Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity of Tetrandrine in Both Sensitive and Resistant Cancer Cells
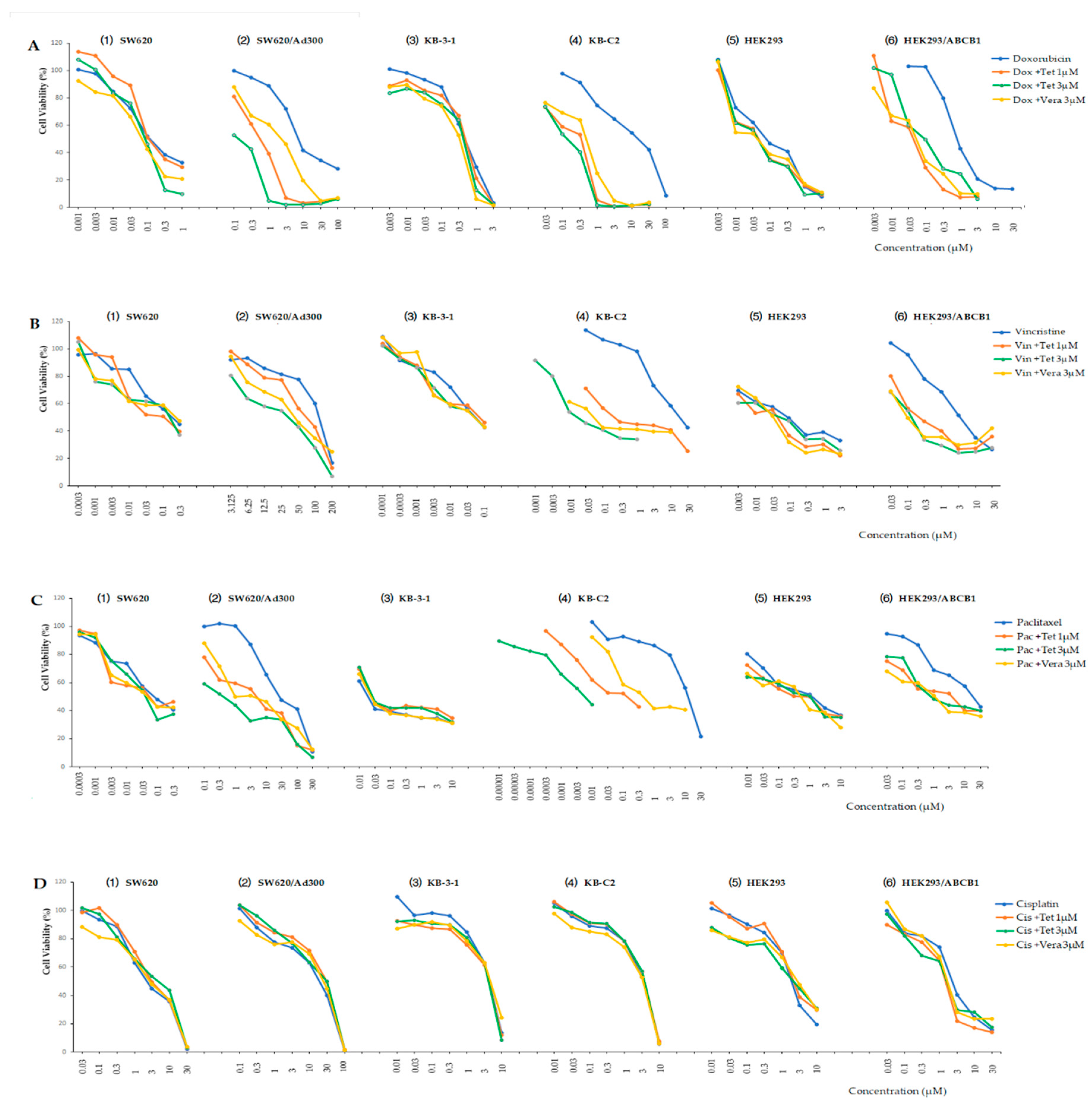
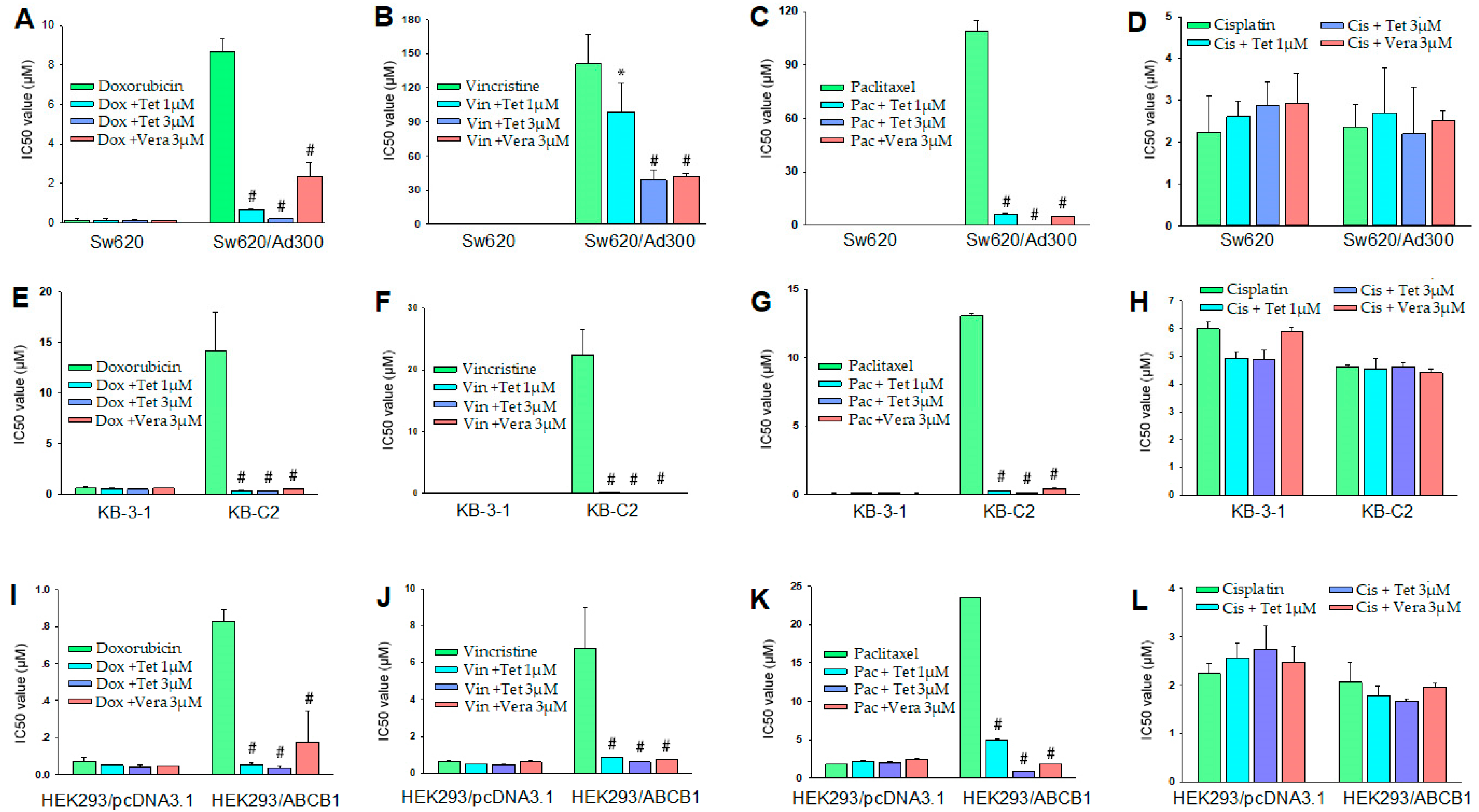
2.2. Reversal Effect of Tetrandrine in ABCB1 Overexpressing Cancer Cells
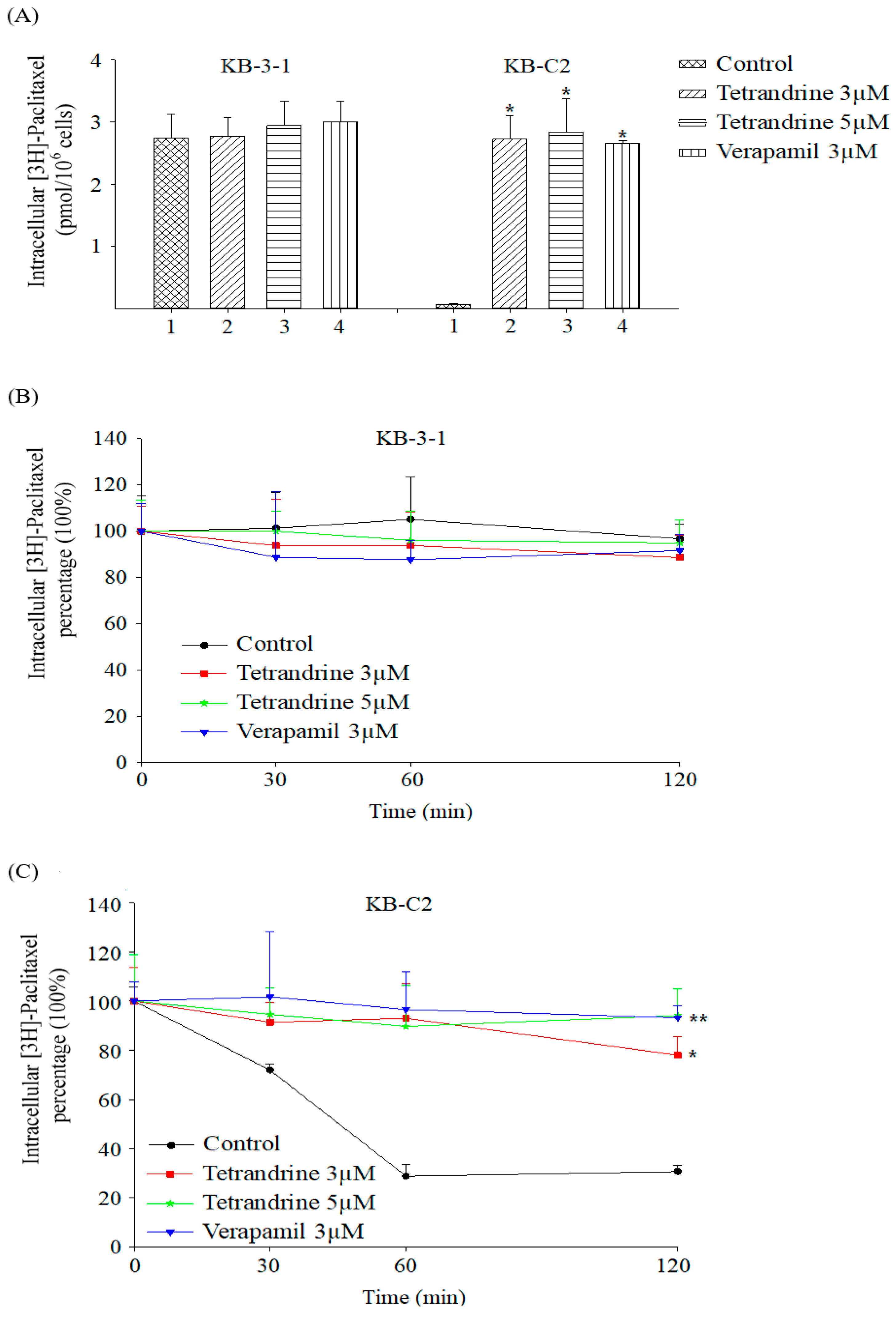
2.3. Tetrandrine Prevents Efflux and Increases Intracellular Accumulation of [3H]-Paclitaxel in ABCB1 Overexpressing Cells
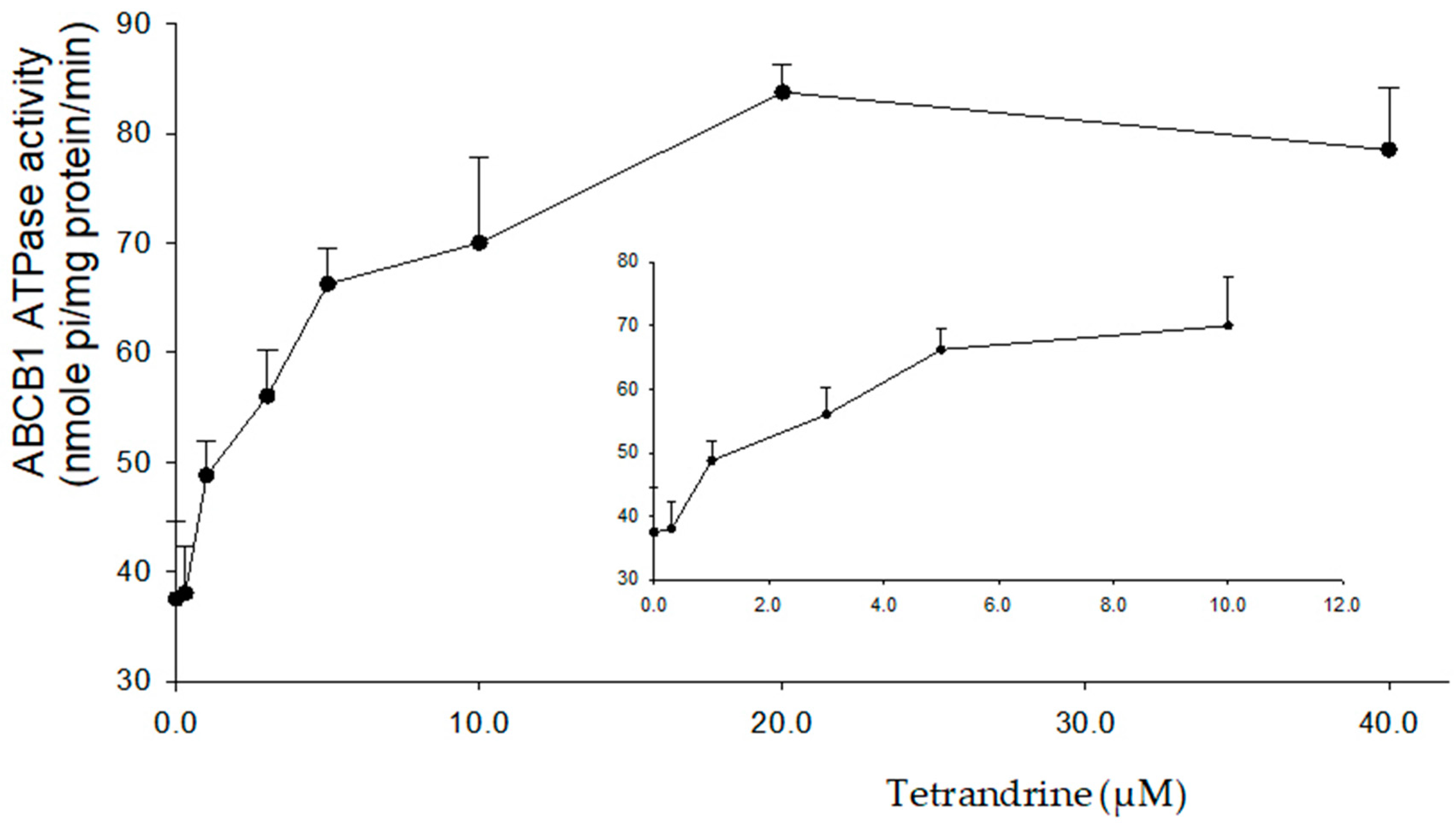
2.4. Tetrandrine Stimulates the ATPase Activity of ABCB1
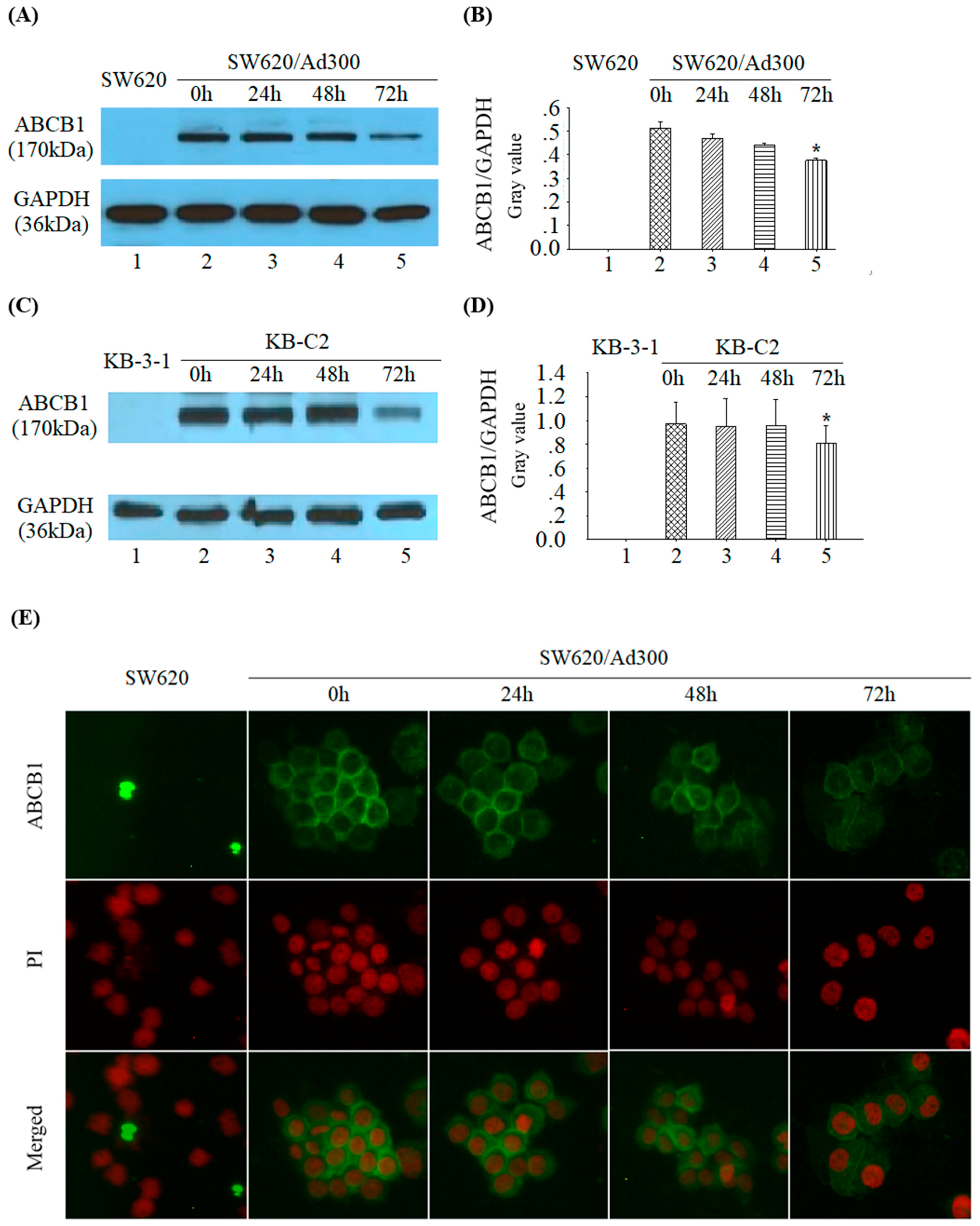
2.5. Tetrandrine Reduces ABCB1 Protein Expression without Changing its Cellular Localization
2.6. Tetrandrine Does Not Alter the mRNA Expression of ABCB1
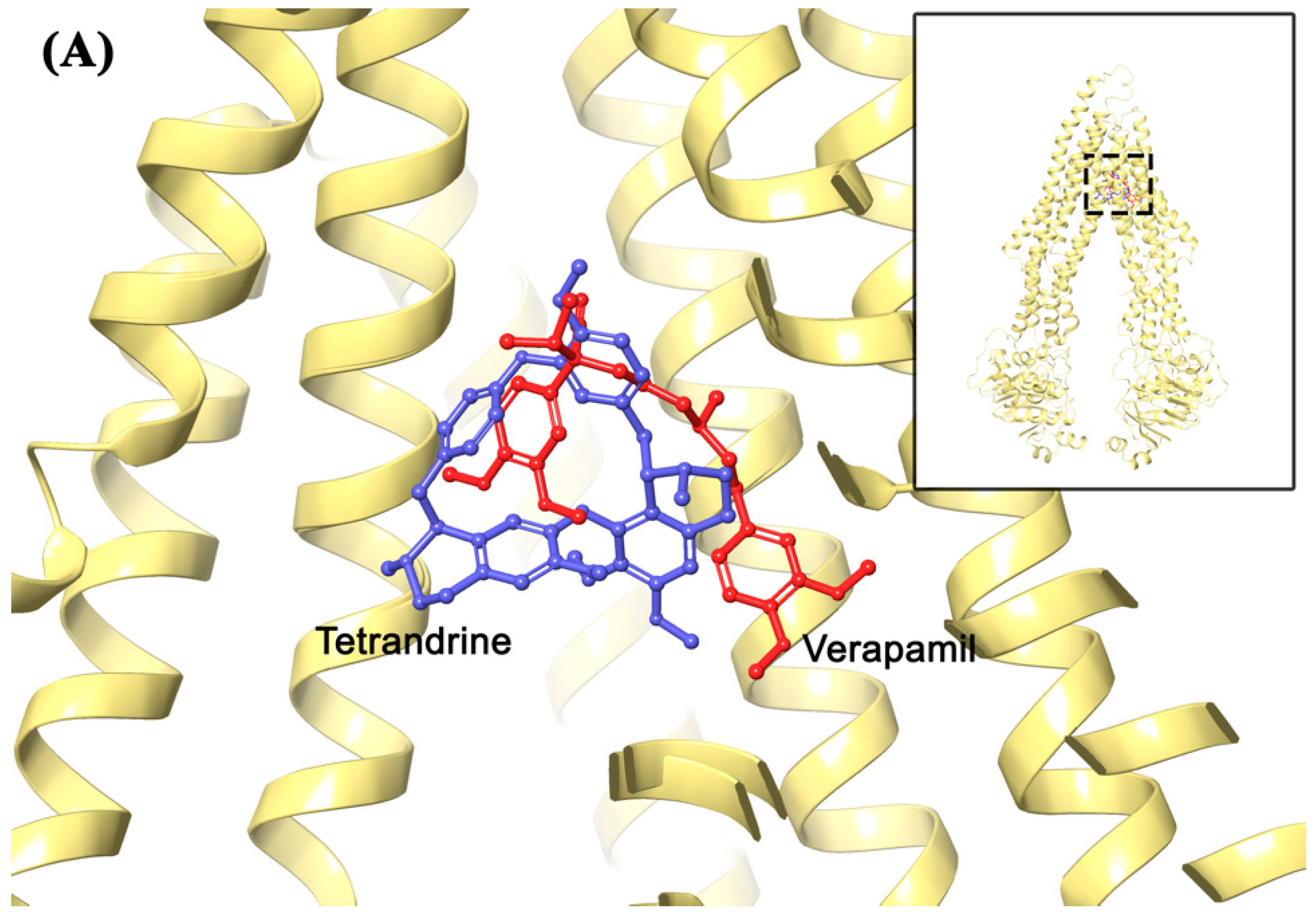
2.7. Molecular Modeling Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cells Culture
4.3. MTT Assay
4.4. The Efflux and Accumulation Assay for [3H]-Paclitaxel
4.5. Extraction of Total Cell Protein and Western Blotting Analysis
4.6. Analysis of ABCB1 ATPase Activity
4.7. Immunofluorescence
4.8. Detecting the mRNA Expression by RT-PCR
4.9. Molecular Modeling
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome. Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Sodani, K.; Wang, S.R.; Kuang, Y.H.; Ashby, C.R., Jr.; Chen, X.; Chen, Z.S. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem. Pharmacol. 2009, 78, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58, Review. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; Setinek, U.; Hollaus, P.; Zidek, T.; Steiner, E.; Elbling, L.; Cantonati, H.; Attems, J.; Gsur, A.; Micksche, M. Multidrug resistance markers p-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: Prognostic implications. J. Cancer Res. Clin. Oncol. 2005, 131, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Rudas, M.; Filipits, M.; Taucher, S.; Stranzl, T.; Steger, G.G.; Jakesz, R.; Pirker, R.; Pohl, G. Expression of MRP1, LRP and Pgp in breast carcinoma patients treated with preoperative chemotherapy. Breast Cancer Res. Treat. 2003, 81, 149–157. [Google Scholar] [CrossRef]
