Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD
Abstract
1. Introduction
2. Results and Discussion
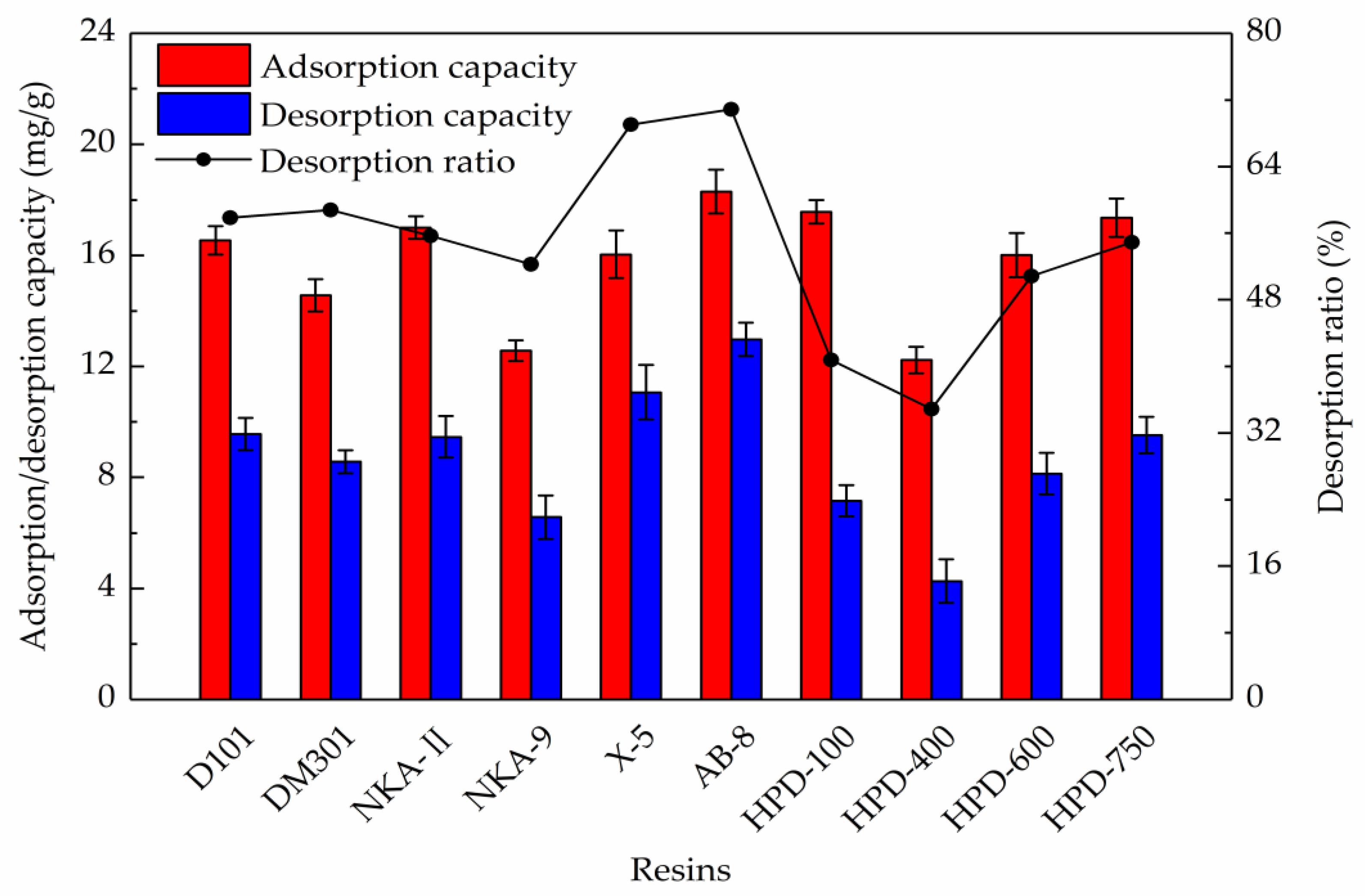
2.1. Resin Selection
2.2. Effect of Sample pH on Adsorption Capacity
2.3. Adsorption Isotherms
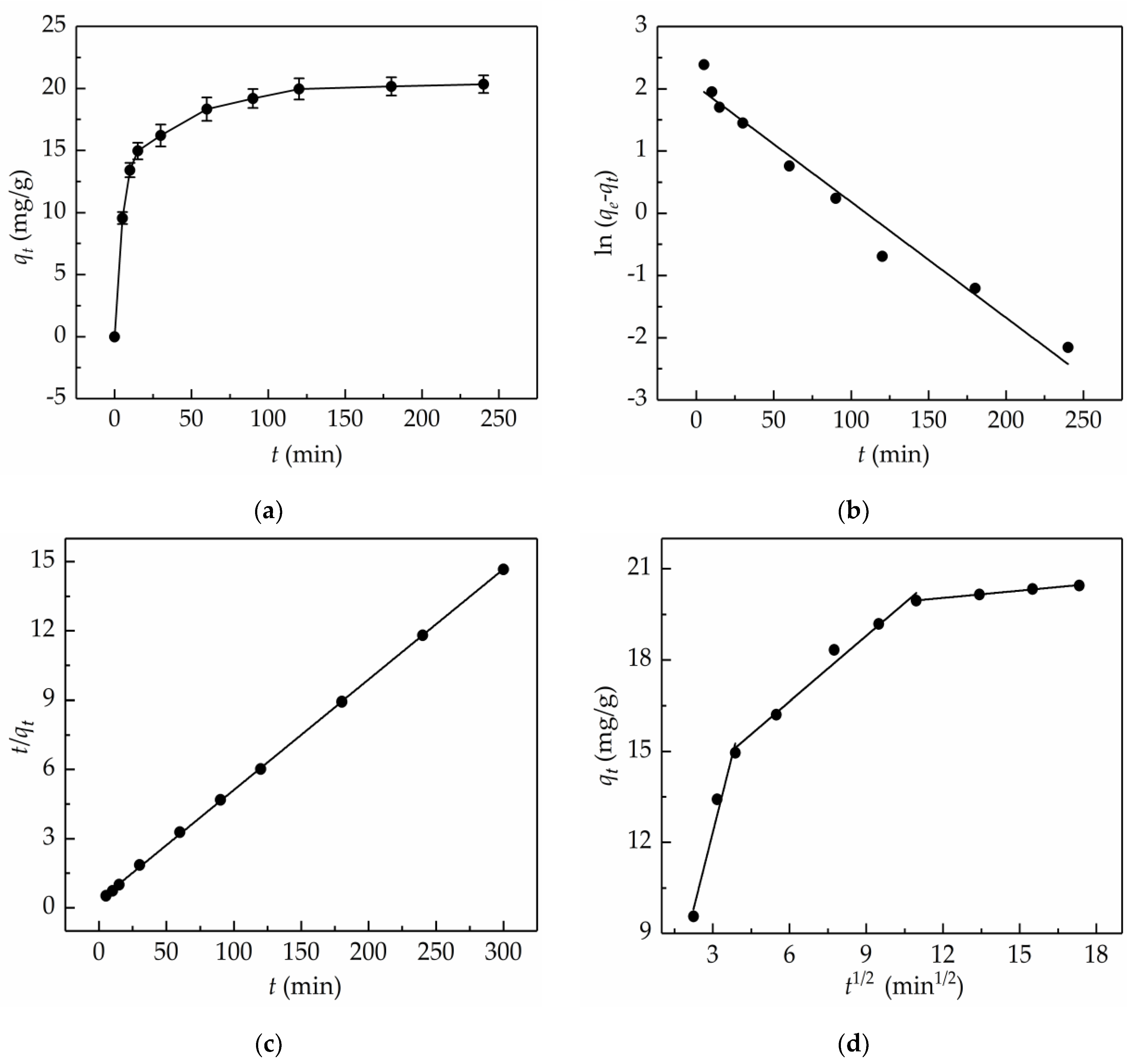
2.4. Adsorption Kinetics
2.5. Column Chromatography
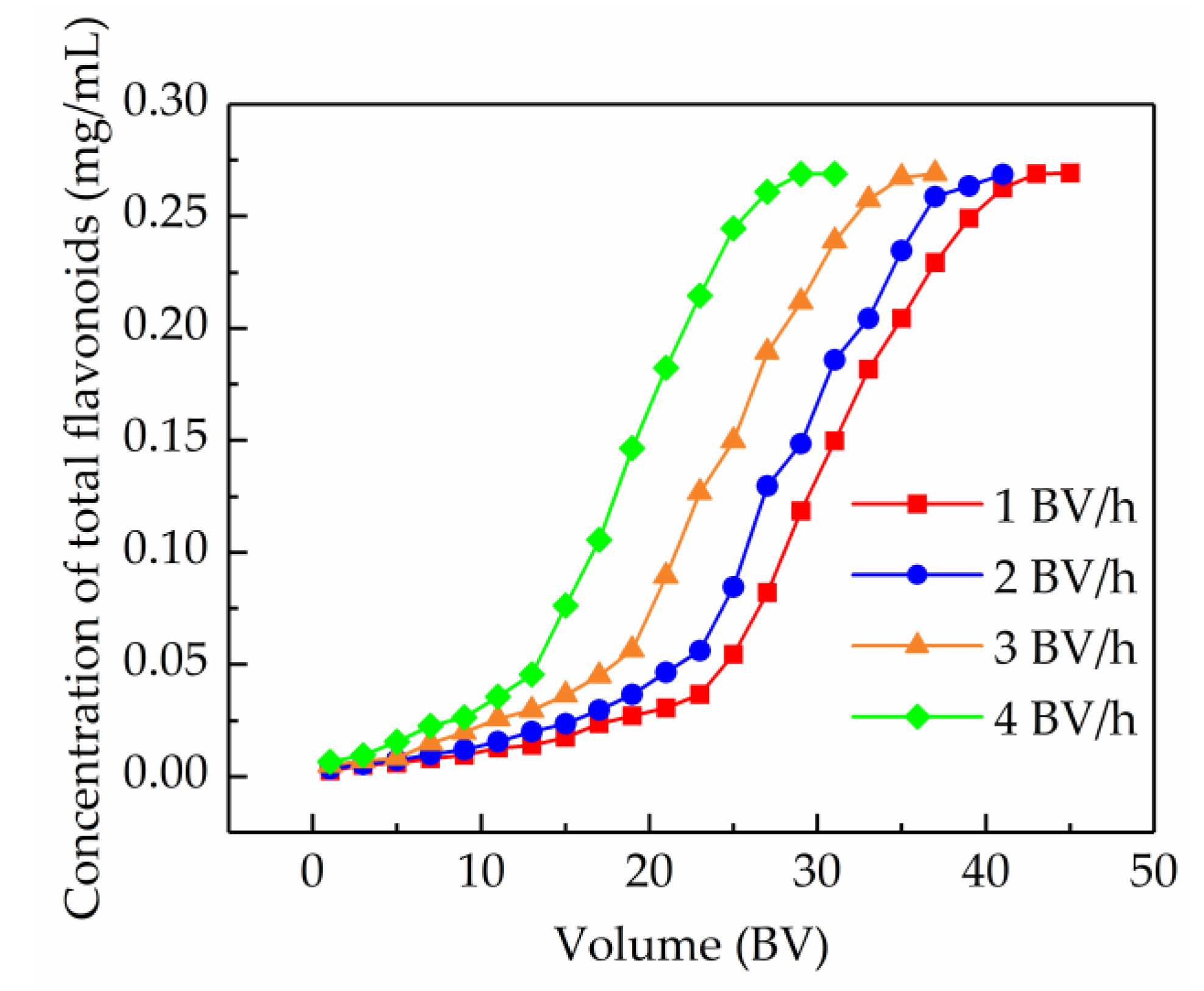
2.5.1. Dynamic Breakthrough Curves
2.5.2. Effect of Ethanol Concentration on Desorption Ratio
2.5.3. Dynamic Desorption Curves
2.6. Preparative Purification of Total Flavonoids Under Optimized Conditions
2.7. Comparative Analysis of Flavonoid Profiles by HPLC-PAD
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Sample Solutions
3.3. Determination of Total Flavonoids Content
3.4. Comparison of Adsorption Capacity, Desorption Capacity and Ratio
3.5. Adsorption Isotherms
3.6. Adsorption Kinetics
3.7. Optimization of Resin Column Chromatography Conditions
3.8. HPLC-PAD Analysis of S. tonkinensis Extract Before and After Purification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoo, H.; Ryu, K.H.; Bae, S.K.; Kim, J. Simultaneous determination of trifolirhizin, (-)-maackiain, (-)-sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxy benzofuran from Sophora tonkinensis in rat plasma by liquid chromatography with tandem mass spectrometry and its application to a pharmacokinetic study. J. Sep. Sci. 2014, 37, 3235–3244. [Google Scholar] [PubMed]
- Tang, L.; Dong, L.N.; Peng, X.J.; Li, Y.; Shi, J.; Zhou, F.Y.; Liu, Z.Q. Pharmacokinetic characterization of oxymatrine and matrine in rats after oral administration of radix Sophorae tonkinensis extract and oxymatrine by sensitive and robust UPLC-MS/MS method. J. Pharm. Biomed. Anal. 2013, 83, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, Y.M.; Chae, H.S.; Choi, Y.H.; Ahn, H.C.; Yoo, H.; Kang, M.; Kim, J.; Chin, Y.W. Prenylated flavonoids