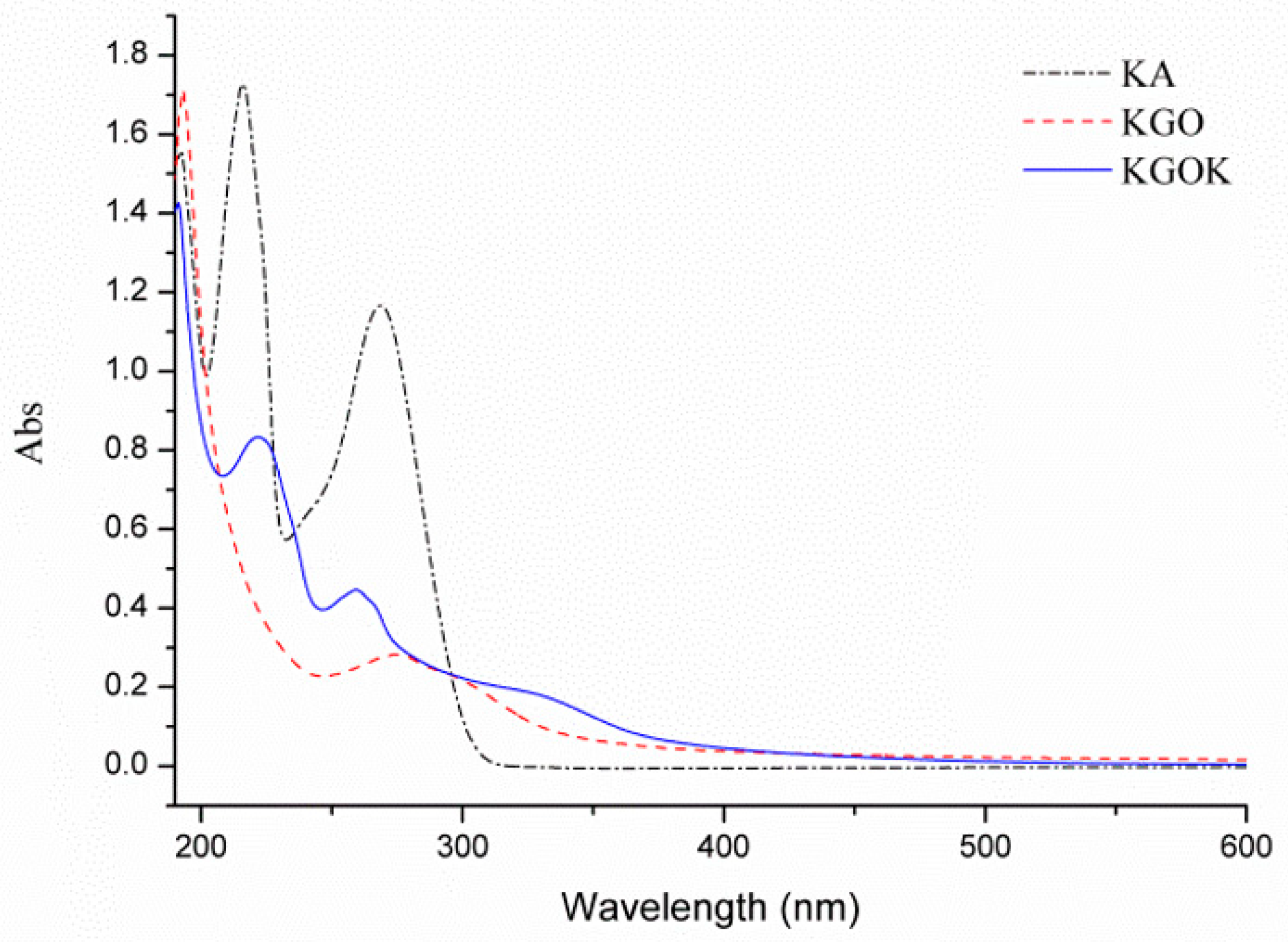
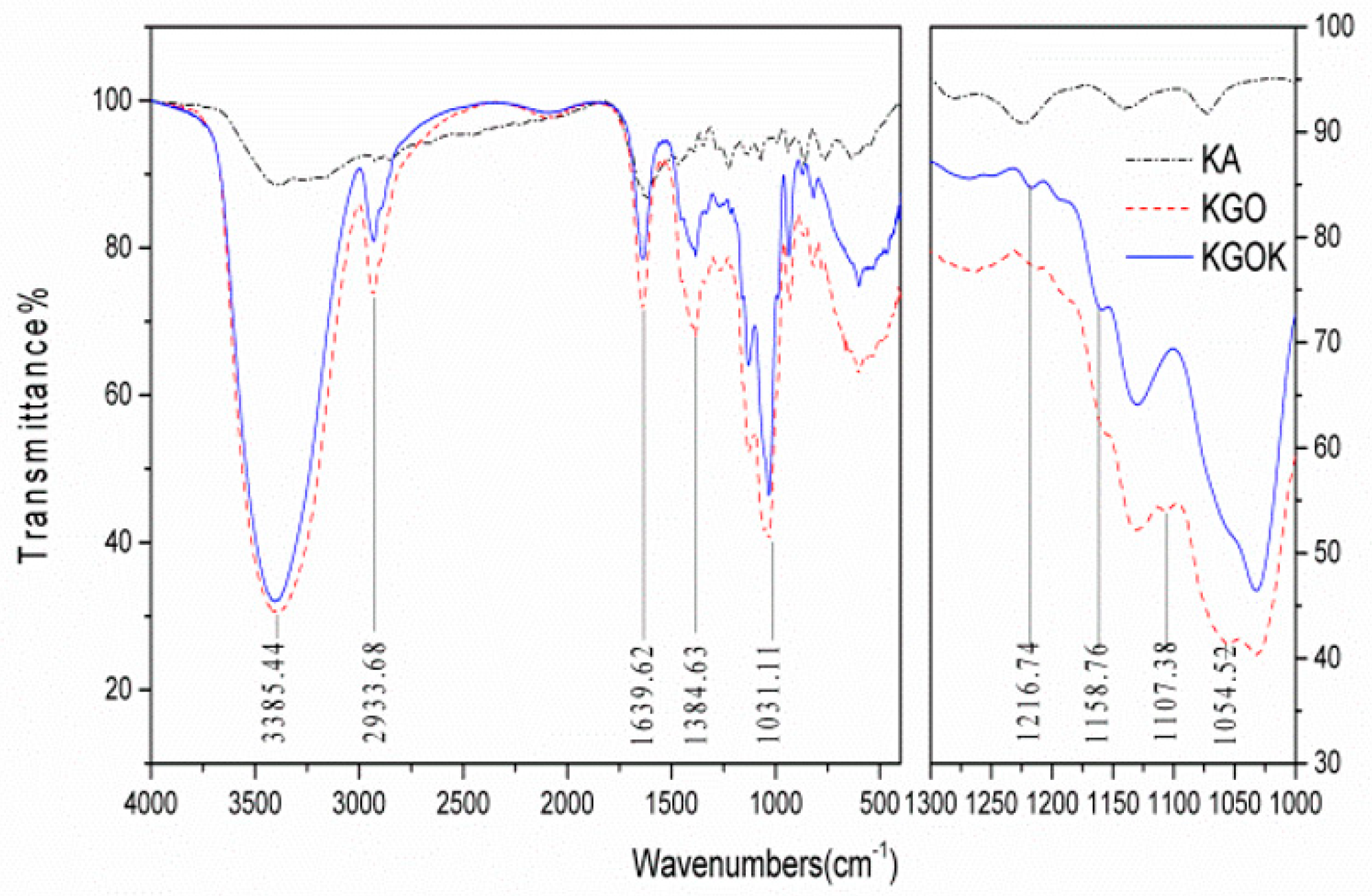
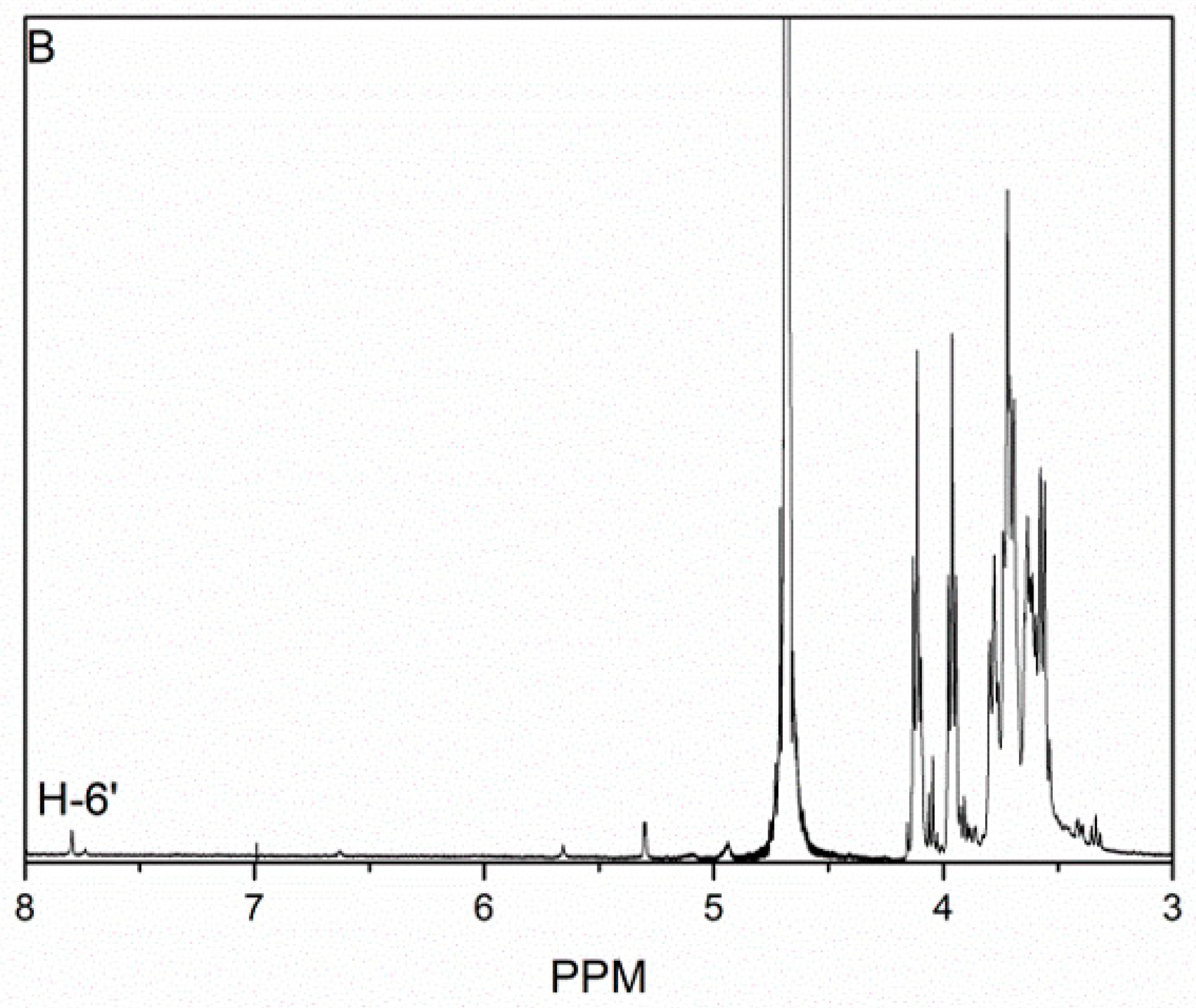
3.1. Characterization of KGOK
3.2. Antimicrobial Activity
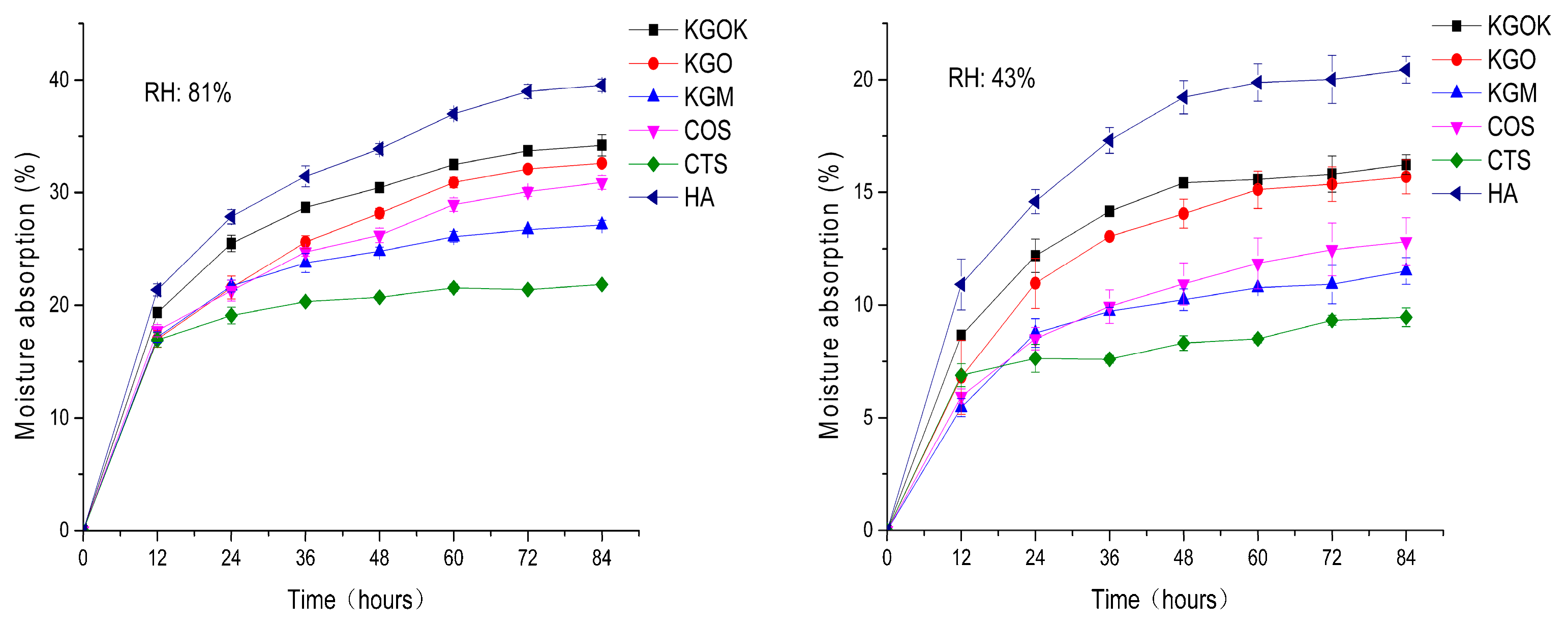
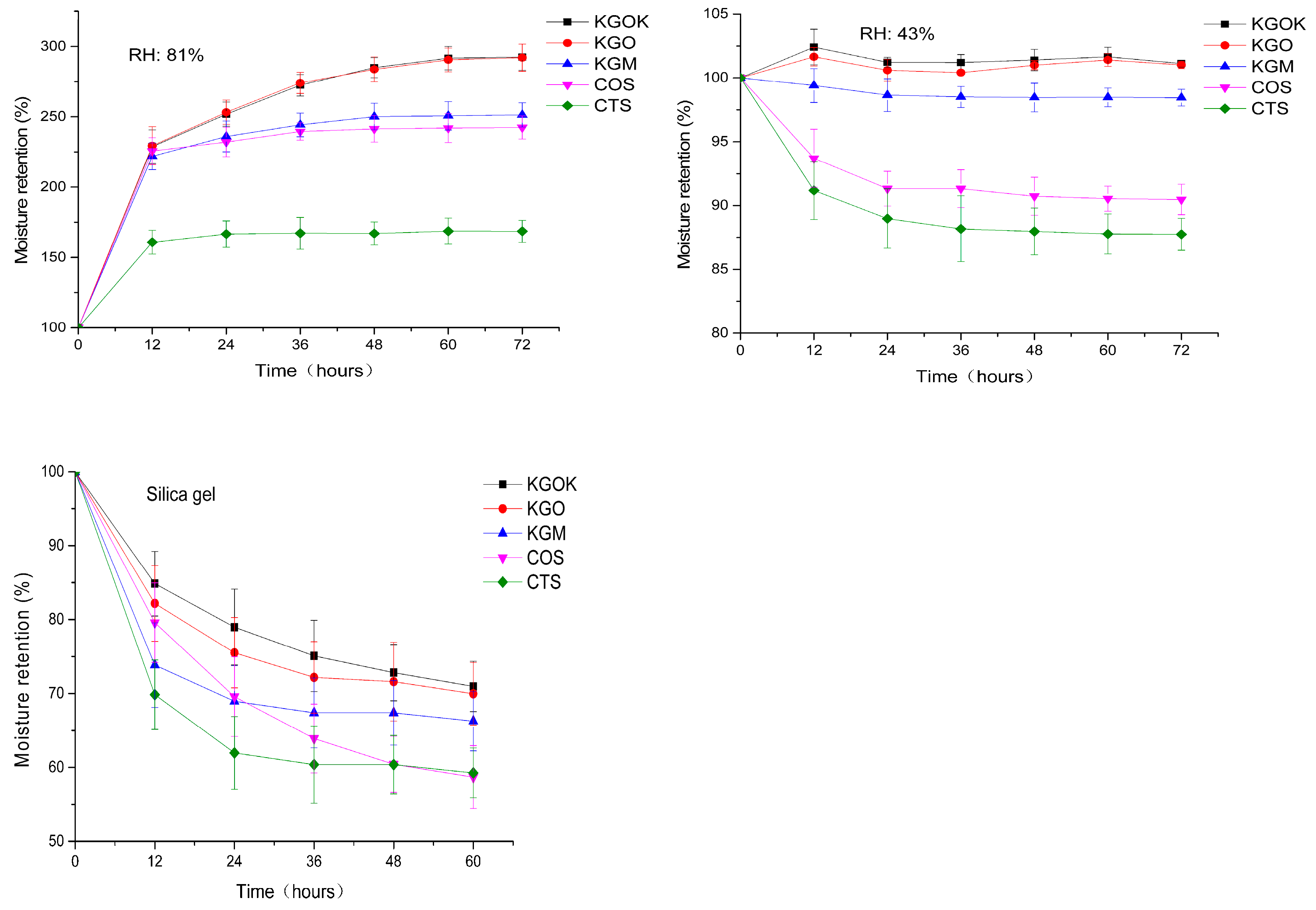
3.3. Moisture Absorption and Retention Properties
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chantarasataporn, P.; Yoksan, R.; Visessanguan, W.; Chirachanchai, S. Water-based nano-sized chitin and chitosan as seafood additive through a case study of Pacific white shrimp (Litopenaeus vannamei). Food Hydrocoll. 2013, 32, 341–348. [Google Scholar] [CrossRef]
- Chouljenko, A.; Chotiko, A.; Bonilla, F.; Moncada, M.; Reyes, V.; Sathivel, S. Effects of vacuum tumbling with chitosan nanoparticles on the quality characteristics of cryogenically frozen shrimp. LWT-Food Sci. Technol. 2017, 75, 114–123. [Google Scholar] [CrossRef]
- Jian, W.; Chen, Y.H.; Wang, L.; Tu, L.; Xiong, H.; Sun, Y.M. Preparation and cellular protection against oxidation of Konjac oligosaccharides obtained by combination of γ -irradiation and enzymatic hydrolysis. Food Res. Int. 2018, 107, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Q.; Zhang, J.; Zhou, X.; Lyu, F.; Zhao, P.; Ding, Y. Preparation, composition analysis and antioxidant activities of konjac oligo-glucomannan. Carbohydr. Polym. 2015, 130, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.M.; Mendes, T.S.; Vicente, A.A. Effect of chitosan-based solutions applied as edible coatings and water glazing on frozen salmon preservation—A pilot-scale study. J. Food Eng. 2013, 119, 316–323. [Google Scholar] [CrossRef]