- Ge, J.; Chen, Z.; Wu, S.; Chen, J.; Li, X.; Li, J.; Yin, J.; Chen, Z. Expression levels of insulin-like growth factor-1 and multidrug resistance-associated protein-1 indicate poor prognosis in patients with gastric cancer. Digestion 2009, 80, 148–158. [Google Scholar] [CrossRef]
- Huang, W.T.; Huang, C.C.; Weng, S.W.; Eng, H.L. Expression of the multidrug resistance protein MRP and the lung-resistance protein LRP in nasal NK/T cell lymphoma: Further exploring the role of P53 and WT1 gene. Pathology 2009, 41, 127–132. [Google Scholar] [CrossRef]
- Saglam, A.; Hayran, M.; Uner, A.H. Immunohistochemical expression of multidrug resistance proteins in mature T/NK-cell lymphomas. APMIS 2008, 116, 791–800. [Google Scholar] [CrossRef]
- Sullivan, G.F.; Yang, J.M.; Vassil, A.; Yang, J.; Bash-Babula, J.; Hait, W.N. Regulation of expression of the multidrug resistance protein MRP1 by p53 in human prostate cancer cells. J. Clin. Investig. 2000, 105, 1261–1267. [Google Scholar] [CrossRef]
- Ohishi, Y.; Oda, Y.; Uchiumi, T.; Kobayashi, H.; Hirakawa, T.; Miyamoto, S.; Kinukawa, N.; Nakano, H.; Kuwano, M.; Tsuneyoshi, M. ATP-binding cassette superfamily transporter gene expression in human primary ovarian carcinoma. Clin. Cancer Res. 2002, 8, 3767–3775. [Google Scholar] [PubMed]
- Schaich, M.; Soucek, S.; Thiede, C.; Ehninger, G.; Illmer, T.; SHG AML96 Study Group. MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br. J. Haematol. 2005, 128, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.S.; Peklak-Scott, C.; Bishwokarma, B.; Kute, T.E.; Smitherman, P.K.; Townsend, A.J. Multidrug resistance protein 1 (MRP1, ABCC1) mediates resistance to mitoxantrone via glutathione dependent drug efflux. Mol. Pharmacol. 2006, 69, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, F.; Xu, T.; Sun, J. Tetrandrine prevents multidrug resistance in the osteosarcoma cell line, U-2OS, by preventing Pgp overexpression through the inhibition of NF-κB signaling. Int. J. Mol. Med. 2017, 39, 993–1000. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, T.; Tan, Z.; Wu, X.; Li, M.; Jiang, L.; Bao, R.; Shu, Y.; Lu, A.; Liu, Y. Tetrandrine induces apoptosis in gallbladder carcinoma in vitro. Int. J. Clin. Pharmacol. Ther. 2014, 52, 900–905. [Google Scholar] [CrossRef]
- Sun, X.C.; Cheng, H.Y.; Deng, Y.X.; Shao, R.G.; Ma, J. Tetrandrine: A potent abrogator of G2 checkpoint function in tumor cells and its mechanism. Biomed. Environ. Sci. 2007, 20, 495–501. [Google Scholar]
- Qiu, W.; Zhang, A.L.; Tian, Y. Tetrandrine triggers an alternative autophagy in DU145 cells. Oncol. Lett. 2017, 13, 3734–3738. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, X.Y. Tetrandrine reversed the resistance of tamoxifen in human breast cancer MCF-7/TAM cells: An experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 488–491. [Google Scholar]
- Chen, L.M.; Liang, Y.J.; Zhang, X.; Su, X.D.; Dai, C.L.; Wang, F.P.; Yan, Y.Y.; Tao, L.Y.; Fu, L.W. Reversal of ABCB1-mediated multidrug resistance by Bromo tetrandrine in vivo is associated with enhanced accumulation of chemotherapeutical drug in tumor tissue. Anticancer Res. 2009, 29, 4597–4604. [Google Scholar]
- Choi, S.U.; Park, S.H.; Kim, K.H.; Choi, E.J.; Kim, S.; Park, W.K.; Zhang, Y.H.; Kim, H.S.; Jung, N.P.; Lee, C.O. The bisbenzylisoquinoline alkaloids, tetrandine and fangchinoline, enhance the cytotoxicity of multidrug resistance-related drugs via modulation of P-glycoprotein. Anticancer Drugs 1998, 9, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Hirano, T.; Tanaka, S.; Onda, K.; Oka, K. Persistent reversal of P-glycoprotein-mediated daunorubicin resistance by tetrandrine in multidrug-resistant human T lymphoblastoid leukemia MOLT-4 cells. J. Pharm. Pharmacol. 2003, 55, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, F.P.; Wei, H.; Liu, G. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrine. Cancer Chemother. Pharmacol. 2005, 55, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.P.; Wang, L.; Yang, J.S.; Nomura, M.; Miyamoto, K. Reversal of P-glycoprotein-dependent resistance to vinblastine by newly synthesized bisbenzylisoquinoline alkaloids in mouse leukemia P388 cells. Biol. Pharm. Bull. 2005, 28, 1979–1982. [Google Scholar] [CrossRef]