from the roots and rhizomes of Sophora tonkinensis and their effects on the expression of inflammatory mediators and proprotein convertase subtilisin/kexin type 9. J. Nat. Prod. 2019, 82, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Deng, S.H.; Huang, M.; Wang, J.L.; Chen, L.; Xiong, M.R.; Yang, J.; Zheng, S.J.; Ma, X.H.; Zhao, P.; et al. Chemical constituents from Sophora tonkinensis and their glucose transporter 4 translocation activities. Bioorg. Med. Chem. Lett. 2017, 27, 1463–1466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, R.Y.; Lan, Y.S.; Huang, Z.J.; Shao, C.L.; Liang, H.; Chen, Z.F.; Li, J. Isoflavonoids from Sophora tonkinensis. Chem. Nat. Compd. 2014, 48, 674–676. [Google Scholar] [CrossRef]
- Li, X.N.; Sha, N.; Yan, H.X.; Pang, X.Y.; Guan, S.H.; Yang, M.; Hua, H.M.; Wu, L.J.; Guo, D.A. Isoprenylated flavonoids from the roots of Sophora tonkinensis. Phytochem. Lett. 2008, 1, 163–167. [Google Scholar] [CrossRef]
- Ding, P.; Chen, D. Three cyclized isoprenylated flavonoids from the roots and rhizomes of Sophora tonkinensis. Helv. Chim. Acta 2007, 90, 2236–2244. [Google Scholar] [CrossRef]
- Deng, Y.H.; Xu, K.P.; Zhou, Y.J.; Li, F.S.; Zeng, G.Y.; Tan, G.S. A new flavonol from Sophora tonkinensis. J. Asian Nat. Prod. Res. 2007, 9, 45–48. [Google Scholar] [CrossRef]
- Wu, C.; He, L.; Yi, X.; Qin, J.; Li, Y.; Zhang, Y.; Wang, G. Three new alkaloids from the roots of Sophora tonkinensis. J. Nat. Med. 2019, 73, 667–671. [Google Scholar] [CrossRef]
- Pan, Q.M.; Zhang, G.J.; Huang, R.Z.; Pan, Y.M.; Wang, H.S.; Liang, D. Cytisine-type alkaloids and flavonoids from the rhizomes of Sophora tonkinensis. J. Asian Nat. Prod. Res. 2016, 18, 429–435. [Google Scholar] [CrossRef]
