New Routes to Pyridino[2,3-d]pyrimidin-4-one and Pyridino-[2,3-d]triazolino[4,5-a]pyrimidin-5-one Derivatives
Abstract
:Introduction
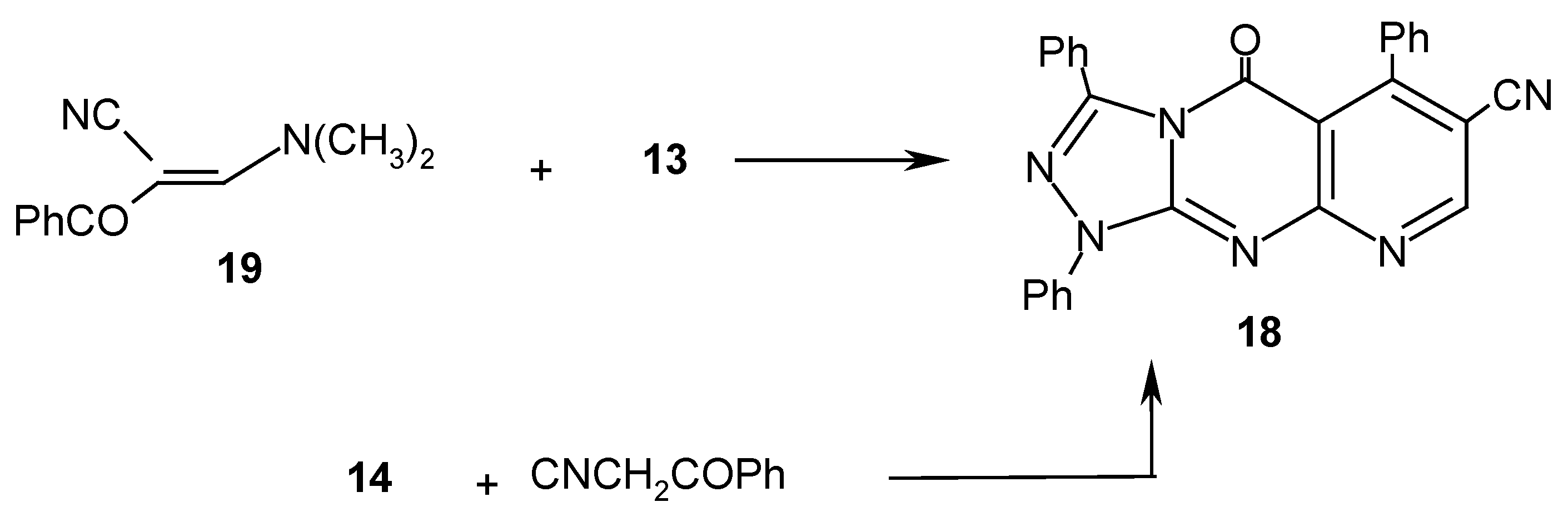
Results and Discussion
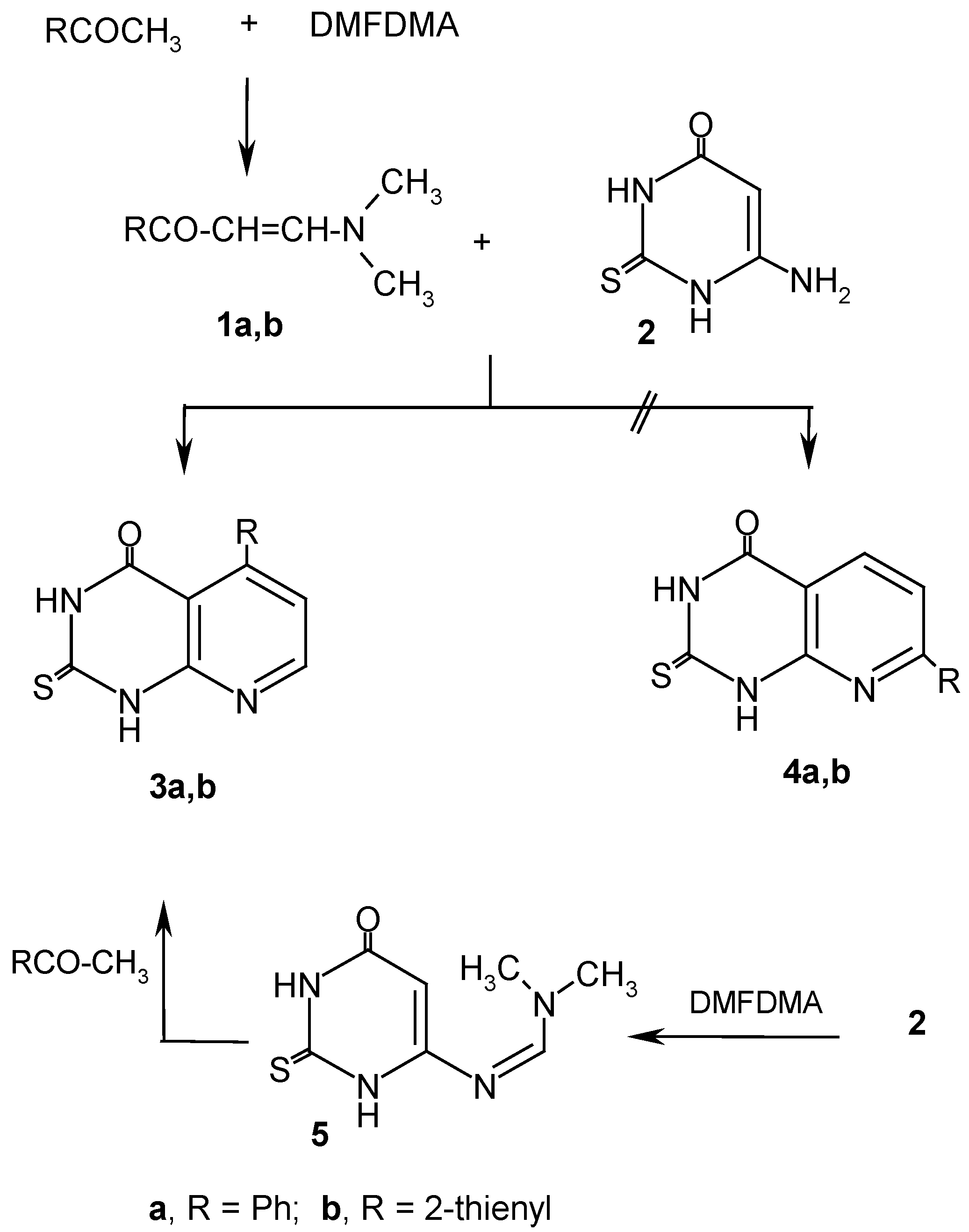
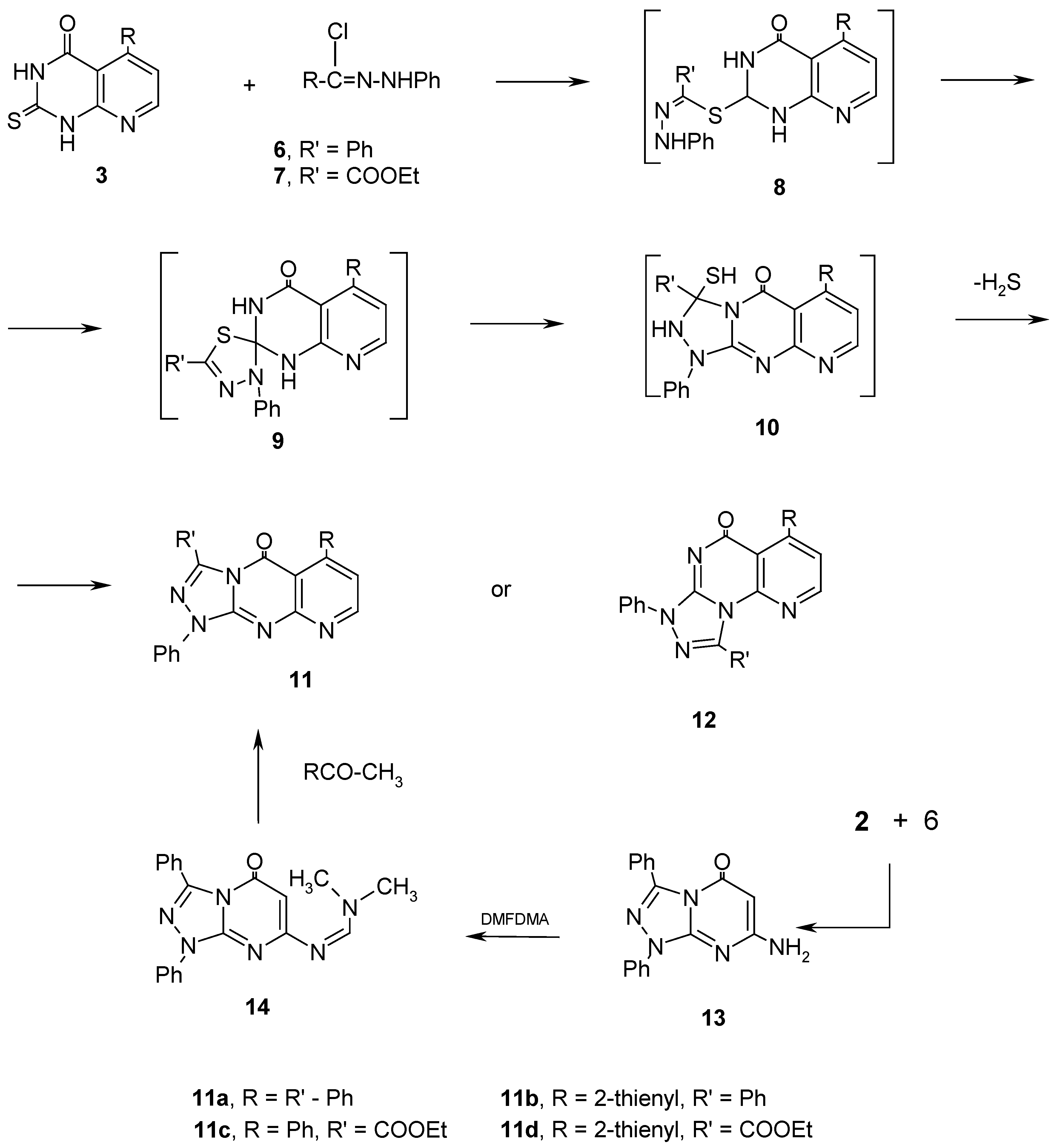
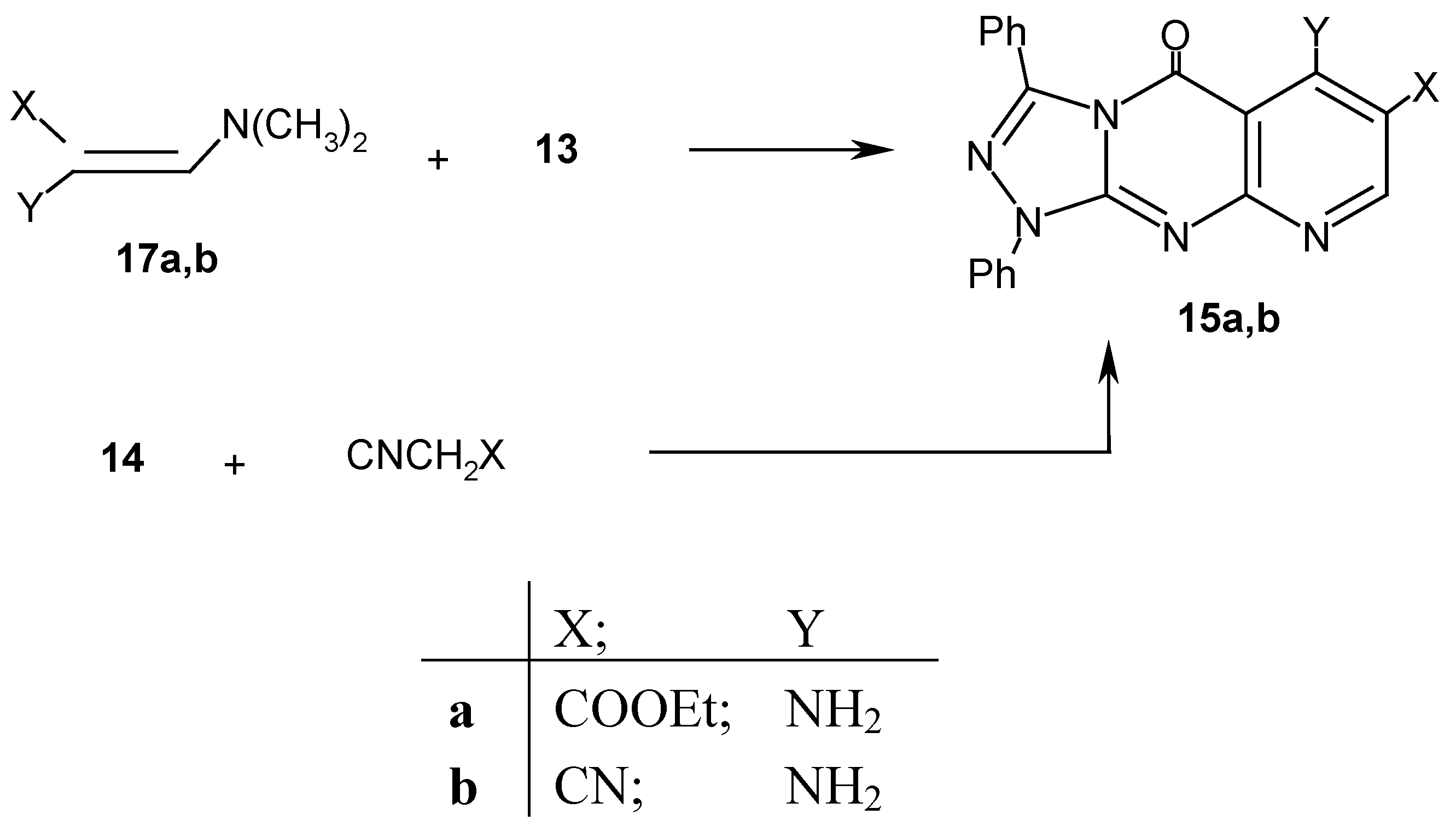
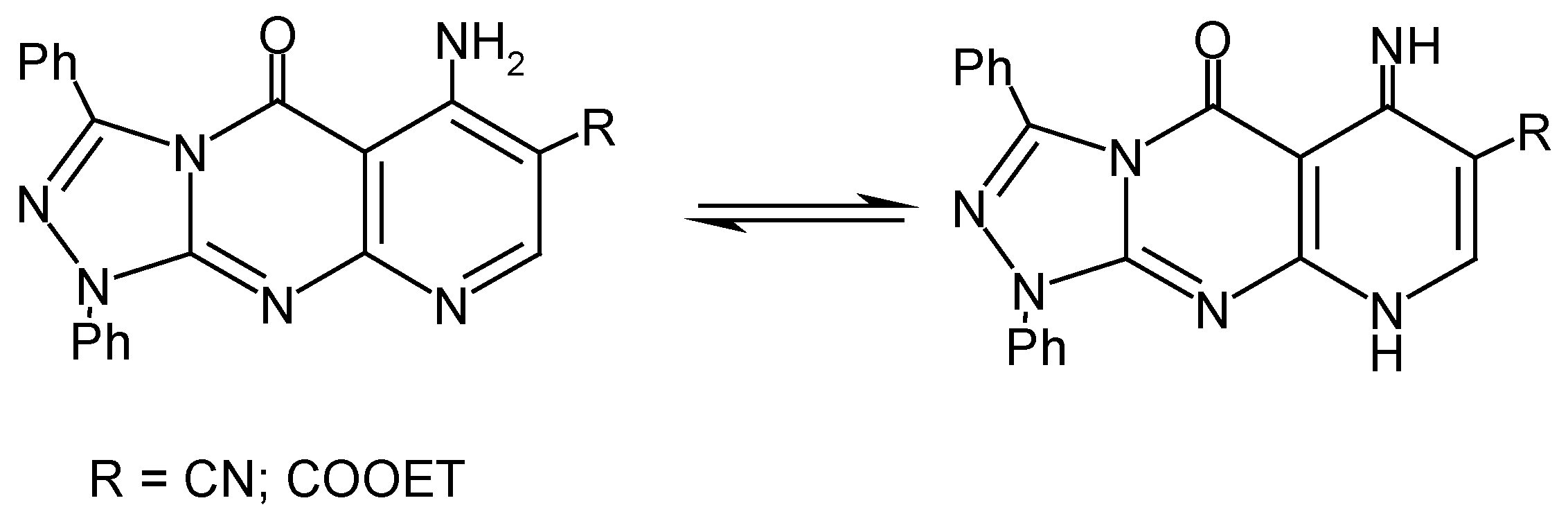
Experimental
General
| Compd. no. | Color | Yield (%) | m.p. °C solvent | Mol. formula (Mol. Wt) | Analysis Calcd (Found) | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | |||||
| 3a | yellowish | 75 | 260-261 | C13H9N3OS | 61.15 | 3.55 | 16.47 | 12.54 |
| white | DMF | 255.10 | 61.40 | 3.71 | 16.53 | 12.59 | ||
| 3b | yellow | 80 | 254-255 | C11H7N3OS2 | 50.56 | 2.70 | 16.09 | 24.51 |