- Shen, H.; Xu, W.; Chen, Q.; Wu, Z.; Tang, H.; Wang, F. Tetrandrine prevents acquired drug resistance of K562 cells through inhibition of mdr1 gene transcription. J. Cancer Res. Clin. Oncol. 2010, 136, 659–665. [Google Scholar] [CrossRef]
- Sun, Y.F.; Wink, M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine 2014, 21, 1110–1119. [Google Scholar] [CrossRef]
- Fanelli, M.; Hattinger, CM.; Vella, S.; Tavanti, E.; Michelacci, F.; Gudeman, B.; Barnett, D.; Picci, P.; Serra, M. Targeting ABCB1 and ABCC1 with their Specific Inhibitor CBT-1® can Overcome Drug Resistance in Osteosarcoma. Curr. Cancer Drug Targets 2016, 16, 261–274. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Tan, B.B.; Fan, L.Q.; Liu, Q.W.; Jiao, Z.K.; Zhao, X.F.; Hao, Y.J. Effect and mechanisms of TET on human gastric carcinoma cell line SGC7901 and SGC7901/ADR. Zhongguo Zhong Xi Yi Jie He Za Zhi 2014, 34, 66–70. [Google Scholar]
- Karthikeyan, S.; Hoti, S.L. Development of Fourth Generation ABC Inhibitors from Natural Products: A Novel Approach to Overcome Cancer Multidrug Resistance. Anticancer Agents Med. Chem. 2015, 15, 605–615. [Google Scholar] [CrossRef]
- Raderer, M.; Scheithauer, W. Clinical Trials of Agents that Reverse Multidrug Resistance. A literature review. Cancer 1993, 72, 3553–3563. [Google Scholar] [CrossRef]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, J. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981, 41, 1967–1972. [Google Scholar] [PubMed]
- Vezmar, M.; Georges, E. Reversal of MRP-mediated doxorubicin resistance with quinoline-based drugs. Biochem. Pharmacol. 2000, 59, 1245–1252. [Google Scholar] [CrossRef]