- Torti, M.J.; Sims, C.A.; Adams, C.M.; Sarnoski, P.J. Polysaccharides as Alternative Moisture Retention Agents for Shrimp. J. Food Sci. 2016, 81, S728–S735. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, B.; Ma, L.K.; Sun, J.P. Cryoprotective Effects of Trehalose, Alginate, and its Oligosaccharide on Quality of Cooked-Shrimp (Litopenaeus vannamei) During Frozen Storage. J Food Process. Preserv. 2017, 41, e12825. [Google Scholar]
- Nishinari, K.; Williams, P.A.; Phillips, G.O. Review of the physico-chemical characteristics and properties of konjac mannan. Food Hydrocoll. 1992, 6, 199–222. [Google Scholar] [CrossRef]
- Pérols, C.; Piffaut, B.; Scher, J.; Ramet, J.P.; Poncelet, D. The potential of enzyme entrapment in konjac cold-melting gel beads. Enzym. Microb. Technol. 1997, 20, 57–60. [Google Scholar] [CrossRef]
- Zhu, F. Modifications of konjac glucomannan for diverse applications. Food Chem. 2018, 256, 419–426. [Google Scholar] [CrossRef]
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Xie, B.J.; Gan, X. Advance in the applications of konjac glucomannan and its derivatives. Carbohydr. Polym. 2005, 60, 27–31. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Ding, Y. Optimization of adding konjac glucomannan to improve gel properties of low-quality surimi. Carbohydr. Polym. 