Cytoprotective Compounds Interfere with the Nutraceutical Potential of Bread Supplemented with Green Coffee Beans
Abstract
1. Introduction
2. Materials and Methods
2.1. Gastrointestinal Processing and Preparation of Extracts
Plant Materials
2.2. Bread Preparation
2.3. Gastrointestinal Processing and Preparation of Extracts
2.4. Cell Cultures and Medium Supplementation
2.5. Cell Proliferation
2.6. Cell Motility
2.7. Phenolics Content
2.8. Analysis of Caffeine and Phenolic acids
2.9. Xanthine Oxidase Inhibition
2.10. Statistics
3. Results and Discussion
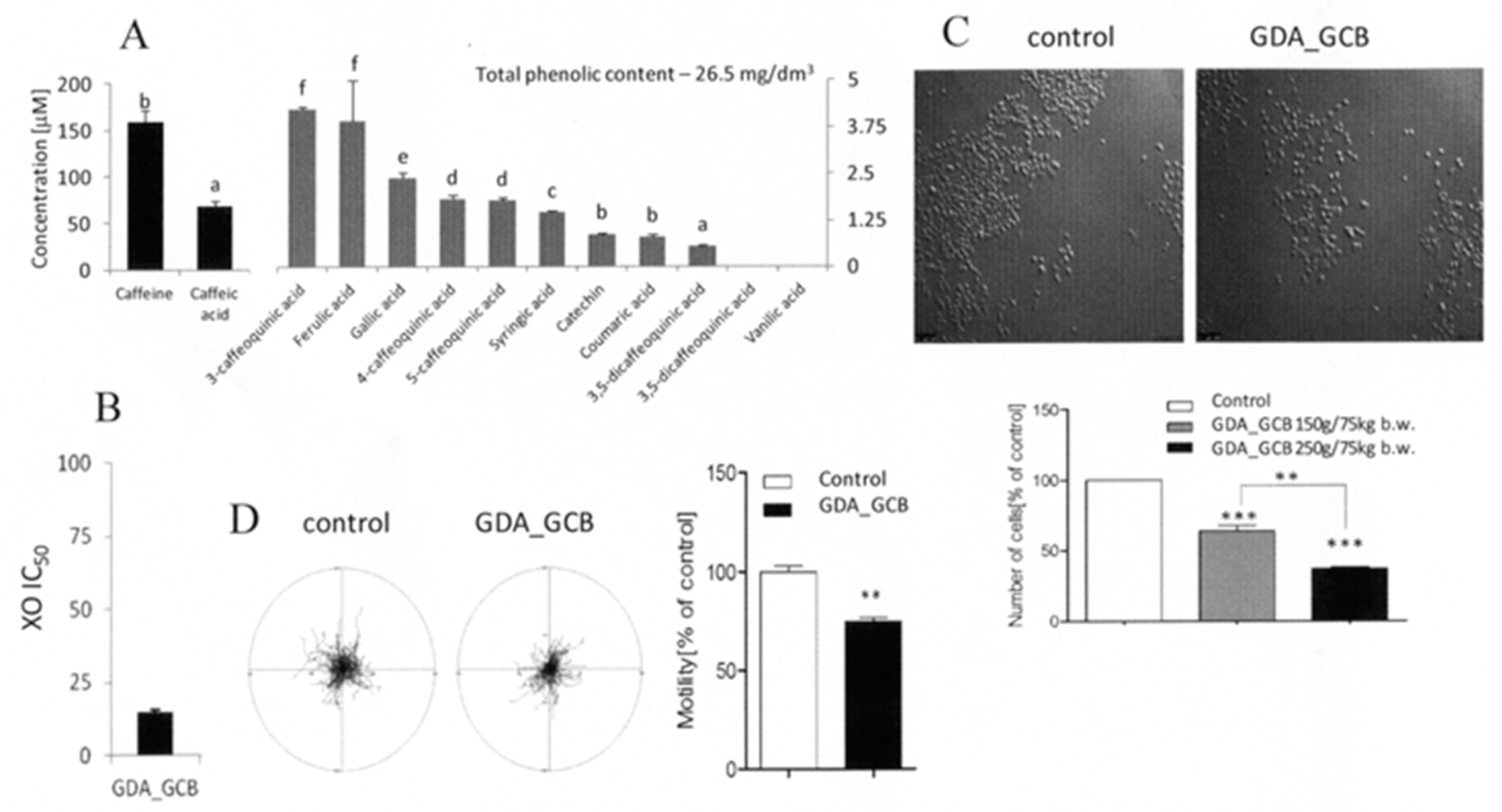
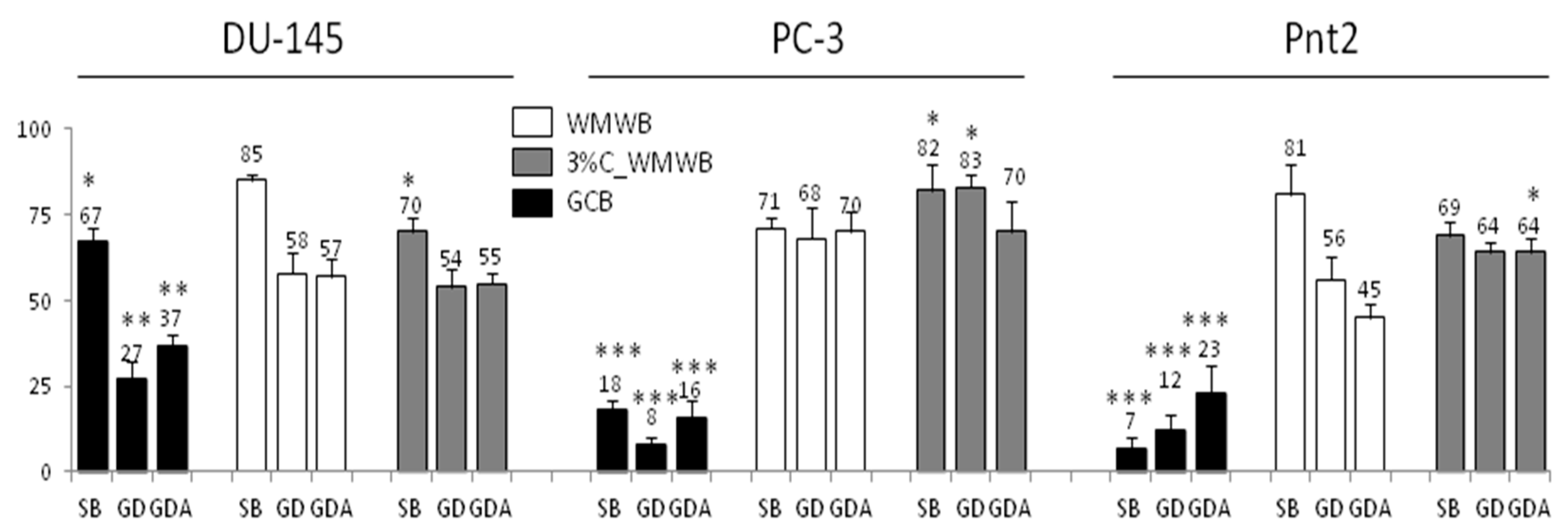
3.1. Bioavailable GCB Compounds Exert Cytostatic Effects on Prostate Cancer Cells
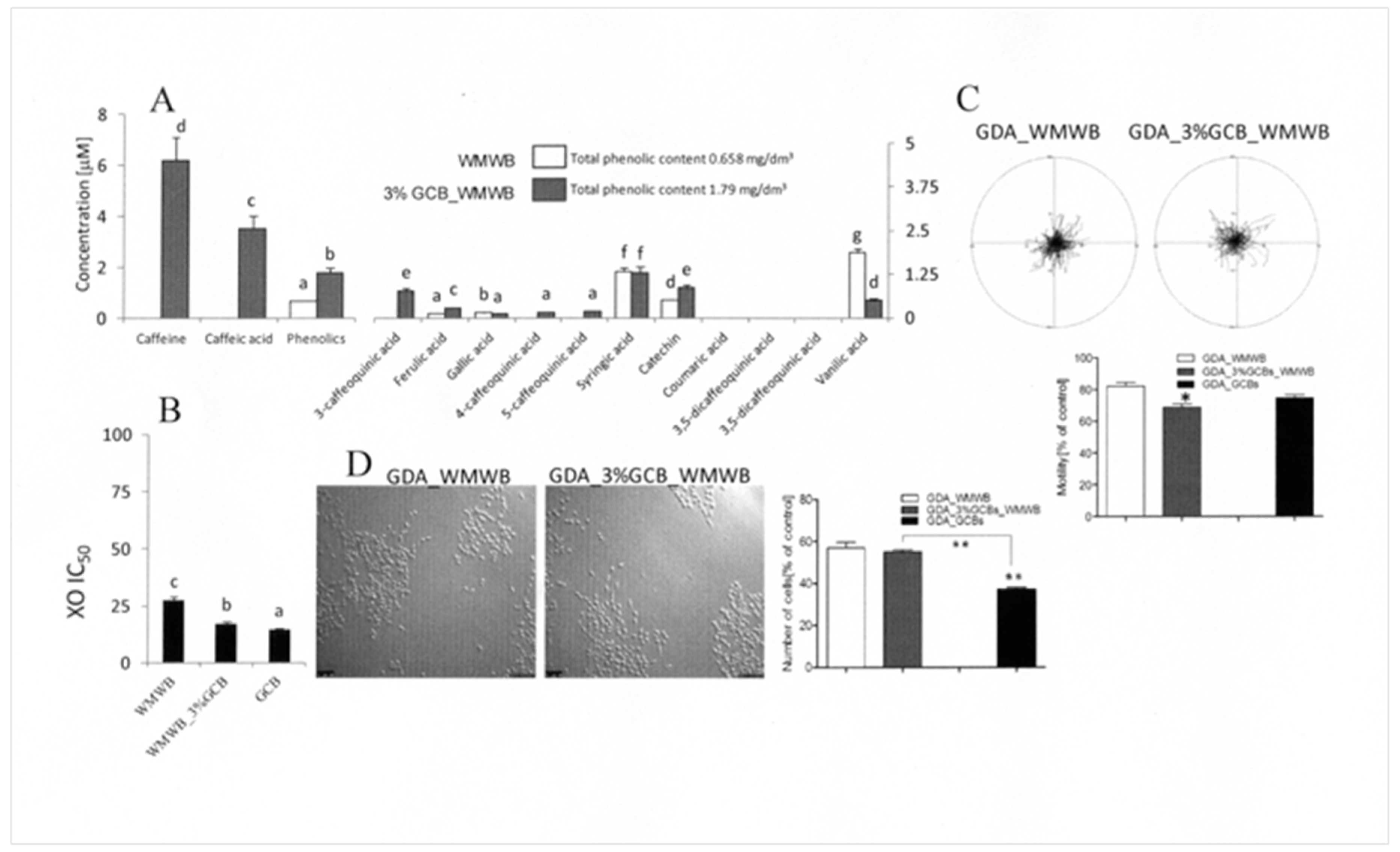
3.2. GCB Supplementation Slightly Affects the Cytostatic Properties of Bioavailable WMWB Compounds
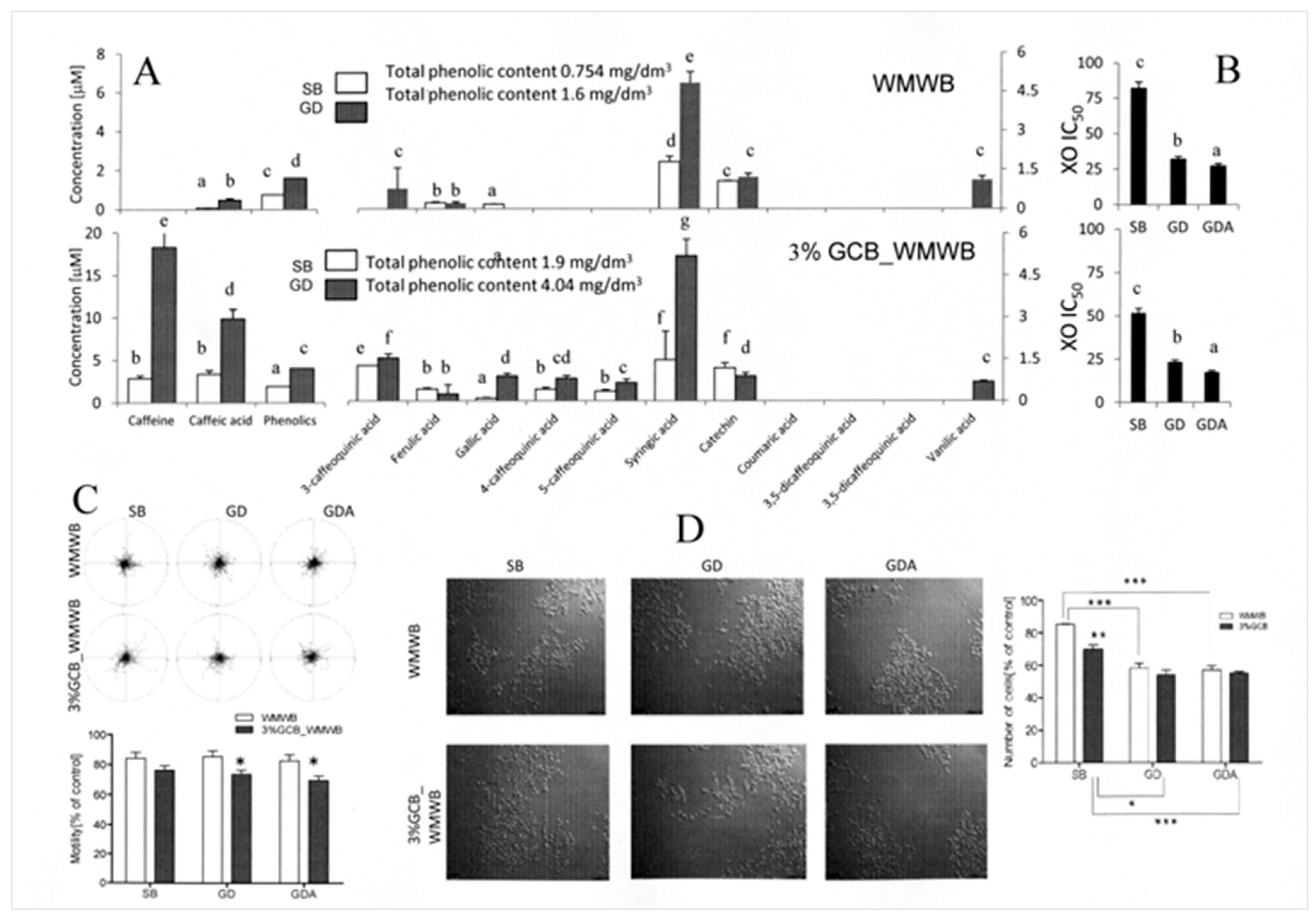
3.3. Bioaccessible WMWB Compounds Interfere with Cytostatic GCB Compounds
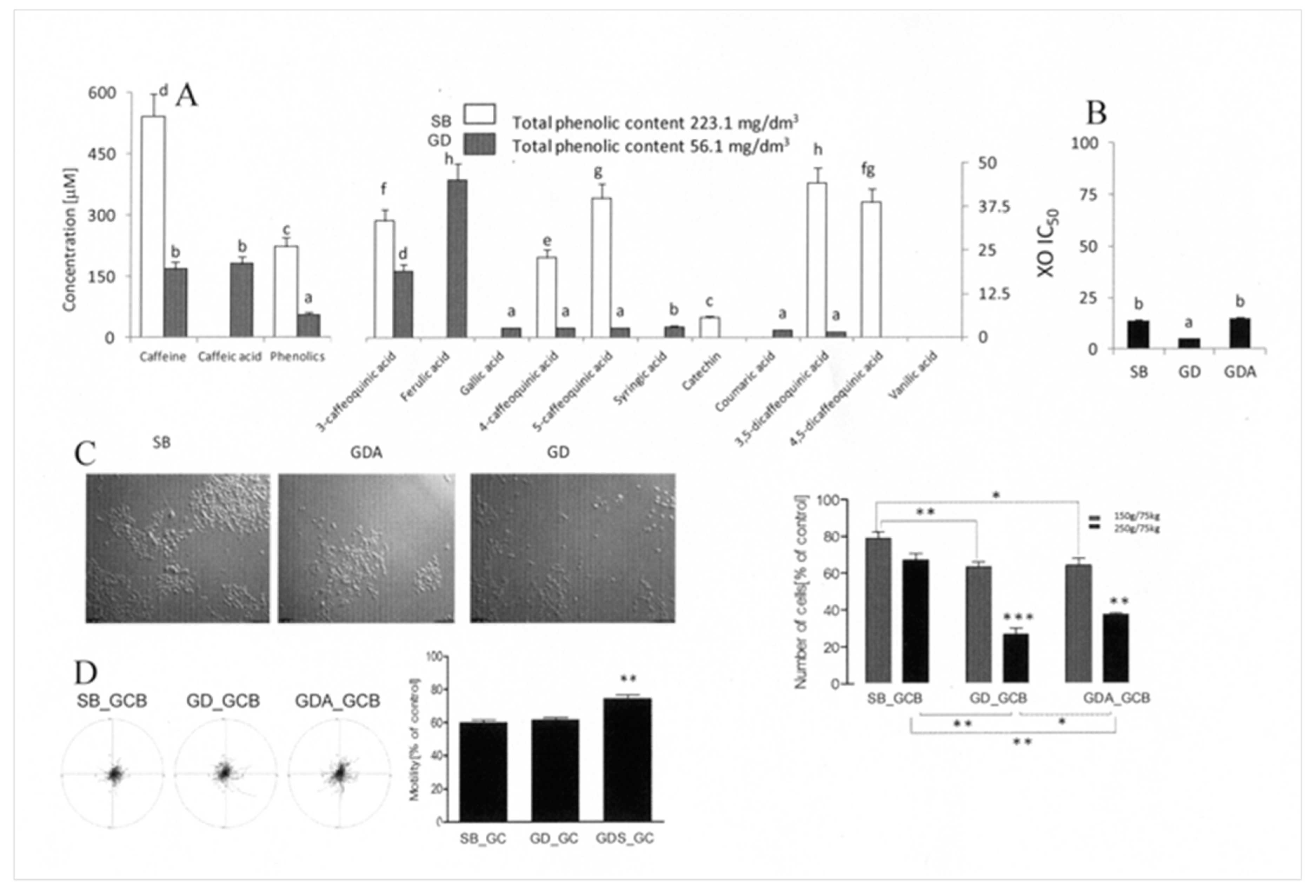
3.4. Gastrointestinal Digestion Attenuates the Activity of Cytoprotective GCB Compounds
3.5. Prostate Cells Differ in Their Reactivity to Cytostatic/Cytoprotective WMWB/GCB Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Mehari, B.; Redi-Abshiro, M.; Chandravanshi, B.S.; Combrinck, S.; Atlabachew, M.; McCrindle, R. Profiling of phenolic compounds using UPLC-MS for determining the geographical origin of green coffee beans from Ethiopia. J. Food Compos. Anal. 2016, 45, 16–25. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Kordowska-Wiater, M.; Domań-Pytka, M. Influence of elicitation and germination conditions on biological activity of wheat sprouts. J. Chem. 2015, 2015, 649709. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Pałecz, B.; Rachwał-Rosiak, D.; Belica, S.; Den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H. Interactions of free and encapsulated hydroxycinnamic acids from green coffee with egg ovalbumin, whey and soy protein hydrolysates. LWT-Food Sci. Technol. 2016, 65, 823–831. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Pecio, Ł.; Różyło, R.; Świeca, M.; Krzykowski, A.; Rudy, S. Ground green coffee beans as a functional food supplement–Preliminary study. LWT-Food Sci. Technol. 2015, 63, 691–699. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Kowalska, I.; Pecio, Ł.; Durak, A.; Sęczyk, Ł. Lipoxygenase inhibitors and antioxidants from green coffee—mechanism of action in the light of potential bioaccessibility. Food Res. Int. 2014, 61, 48–55. [Google Scholar] [CrossRef]
- Onakpoya, I.; Terry, R.; Ernst, E. The use of green coffee extract as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. Gastroenterol. Res. Pract. 2011, 2011, 382852. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Kaszuba, K.; Piwowarczyk, K.; Świeca, M.; Dziki, D.; Czyż, J. Onion skin—Raw material for the production of supplement that enhances the health-beneficial properties of wheat bread. Food Res. Int. 2015, 73, 97–106. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. J. Funct. Foods 2012, 4, 872–882. [Google Scholar] [CrossRef]
- Izli, N.; Yildiz, G.; Ünal, H.; Işik, E.; Uylaşer, V. Effect of different drying methods on drying characteristics, colour, total phenolic content and antioxidant capacity of Goldenberry (Physalis peruviana L.). Int. J. Food Sci. Technol. 2014, 49, 9–17. [Google Scholar] [CrossRef]
- Wang, A.; Wang, S.; Zhu, C.; Huang, H.; Wu, L.; Wan, X.; Yang, X.; Zhang, H.; Miao, R.; He, L.; et al. Coffee and cancer risk: A meta-analysis of prospective observational studies. Sci. Rep. 2016, 6, 33711. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitrodigestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B. Wheat bread enriched with green coffee–In vitro bioaccessibility and bioavailability of phenolics and antioxidant activity. Food Chem. 2017, 221, 1451–1457. [Google Scholar] [CrossRef]