- Kirk, J.; Houlbrook, S.; Stuart, N.S.; Stratford, I.J.; Harris, A.L.; Carmichael, J. Differential modulation of doxorubicin toxicity to multidrug and intrinsically drug resistant cell lines by anti-estrogens and their major metabolites. Br. J. Cancer 1993, 67, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Foxwell, B.M.; Mackie, A.; Ling, V.; Ryffel, B. Identification of the multidrug resistance-related P-glycoprotein as a cyclosporine binding protein. Mol. Pharmacol. 1989, 36, 543–546. [Google Scholar]
- Bijie, H.; Kulpradist, S.; Manalaysay, M.; Soebandrio, A. In vitro activity, pharmacokinetics, clinical efficacy, safety and pharmacoeconomics of ceftriaxone compared with third and fourth generation cephalosporins: Review. J. Chemother. 2005, 17, 3–24. [Google Scholar]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Nobili, S.; Landini, I.; Giglioni, B.; Mini, E. Pharmacological strategies for overcoming multidrug resistance. Curr. Drug Targets 2006, 7, 861–879. [Google Scholar] [CrossRef]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef]
- Mistry, P.; Stewart, A.J.; Dangerfield, W.; Okiji, S.; Liddle, C.; Bootle, D.; Plumb, J.A.; Templeton, D.; Charlton, P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001, 61, 749–758. [Google Scholar]
- Dantzig, A.H.; Shepard, R.L.; Cao, J.; Law, K.L.; Ehlhardt, W.J.; Baughman, T.M.; Bumol, T.F.; Starling, J.J. Reversal of p-glycoprotein mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996, 56, 4171–4179. [Google Scholar]
- Werle, M.; Takeuchi, H.; Bernkop-Schnürch, A. New-generation efflux pump inhibitors. Expert Rev. Clin. Pharmacol. 2008, 1, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Vergely, C.; Vignaud, P.; Grand-Perret, T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridone carboxamide derivative. Cancer Res. 1993, 53, 4595–4602. [Google Scholar] [PubMed]
- Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Chen, Z.S. Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Leonard, G.D.; Fojo, T.; Bates, S.E. The role of ABC transporters in clinical practice. Oncologist 2003, 8, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Cao, H.; Qi, X.; Li, H.; Ye, P.; Wang, Z.; Wang, D.; Zhou, H.; Xue, J.; Sun, M. Research Progress in Reversal of Tumor Multi-drug Resistance via Natural Products. Anticancer Agents Med. Chem. 2017, 17, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Pan, Q.C. A comparative study on effect of two bisbenzylisoquinolines, tetrandrine and berbamine, on reversal of multidrug resistance. Yao Xue Xue Bao 1997, 32, 245–250. [Google Scholar]
- Xu, J.Y.; Zhou, Q.; Shen, P.; Tang, W. Reversal effect of TTD on human multidrug resistant KBV200 cell line. J. Exp. Clin. Cancer Res. 1999, 18, 549–552. [Google Scholar]
- Fu, R.B.; Wu, P.S.; Dai, T.Y.; Lai, W.Y.; Qiu, J. Construction and expression analysis of recombinant vector pcDNA3.1+-HIF-1alpha. Di Yi Jun Yi Da Xue Xue Bao 2003, 23, 1134–1136, Chinese. [Google Scholar]
- Gupta, P.; Zhang, Y.K.; Zhang, X.Y.; Wang, Y.J.; Lu, K.W.; Hall, T.; Peng, R.; Yang, D.H.; Xie, N.; Chen, Z.S. Voruciclib, a Potent CDK4/6 Inhibitor, Antagonizes ABCB1 and ABCG2-Mediated Multi-Drug Resistance in Cancer Cells. Cell Physiol. Biochem. 2018, 45, 1515–1528. [Google Scholar] [CrossRef]
- Kenneth, J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real Time Quantitative PCR and the 2−DDCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are commercially available. |
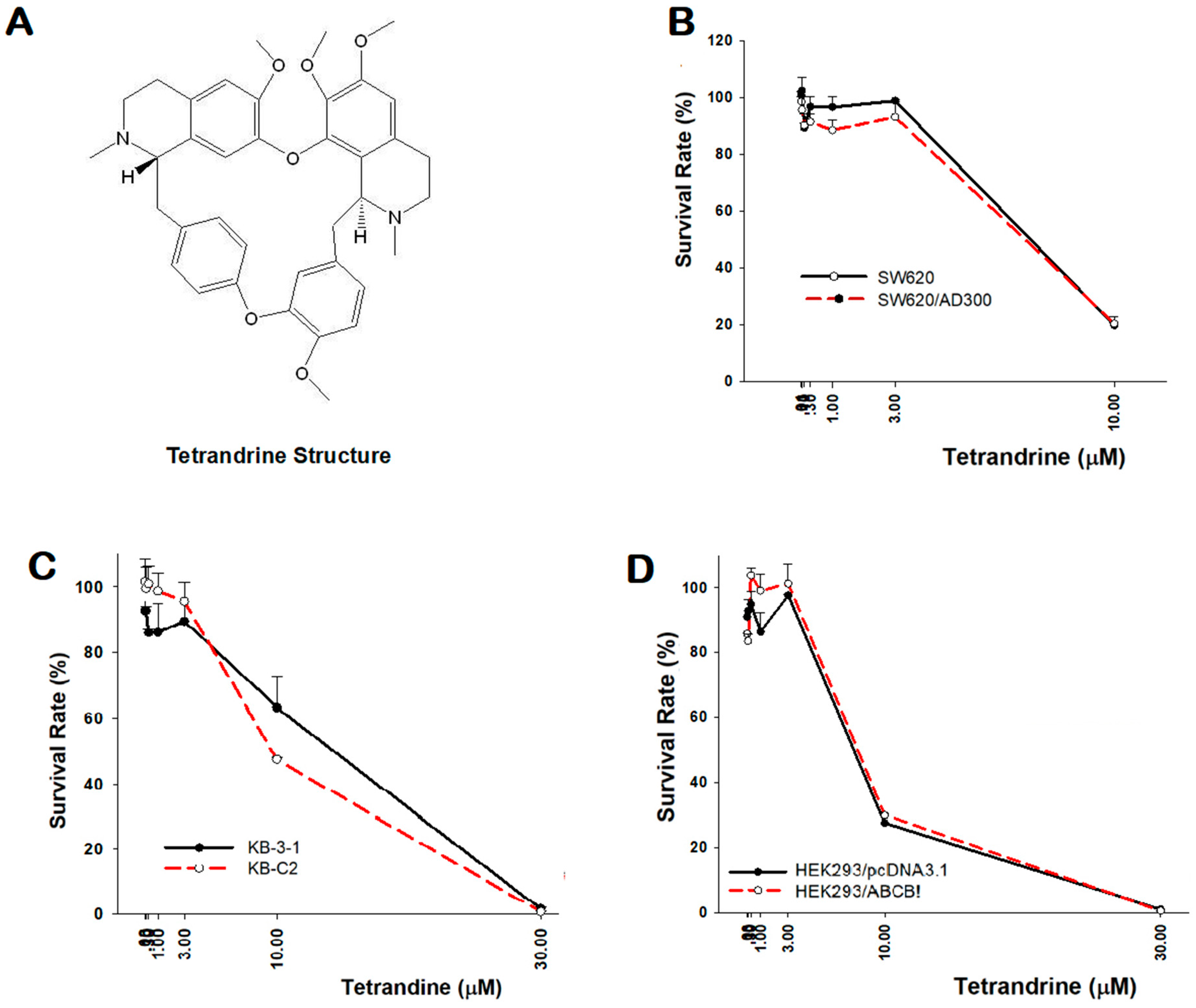


| IC50 ± SD (µM) a [Resistance folds] b | |||||
|---|---|---|---|---|---|
| SW620 | SW620/Ad300 | KB-3-1 | KB-C2 | HEK293/pcDNA3.1 | HEK293/ABCB1 |
| 6.848 ± 0.338 | 7.094 ± 0.569 [1.0] | 14.315 ± 1.676 | 9.68 ± 0.085 [0.8] | 6.65 ± 0.552 | 7.57 ± 0.339 [1.1] |
| Treatment | IC50 ± SD a (μM, Resistance Fold b) | |
| SW620 | SW620/Ad300 | |
| Doxorubicin | 0.135 ± 0.066 [1.0] | 8.665 ± 0.686 [64.2] |
| +Tetrandrine 1 μM | 0.138 ± 0.078 [1.0] | 0.655 ± 0.049 [4.9] # |
| +Tetrandrine 3 μM | 0.119 ± 0.038 [0.9] | 0.197 ± 0.002 [1.5] # |
| +Verapamil 3 μM | 0.108 ± 0.014 [0.8] | 2.370 ± 0.693 [17.6] # |
| Vincristine | 0.268 ± 0.032 [1.0] | 141.060 ± 25.977 [526.3] |