| DMF | 261.08 | 50.54 | 2.52 | 16.37 | 24.45 | |||
| 5 | yellow | 85 | 339-341 | C7H10N4OS | 42.40 | 5.08 | 28.28 | 16.15 |
| DMSO | 198.11 | 42.49 | 5.15 | 28.36 | 16.33 | |||
| 11A | colorless | 78 | 281-282 | C26H17N5O | 75.15 | 4.12 | 16.86 | |
| DMF | 415.19 | 75.25 | 4.23 | 17.06 | ||||
| 11B | yellowish | 80 | 287-289 | C24H15N5OS | 68.38 | 3.58 | 16.62 | 7.59 |
| white | DMF | 421.17 | 61.31 | 3.74 | 16.52 | 7.36 | ||
| 11C | yellow | 76 | 286-288 | C23H17N5O3 | 67.12 | 4.16 | 17.03 | |
| DMF | 411.19 | 67.25 | 4.08 | 17.29 | ||||
| 11D | yellow | 75 | 216-218 | C21H15N5O3S | 60.41 | 3.62 | 16.78 | 7.67 |
| DMF | 417.17 | 60.26 | 3.40 | 16.63 | 7.82 | |||
| 14 | colorless | 90 | 208-209 | C20H18N6O | 67.00 | 5.06 | 23.46 | |
| DMF | 358.20 | 67.24 | 5.35 | 23.58 | ||||