- Pan, Q.M.; Li, Y.H.; Hua, J.; Huang, F.P.; Wang, H.S.; Liang, D. Antiviral matrine-type alkaloids from the rhizomes of Sophora tonkinensis. J. Nat. Prod. 2015, 78, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Chae, H.S.; Kim, Y.M.; Kang, M.; Ryu, K.H.; Ahn, H.C.; Yoon, K.D.; Chin, Y.W.; Kim, J. Flavonoids and arylbenzofurans from the rhizomes and roots of Sophora tonkinensis with IL-6 production inhibitory activity. Bioorg. Med. Chem. Lett. 2014, 24, 5644–5647. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.Y.; Yang, Y.; Zhou, M.; Ye, Q.; Liu, Y.; Gu, J.; Zhang, G.L.; Luo, Y.G. Novel 2-arylbenzofuran dimers and polyisoprenylated flavanones from Sophora tonkinensis. Fitoterapia 2014, 99, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nakashima, K.I.; Hirai, T.; Inoue, M. Anti-inflammatory effects of naturally occurring retinoid X receptor agonists isolated from Sophora tonkinensis Gagnep. via retinoid X receptor/liver X receptor heterodimers. J. Nat. Med. 2019, 73, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Yoo, H.; Kim, Y.M.; Choi, Y.H.; Lee, C.H.; Chin, Y.W. Anti-inflammatory effects of 6, 8-diprenyl-7,4′-dihydroxyflavanone from Sophora tonkinensis on lipopolysaccharide-stimulated RAW 264.7 cells. Molecules 2016, 21, 1049. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, J.H.; Lee, C.; Jin, Q.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Inhibitory constituents of Sophora tonkinensis on nitric oxide production in RAW 264.7 macrophages. Bioorg. Med. Chem. Lett. 2015, 25, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nakashima, K.I.; Hirai, T.; Inoue, M. Neuroprotective effect of naturally occurring RXR agonists isolated from Sophora tonkinensis Gagnep. on amyloid-β-induced cytotoxicity in PC12 cells. J. Nat. Med. 2019, 73, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Deng, S.; Han, Q.; Zhao, P.; Zhou, Q.; Zheng, S.; Ma, X.; Xu, C.; Yang, J.; Yang, X. Hypoglycemic activity and the potential mechanism of the flavonoid rich extract from Sophora tonkinensis Gagnep. in KK-Ay mice. Front. Pharmacol. 2016, 7, 288. [Google Scholar] [CrossRef]