2013, 92, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Cheng, W.; Ye, L.; Du, X.; Zhou, M.; Lin, R.; Geng, S.; Chen, M.; Corke, H.; Cai, Y.Z. Effects of konjac glucomannan on physicochemical properties of myofibrillar protein and surimi gels from grass carp (Ctenopharyngodon idella). Food Chem. 2009, 116, 413–418. [Google Scholar] [CrossRef]
- Simone, A.; van Muiswinkel, G.C.J.; Xu, J.; Schols, H.A.; Voragen, A.G.J.; Gruppen, H. Enzymatic production and characterization of konjac glucomannan oligosaccharides. J. Agric. Food Chem. 2011, 59, 12658–12666. [Google Scholar]
- Aytemir, M.D.; Ozçelik, B. A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur. J. Med. Chem. 2010, 45, 4089–4095. [Google Scholar] [CrossRef]
- Emami, S.; Ghafouri, E.; Faramarzi, M.A.; Samadi, N.; Irannejad, H.; Foroumadi, A. ChemInform Abstract: Mannich Bases of 7-Piperazinylquinolones and Kojic Acid Derivatives: Synthesis, in vitro Antibacterial Activity and in silico Study. Eur. J. Med. Chem. 2014, 45, 185–191. [Google Scholar] [CrossRef]
- Liu, X.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J. Agric. Food Chem. 2014, 62, 297–303. [Google Scholar] [CrossRef]
- Sik, H.; Chang, S.L.; Mi, S.; Yong, A.; Hong, D.; Song, S.S.; Park, Y.H.; Park, S.N. Studies on Tyrosinase Inhibitory and Antioxidant Activities of Benzoic Acid Derivatives Containing Kojic Acid Moiety. Bull. Korean Chem. Soc. 2011, 32, 4411–4414. [Google Scholar]