| +Tetrandrine 1 μM | 0.274 ± 0.029 [1.0] | 98.797 ± 25.025 [368.6] * |
| +Tetrandrine 3 μM | 0.348 ± 0.039 [1.3] | 38.710 ± 8.976 [144.4] # |
| +Verapamil 3 μM | 0.360 ± 0.015 [1.3] | 42.144 ± 2.625 [157.2] # |
| Paclitaxel | 0.019 ± 0.001 [1.0] | 108.990 ± 5.996 [5736.3] |
| +Tetrandrine 1 μM | 0.015 ± 0.002 [0.8] | 6.030 ± 0.749 [317.4] # |
| +Tetrandrine 3 μM | 0.018 ± 0.001 [1.0] | 0.373 ± 0.047 [19.6] # |
| +Verapamil 3 μM | 0.024 ± 0.001 [1.3] | 4.790 ± 0.509 [252.1] # |
| Cisplatin | 2.245 ± 0.869 [1.0] | 2.354 ± 0.558 [1.1] |
| +Tetrandrine 1 μM | 2.614 ± 0.361 [1.2] | 2.701 ± 1.563 [1.2] |
| +Tetrandrine 3 μM | 2.882 ± 0.556 [1.3] | 2.198 ± 1.115 [1.0] |
| +Verapamil 3 μM | 2.925 ± 0.728 [1.3] | 2.512 ± 0.247 [1.1] |
| Treatment | IC50 ± SD a (μM, Resistance Fold b) | |
| KB-3-1 | KB-C2 | |
| Doxorubicin | 0.573 ± 0.137 [1.0] | 14.115 ± 3.854 [24.6] |
| +Tetrandrine 1 μM | 0.545 ± 0.035 [1.0] | 0.319 ± 0.057 [0.6] # |
| +Tetrandrine 3 μM | 0.470 ± 0.014 [0.8] | 0.277 ± 0.008 [0.5] # |
| +Verapamil 3 μM | 0.585 ± 0.007 [1.0] | 0.520 ± 0.028 [0.9] # |
| Vincristine | 0.068 ± 0.001 [1.0] | 22.430 ± 4.059 [329.9] |
| +Tetrandrine 1 μM | 0.071 ± 0.014 [1.0] | 0.258 ± 0.002 [3.8] # |
| +Tetrandrine 3 μM | 0.060 ± 0.003 [0.9] | 0.015 ± 0.001 [0.2] # |
| +Verapamil 3 μM | 0.066 ± 0.002 [1.0] | 0.056 ± 0.007 [0.8] # |
| Paclitaxel | 0.029 ± 0.005 [1.0] | 13.070 ± 0.203 [450.7] |
| +Tetrandrine 1 μM | 0.033 ± 0.009 [1.1] | 0.231 ± 0.014 [7.9] # |
| +Tetrandrine 3 μM | 0.031 ± 0.004 [1.1] | 0.083 ± 0.002 [2.9] # |
| +Verapamil 3 μM | 0.027 ± 0.006 [0.9] | 0.422 ± 0.071 [14.6] # |
| Cisplatin | 5.995 ± 0.262 [1.0] | 4.615 ± 0.092 [0.8] |
| +Tetrandrine 1 μM | 4.925 ± 0.247 [0.8] | 4.540 ± 0.382 [0.8] |
| +Tetrandrine 3 μM | 4.905 ± 0.318 [0.8] | 4.620 ± 0.141 [0.8] |
| +Verapamil 3 μM | 5.890 ± 0.169 [1.0] | 4.410 ± 0.127 [0.7] |
| Treatment | IC50 ± SD a (μM, Resistance Fold b) | |
| HEK293/pcDNA3.1 | HEK293/ABCB1 | |
| Doxorubicin | 0.072 ± 0.024 [1.0] | 0.829 ± 0.060 [11.5] |
| +Tetrandrine 1 μM | 0.051 ± 0.001 [0.7] | 0.056 ± 0.012 [0.8] # |