- Ding, P.L.; He, C.M.; Cheng, Z.H.; Chen, D.F. Flavonoids rather than alkaloids as the diagnostic constituents to distinguish Sophorae Flavescentis Radix from Sophorae Tonkinensis Radix et Rhizoma: An HPLC fingerprint study. Chin. J. Nat. Med. 2018, 16, 951–960. [Google Scholar] [CrossRef]
- He, C.M.; Cheng, Z.H.; Chen, D.F. Qualitative and quantitative analysis of flavonoids in Sophora tonkinensis by LC/MS and HPLC. Chin. J. Nat. Med. 2013, 11, 690–698. [Google Scholar] [CrossRef]
- Liu, E.H.; Qi, L.W.; Cao, J.; Li, P.; Li, C.Y.; Peng, Y.B. Advances of modern chromatographic and electrophoretic methods in separation and analysis of flavonoids. Molecules 2008, 13, 2521–2544. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Y.; Chen, H.; Hao, Z.; Wang, J.; Guan, Y.; Zhang, Y.; Feng, W.; Zheng, X. Isolation of two new prenylated flavonoids from Sinopodophyllum emodi fruit by silica gel column and high-speed counter-current chromatography. J. Chromatogr. B 2014, 969, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhan, Y.; Liu, L.; Xu, Y.; Xu, T.; Liu, T. Preparative isolation and purification of five flavonoid glycosides and one benzophenone galloyl glycoside from Psidium guajava by high-speed counter-current chromatography (HSCCC). Molecules 2013, 18, 15648–15661. [Google Scholar] [CrossRef] [PubMed]
- He, J.Z.; Shao, P.; Liu, J.H.; Ru, Q.M. Supercritical carbon dioxide extraction of flavonoids from Pomelo (Citrus grandis (L.) Osbeck) peel and their antioxidant activity. Int. J. Mol. Sci. 2012, 13, 13065–13078. [Google Scholar] [CrossRef]