- Moreno, R.E.; Espigares, R.E.; Navarro, V.C.; Fernandezcrehuet, N.M.; Moreno, A.O. Microbial contamination of bivalve mollusks used for human consumption. J. Food Saf. 2011, 31, 257–261. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; López-Cabo, M.; Saá-Ibusquiza, P.; Rodríguez-Herrera, J.J. Incidence and characterization of Staphylococcus aureus in fishery products marketed in Galicia (Northwest Spain). Int. J. Food Microbiol. 2012, 157, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, C.; Song, M.; Xu, X.; Yang, P.; Paoli, G.; Shi, X. Phenotypic and Genotypic Antimicrobial Resistance Traits of Foodborne Staphylococcus aureus Isolates from Shanghai. J. Food Sci. 2014, 79, M635–M642. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, C.; Wu, T.; Yuan, C.; Hu, Y. Antioxidant and antibacterial properties of coating with chitosan-citrus essential oil and effect on the quality of Pacific mackerel during chilled storage. Food Sci. Nutr. 2019, 7, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Jonas, A.M.; Glinel, K.; Behrens, A.; Anselmo, A.C.; Langer, R.; Jaklenec, A. Controlling the Growth of Staphylococcus epidermidis by Layer-by-Layer Encapsulation. ACS Appl. Mater. Interfaces 2018, 10, 16250–16259. [Google Scholar] [CrossRef]
- Chemical aspects of the preservation and safety control of sea foods. RSC Adv. 2015, 5, 31010–31017. [CrossRef]