| 15a | yellow | 75 | 350-352 | C23H18N6O3 | 64.76 | 4.25 | 19.71 | |
| DMF | 426.20 | 64.73 | 4.21 | 19.86 | ||||
| 15b | colorless | 80 | 335-337 | C21H13N7O | 66.46 | 3.45 | 25.85 | |
| DMF | 379.17 | 66.78 | 3.62 | 25.70 | ||||
| 18 | yellowish | 82 | 327-328 | C27H16N6O | 73.61 | 3.66 | 19.09 | |
| white | DMF | 440.19 | 73.79 | 3.51 | 19.24 | |||
| Compd. no. | IR (ν/cm-1) | M+ | 1H-NMR (δ/ppm) |
|---|---|---|---|
| 3a | 1686 (CO), 3255 (NH) | 255 | 7.56-7.59 (m, 3H, aromatic-H), 7.91 (d, 1H, pyridine), 8.18-8.22 (m, 2H, aromatic-H), 8.63 (d, 1H, pyridine), 12.62 (s, 1H, NH), 13.17 (s, 1H, NH). |
| 3b | 1680 (CO), 3343 (NH) | 261 | 7.25-9.22 (m, 5H, aromatic-H), 12.86 (s, 1H, NH), 13.32 (s, 1H, NH). |