- Wen, L.; Lin, Y.; Lv, R.; Yan, H.; Yu, J.; Zhao, H.; Wang, X.; Wang, D. An efficient method for the preparative isolation and purification of flavonoids from leaves of Crataegus pinnatifida by HSCCC and Pre-HPLC. Molecules 2017, 22, 767. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Guo, Q.-S.; Wang, G.-S. Preparative separation and purification of the total flavonoids in Scorzonera austriaca with macroporous resins. Molecules 2016, 21, 768. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, H.; Shao, Q.; Bilia, A.R.; Wang, Y.; Zhao, X. Enrichment and purification of the bioactive flavonoids from flower of Abelmoschus manihot (L.) Medic using macroporous resins. Molecules 2018, 23, 2649. [Google Scholar] [CrossRef]
- Ren, J.Y.; Zheng, Y.M.; Lin, Z.H.; Han, X.; Liao, W.Z. Macroporous resin purification and characterization of flavonoids from Platycladus orientalis (L.) Franco and their effects on macrophage inflammatory response. Food Funct. 2017, 8, 86–95. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, L.; Ur Rashid, H.; Liang, H.; Liu, X.; Xie, P. Ultrasonic-assisted reductive extraction of matrine from sophorae tonkinesis and its purification by macroporous resin column chromatography. Sep. Sci. Technol. 2018, 53, 745–755. [Google Scholar] [CrossRef]
- Li, J.; Chase, H.A. Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat. Prod. Rep. 2010, 27, 1493–1510. [Google Scholar] [CrossRef]
- Aksu, Z.; Kabasakal, E. Batch adsorption of 2, 4-dichlorophenoxy-acetic acid (2, 4-D) from aqueous solution by granular activated carbon. Sep. Purif. Technol. 2004, 35, 223–240. [Google Scholar] [CrossRef]
- Huang, J.H.; Liu, Y.F.; Wang, X.G. Selective sorption of tannin from flavonoids by organically modified attapulgite clay. J. Hazard. Mater. 2008, 160, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Martins, A.C.; Moraes, J.C.G.; Garcia, E.E.; Gauze, G.F.; Costa, W.F.; Almeida, V.C. Kinetic and equilibrium studies: adsorption of food dyes acid yellow 6, acid yellow 23, and acid red 18 on activated carbon from flamboyant pods. Chem. Eng. J. 2012, 181–182, 243–250. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A. Acid washed black cumin seed powder preparation for adsorption of methylene blue dye from aqueous solution: Thermodynamic, kinetic and isotherm studies. J. Mol. Liq. 2018, 264, 275–284. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, J.H. Isotherm, kinetic and thermodynamic characteristics of adsorption of paclitaxel onto Diaion HP-20. Process Biochem. 2016, 51, 917–924. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Zu, Y.G.; Fu, Y.J.; Ma, W.; Zhang, D.Y.; Kong, Y.; Li, X.J. Preliminary purification and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresour. Technol. 2010, 101, 4667–4675. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
Sample Availability:Sophora tonkinensis Gagnep. and flavonoids standards are available from the authors. |





| Model | T (K) | Equations | Parameters | ||
|---|---|---|---|---|---|
| KL (L/mg) | qm (mg/g) | R2 | |||
| Langmuir | 298.15 | 0.0527 | 24.33 | 0.9986 | |
| 308.15 | 0.0472 | 22.57 | 0.9981 | ||
| 318.15 | 0.0431 | 20.96 | 0.9977 | ||
| KF [mg/g(L/mg)1/n] | n | R2 | |||
| Freundlich | 298.15 | 3.3953 | 2.5760 | 0.9215 | |
| 308.15 | 3.1769 | 2.6546 | 0.9432 | ||
| 318.15 | 2.9300 | 2.6954 | 0.9439 | ||
| KT (L/mg) | BT (J/mol) | R2 | |||
| Temkin | 298.15 | 0.5656 | 5.0133 | 0.9696 | |
| 308.15 | 0.5330 | 4.5858 | 0.9736 | ||
| 318.15 | 0.4865 | 4.2617 | 0.9704 | ||
| Kinetics Model | Regression Equations | Parameters |
|---|---|---|
| Pseudo-first-order | k1 = 0.0186 min−1 Qe = 7.70 mg/g R2 = 0.9696 | |
| Pseudo-second-order | k2 = 6.9469 × 10−3 g/(mg·min) Qe = 20.92 mg/g R2= 0.9999 | |
| Intra-particle diffusion (first stage) | ki = 3.3432 mg/(g·min1/2) I = 2.3129 mg/g R2 = 0.9721 | |
| (second stage) | Ki = 0.7191 mg/(g·min1/2) I = 12.3295 mg/g R2 = 0.9843 | |
| (third stage) | Ki = 0.0797 mg/(g·min1/2) I = 19.0885 mg/g R2 = 0.9966 |
| Compounds | Retention Time (min) | Content (%) | Recovery | |