- Böhme, K.; Fernández-No, I.C.; Gallardo, J.M.; Cañas, B.; Calo-Mata, P. Safety Assessment of Fresh and Processed Seafood Products by MALDI-TOF Mass Fingerprinting. Food Bioprocess Technol. 2011, 4, 907–918. [Google Scholar] [CrossRef]
- Ye, X.; Kennedy, J.F.; Li, B.; Xie, B.J. Condensed state structure and biocompatibility of the konjac glucomannan/chitosan blend films. Carbohydr. Polym. 2006, 64, 532–538. [Google Scholar] [CrossRef]
- Bagge, D.; Hjelm, M.C.; Huber, I.; Grami, L. Shewanella putrefaciens adhesion and biofilm formation on food processingsurfaces. Appl. Environ. Microbiol. 2001, 67, 2319–2325. [Google Scholar] [CrossRef]
- Aytemir, M.D.; Özçelik, B. Synthesis and biological activities of new Mannich bases of chlorokojic acid derivatives. Med. Chem. Res. 2010, 20, 443–452. [Google Scholar] [CrossRef]
- Synytsya, A.; Blafkova, P.; Synytsya, A.; Copikova, J.; Spevacek, J.; Uher, M. Conjugation of kojic acid with chitosan. Carbohydr. Polym. 2008, 72, 21–31. [Google Scholar] [CrossRef]
- Casey, J.T.; O’Cleirigh, C.; Walsh, P.K.; O’Shea, D.G. Development of a robust microliter plate-based assay method for assessment of bioactivity. J. Microbiol. Methods 2004, 58, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, Z.; Zhou, Y.; Zhou, L.; Xue, Q.; Miao, F.; Qin, S. Synthesis and moisture absorption and retention activities of a carboxymethyl and a quaternary ammonium derivative