- Piwowarczyk, K.; Wybieralska, E.; Baran, J.; Borowczyk, J.; Rybak, P.; Kosińska, M.; Włodarczyk, A.J.; Michalik, M.; Siedlar, M.; Madeja, Z.; et al. Fenofibrate enhances barrier function of endothelial continuum within the metastatic niche of prostate cancer cells. Expert Opin. Ther. Targets 2015, 19, 163–176. [Google Scholar] [CrossRef]
- Piwowarczyk, K.; Paw, M.; Ryszawy, D.; Rutkowska-Zapała, M.; Madeja, Z.; Siedlar, M.; Czyż, J. Connexin43high prostate cancer cells induce endothelial connexin43 up-regulation through the activation of intercellular ERK1/2-dependent signaling axis. Eur. J. Cell Biol. 2017, 96, 337–346. [Google Scholar] [CrossRef]
- Koczurkiewicz, P.; Podolak, I.; Skrzeczyńska-Moncznik, J.; Sarna, M.; Wójcik, K.A.; Ryszawy, D.; Galanty, A.; Lasota, S.; Madeja, Z.; Czyz, J.; et al. Triterpene saponosides from Lysimachia ciliata differentially attenuate invasive potential of prostate cancer cells. Chem. Biol. Interact. 2013, 206, 6–17. [Google Scholar] [CrossRef]
- Daniel-Wójcik, A.; Misztal, K.; Bechyne, I.; Sroka, J.; Miekus, K.; Madeja, Z.; Czyz, J. Cell motility affects the intensity of gap junctional coupling in prostate carcinoma and melanoma cell populations. Int. J. Oncol. 2008, 33, 309–315. [Google Scholar]