|---|---|---|---|---|
| Before Purification | After Purification | |||
| Rutin | 28.362 | 0.014% | 0.036% | 83.47% |
| Trifolirhizin | 37.825 | 0.756% | 3.198% | 86.51% |
| Quercitrin | 40.641 | 0.026% | 0.032% | 84.22% |
| Quercetin | 42.103 | 0.007% | 0.067% | 91.53% |
| Maackiain | 48.759 | 1.617% | 7.842% | 87.16% |
| Formononetin | 49.537 | 0.067% | 0.842% | 85.90% |
| Resins | Particle Size (mm) | Surface Area (m2/g) | Average Pore Diameter (nm) | Polarity |
|---|---|---|---|---|
| NKA-9 | 0.3~1.25 | 170~250 | 15.5~16.5 | Polar |
| NKA-II | 0.3~1.25 | 160~200 | 14.5~15.5 | Polar |
| HPD-600 | 0.3~1.25 | 550~600 | 8.0~9.0 | Polar |
| DM301 | 0.3~1.25 | 330~380 | 9.0~11.0 | Middle polar |
| HPD-400 | 0.3~1.25 | 500~550 | 7.5~8.0 | Middle polar |
| HPD-750 | 0.3~1.25 | 650~700 | 8.5~9.0 | Middle polar |
| AB-8 | 0.3~1.25 | 450~530 | 13.0~14.0 | Weak polar |
| HPD-100 | 0.3~1.25 | 650~700 | 8.5~9.0 | Non-polar |
| D101 | 0.3~1.25 | 600~700 | 10.0~12.0 | Non-polar |
| X-5 | 0.3~1.25 | 500~600 | 21.0~23.0 | Non-polar |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Hu, W.; Xiu, Z.; Jiang, A.; Men, L.; Hao, K.; Sun, X.; Cao, D. Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules 2019, 24, 3200. https://doi.org/10.3390/molecules24173200
Hou M, Hu W, Xiu Z, Jiang A, Men L, Hao K, Sun X, Cao D. Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules. 2019; 24(17):3200. https://doi.org/10.3390/molecules24173200
Chicago/Turabian StyleHou, Mengyang, Wenzhong Hu, Zhilong Xiu, Aili Jiang, Lei Men, Kexin Hao, Xingsheng Sun, and Duo Cao. 2019. "Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD" Molecules 24, no. 17: 3200. https://doi.org/10.3390/molecules24173200
APA StyleHou, M., Hu, W., Xiu, Z., Jiang, A., Men, L., Hao, K., Sun, X., & Cao, D. (2019). Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules, 24(17), 3200. https://doi.org/10.3390/molecules24173200