of alpha,alpha-trehalose. Carbohydr. Res. 2010, 345, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Lans, A.M.; Vodovotz, Y. Effect of galacto-oligosaccharide purity on water sorption and plasticization behavior. Food Chem. 2018, 268, 9–14. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, H.; Zhang, A.; Cui, G.; Chen, H.; Duan, J.; Wang, S. A decrease in moisture absorption–retention capacity of N-deacetylation of hyaluronic acid. Glycoconj. J. 2013, 30, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liang, Q.; Du, X. Effects of molecular characteristics of on konjac glucomannan glass transitions of potato amylose, amylopection and their mixtures. J. Sci. Food Agric. 2011, 91, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, T.; Xue, W.; Li, N.; Chen, L.; Dai, S.; Zhu, Z. Preparation, structure analysis and ACE inhibitory activity of konjac oligosaccharide. Ind. Crops Prod. 2018, 124, 812–821. [Google Scholar] [CrossRef]
- Marwaha, S.S.; Kaur, J.; Sodhi, G.S. Organomercury(II) complexes of kojic acid and maltol: Synthesis, characterization, and biological studies. J. Inorg. Biochem. 1994, 54, 67–74. [Google Scholar] [CrossRef]
- Hong, J.H.; Yong, H.C. Physico-chemical properties of protein-bound polysaccharide from Agaricus blazei Murill prepared by ultrafiltration and spray drying process. Int. J. Food Sci. Technol. 