| 5 | 1648 (CO), 3256 (NH) | 198 | 2.99 (s, 3H, CH3); 3.11 (s, 3H, CH3), 5.24 (s, 1H, N=CH), 8.14 (s, 1H, pyrimidine), 11.61 (s, 1H, NH), 11.79 (s, 1H, NH). |
| 11a | 1710 (CO) | 415 | 7.88 (d, 1H, pyridine); 7.56-8.00 (m, 15H, aromatic-H); 8.63 (d, 1H, pyridine). |
| 11b | 1702 (CO) | 421 | 7.23-8.49 (m, 13H, aromatic-H); 7.91 (d, 1H, pyridine); 8.73 (d, 1H, pyridine). |
| 11c | 1715 (CO) 1720 (ester CO) | 411 | 1.42 (t, J = 7Hz, 3H, CH3); 4.25 (q, J = 7Hz, 2H, CH2); 7.21-8.24 (m, 10H, aromatic-H); 7.72 (d, 1H, pyridine), 8.50 (d, 1H, pyridine). |
| 11d | 1700 (CO), 1715 (ester CO) | 417 | 1.41 (t, J = 7 Hz, 3H, CH33), 4.28 (q, J = 7 Hz, 2H, CH2), 7.73 (d, 1H, pyridine-H), 5.45-8.11 (m, 8H, aromatic-H), 8.55 (d, 1H, pyridine-H). |
| 14 | 1702 (CO) | 358 | 3.14 (s, 3H, CH3), 3.15 (s, 3H, CH3), 5.66 (s, 1H, N=CH), 7.21-8.40 (m, 10H, aromatic H), 8.59 (s, 1H, pyrimidine H-6). |
| 15a | 1690 (CO), 1715 (ester CO) | 426 | 1.29 (t, J=7Hz, 3H, CH3), 4.20 (q, J= 7Hz, 2H, CH2), 5.85 (s, 1H, NH), 7.46-8.10 (m, 10H, aromatic-H), 8.92 (s, 1H, pyridine), 11.78 (s, 1H, NH). |
| 15b | 1720 (CO), 2229 (CN), 3193, 3400 (2 NH) | 337 | 5.68 (s, 1H, NH), 7.47-8.17 (m, 10H, aromatic-H), 8.66 (s, 1H, pyridine), 11.73 (s, 1H, NH). |
| 18 | 1728 (CO), 2221 (CN) | 440 | 7.48-8.29 (m, 15H, aromatic-H); 9.15 (s, 1H, pyridine) |
References
- Elnagdi, M. H.; Al-Awdi, N.; Erian, A.W. Comprehensive Heterocyclic Chemistry II; Katritzky, A. R., Rees, C. W., Scriven, E. F. V., Eds.; Pergamon Press: London, 1996; Vol 4, p. 431. [Google Scholar]
- Desenko, S. V.; Komykhov, S. A.; Orlov, V. D.; Meier, H. J Hetrocyclic Chem. 1998, 35, 989.