| +Tetrandrine 3 μM | 0.041 ± 0.014 [0.6] | 0.039 ± 0.006 [0.5] # |
| +Verapamil 3 μM | 0.046 ± 0.004 [0.6] | 0.177 ± 0.166 [2.5] # |
| Vincristine | 0.635 ± 0.049 [1.0] | 6.797 ± 2.216 [10.7] |
| +Tetrandrine 1 μM | 0.530 ± 0.014 [0.8] | 0.865 ± 0.035 [1.4] # |
| +Tetrandrine 3 μM | 0.478 ± 0.025 [0.8] | 0.621 ± 0.011 [1.0] # |
| +Verapamil 3 μM | 0.618 ± 0.060 [1.0] | 0.737 ± 0.019 [1.2] # |
| Paclitaxel | 1.825 ± 0.007 [1.0] | 23.425 ± 0.071 [13.0] |
| +Tetrandrine 1 μM | 2.095 ± 0.106 [1.2] | 4.930 ± 0.207 [2.0] # |
| +Tetrandrine 3 μM | 1.950 ± 0.127 [1.1] | 0.880 ± 0.029 [0.5] # |
| +Verapamil 3 μM | 2.380 ± 0.099 [1.3] | 1.833 ± 0.042 [1.0] # |
| Cisplatin | 2.240 ± 0.212 [1.0] | 2.067 ± 0.402 [0.9] |
| +Tetrandrine 1 μM | 2.555 ± 0.304 [1.1] | 1.790 ± 0.192 [0.8] |
| +Tetrandrine 3 μM | 2.735 ± 0.502 [1.2] | 1.667 ± 0.053 [0.7] |
| +Verapamil 3 μM | 2.480 ± 0.325 [1.1] | 1.958 ± 0.094 [0.9] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, D.; Zhang, W.; Gupta, P.; Lei, Z.-N.; Wang, J.-Q.; Cai, C.-Y.; Vera, A.A.D.; Zhang, L.; Chen, Z.-S.; Yang, D.-H. Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression. Molecules 2019, 24, 4383. https://doi.org/10.3390/molecules24234383
Liao D, Zhang W, Gupta P, Lei Z-N, Wang J-Q, Cai C-Y, Vera AAD, Zhang L, Chen Z-S, Yang D-H. Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression. Molecules. 2019; 24(23):4383. https://doi.org/10.3390/molecules24234383
Chicago/Turabian StyleLiao, Dan, Wei Zhang, Pranav Gupta, Zi-Ning Lei, Jing-Quan Wang, Chao-Yun Cai, Albert A. De Vera, Lei Zhang, Zhe-Sheng Chen, and Dong-Hua Yang. 2019. "Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression" Molecules 24, no. 23: 4383. https://doi.org/10.3390/molecules24234383
APA StyleLiao, D., Zhang, W., Gupta, P., Lei, Z.-N., Wang, J.-Q., Cai, C.-Y., Vera, A. A. D., Zhang, L., Chen, Z.-S., & Yang, D.-H. (2019). Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression. Molecules, 24(23), 4383. https://doi.org/10.3390/molecules24234383