2010, 42, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Du, C.; Mou, H.; Cui, J.; Guan, H.; Hwang, H.; Wang, P. Characterizationof high yield exopolysaccharide produced by Phyllobacterium sp. 921 Fexhibiting moisture preserving properties. Int. J. Biol. Macromol. 2017, 101, 562–568. [Google Scholar] [CrossRef]



| Minimal Inhibitory Concentration (MIC) (mg/mL) | ||
|---|---|---|
| Strain Name | KA | KGOK |
| Staphylococcus aureus | 0.5 | 2.0 |
| Staphylococcus epidermidis | 0.5 | 2.0 |
| Shewanella. putrefaciens | 0.5 | 2.0 |
| Escherichia coli | 1.0 | 3.0 |
| Salmonella enterica | 0.5 | 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Xie, W.; Zhao, Y.; Lv, X.; Yang, H.; Zeng, Q.; Zheng, Z.; Yang, X. Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides. Polymers 2019, 11, 1979. https://doi.org/10.3390/polym11121979
Song L, Xie W, Zhao Y, Lv X, Yang H, Zeng Q, Zheng Z, Yang X. Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides. Polymers. 2019; 11(12):1979. https://doi.org/10.3390/polym11121979
Chicago/Turabian StyleSong, Lin, Wancui Xie, Yukun Zhao, Xinyao Lv, Huanbin Yang, Qingpei Zeng, Zuoxing Zheng, and Xihong Yang. 2019. "Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides" Polymers 11, no. 12: 1979. https://doi.org/10.3390/polym11121979
APA StyleSong, L., Xie, W., Zhao, Y., Lv, X., Yang, H., Zeng, Q., Zheng, Z., & Yang, X. (2019). Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides. Polymers, 11(12), 1979. https://doi.org/10.3390/polym11121979