- Quiroga, J.; Insuasty, B.; Graz, S.; Hernandez, P.; Bolafios, A.; Moreno, R.; Hormoza, A.; de Almeidas, H. J Heterocyclic Chem 1998, 35, 1333.
- Greenhill, J. V. Comprehensive Heterocyclic Chemistry II; Katritzky, A. R., Rees, C. W., Eds.; Pergamon Press: London, 1984; Vol 5, p. 305. [Google Scholar]
- Abdelhadi, H. A.; Elwan, N. M.; Abdallah, T. A.; Hassaneen, H. M. Tetrahedron 1996, 52, 3451.
- Hassaneen, H. M.; Abdelhadi, H. A.; Abdallah, T. A. Tetrahedron 2001, 57, 10133.
- Abdelhadi, H. A.; Abdallah, T. A.; Hassaneen, H. M. Heterocycles 1995, 41, 1999.
- Abdallah, T. A.; Abdelhadi, H. A.; Ibrahim, A. A.; Hassaneen, H. M. Synth Comm 2002, 32, 581.
- Awad, E. M.; Elwan, N. M.; Hassaneen, H. M.; Linden, A.; Heimgartner, H. Helv Chim Acta 2002, 85, 320.
- Awad, E. M.; Elwan, N. M.; Hassaneen, H. M.; Linden, A.; Heimgartner, H. Helv Chim Acta 2001, 84, 1172.
- Al–Omran, F.; Abdelkhalik, M. M.; Elnagdi, M. H. Heteroatom Chem 1995, 6, 545.
- Hübsch, W.; Pfleiderer, W. Helv Chim Acta 1988, 71, 1379.
- Wolkof, P. Can. J.Chem. 1975, 53, 1333.
- Eweiss, N. F.; Abdelhamid, A. O. J.Heterocycl Chem. 1980, 17, 1713.
- Mosselhi, A. N. Monatsh Chem. 2002, 133, 1297.
- Sample availability: Samples not available.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hassneen, H.M.; Abdallah, T.A. New Routes to Pyridino[2,3-d]pyrimidin-4-one and Pyridino-[2,3-d]triazolino[4,5-a]pyrimidin-5-one Derivatives. Molecules 2003, 8, 333-341. https://doi.org/10.3390/80300333
Hassneen HM, Abdallah TA. New Routes to Pyridino[2,3-d]pyrimidin-4-one and Pyridino-[2,3-d]triazolino[4,5-a]pyrimidin-5-one Derivatives. Molecules. 2003; 8(3):333-341. https://doi.org/10.3390/80300333
Chicago/Turabian StyleHassneen, Hamdi M., and Tayseer A. Abdallah. 2003. "New Routes to Pyridino[2,3-d]pyrimidin-4-one and Pyridino-[2,3-d]triazolino[4,5-a]pyrimidin-5-one Derivatives" Molecules 8, no. 3: 333-341. https://doi.org/10.3390/80300333
APA StyleHassneen, H. M., & Abdallah, T. A. (2003). New Routes to Pyridino[2,3-d]pyrimidin-4-one and Pyridino-[2,3-d]triazolino[4,5-a]pyrimidin-5-one Derivatives. Molecules, 8(3), 333-341. https://doi.org/10.3390/80300333