- Singleton, V.L.; Rossi, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstics acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Swieca, M.; Baraniak, B. Nutritional and antioxidant potential of lentil sprouts affected by elicitation with temperature stress. J. Agric. Food Chem. 2014, 62, 3306–3313. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Nowak, R. Mechanism of action and interactions between xanthine oxidase inhibitors derived from natural sources of chlorogenic and ferulic acids. Food Chem. 2017, 225, 1451–1457. [Google Scholar] [CrossRef]
- Sweeney, A. P.; Wyllie, S.G.; Shalliker, R. a.; Markham, J.L. Xanthine oxidase inhibitory activity of selected Australian native plants. J. Ethnopharmacol. 2001, 75, 273–277. [Google Scholar] [CrossRef]
- Baeza, G.; Amigo-Benavent, M.; Sarriá, B.; Goya, L.; Mateos, R.; Bravo, L. Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res. Int. 2014, 62, 1038–1046. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sȩczyk, Ł.; Złotek, U.; Rózyło, R.; Kaszuba, K.; Ryszawy, D.; Czyz, J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. BioMed Res. Int. 2014, 2014, 608053. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Waterhouse, G.I.; Quek, S.; Perera, C.O. Physicochemical properties of bread dough and finished bread with added pectin fiber and phenolic antioxidants. J. Food Sci. 2011, 76, 97–107. [Google Scholar] [CrossRef]
- Li Kwok Cheong, J.D.; Croft, K.D.; Henry, P.D.; Matthews, V.; Hodgson, J.M.; Ward, N.C. Green coffee polyphenols do not attenuate features of the metabolic syndrome and improve endothelial function in mice fed a high fat diet. Arch. Biochem. Biophys. 2014, 559, 46–52. [Google Scholar] [CrossRef]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Wilms, L.C.; Beetstra, S.A.J.N.; Heijnen, C.G.M.; Voss, H.-P.; Bast, A. Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. J. Agric. Food Chem. 2002, 50, 1184–1187. [Google Scholar] [CrossRef]
- Kweon, M.-H.; Hwang, H.-J.; Sung, H.-C. Identification and Antioxidant Activity of Novel Chlorogenic Acid Derivatives from Bamboo (Phyllostachys edulis). J. Agric. Food Chem. 2001, 49, 4646–4655. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Cheng, K.-W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Fletcher, R.J.; Bell, I.P.; Lambert, J.P. Public health aspects of food fortification: A question of balance. Proc. Nutr. Soc. 2004, 63, 605–614. [Google Scholar] [CrossRef]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef]
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R.; World Health Organization; Food and Agricultural Organization of the United Nations. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006; ISBN 9241594012. [Google Scholar]
- Takahama, U.; Tanaka, M.; Hirota, S. Flour and Breads and Their Fortification in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123808868. [Google Scholar]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Dröge, W.; Schipper, H.M. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 2007, 6, 361–370. [Google Scholar] [CrossRef]
- Wu, N.; Zu, Y.; Fu, Y.; Kong, Y.; Zhao, J.; Li, X.; Li, J.; Wink, M.; Efferth, T. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from Geranium sibiricum L. J. Agric. Food Chem. 2010, 58, 4737–4743. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E. Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chem. 2005, 92, 193–202. [Google Scholar] [CrossRef]
- Ichida, K.; Amaya, Y.; Okamoto, K.; Nishino, T. Mutations associated with functional disorder of xanthine oxidoreductase and hereditary xanthinuria in humans. Int. J. Mol. Sci. 2012, 13, 15475–15495. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlik-Dziki, U.; Luty, M.; Dziki, D.; Świeca, M.; Piwowarczyk, K.; Złotek, U.; Czyż, J. Cytoprotective Compounds Interfere with the Nutraceutical Potential of Bread Supplemented with Green Coffee Beans. Antioxidants 2019, 8, 228. https://doi.org/10.3390/antiox8070228
Gawlik-Dziki U, Luty M, Dziki D, Świeca M, Piwowarczyk K, Złotek U, Czyż J. Cytoprotective Compounds Interfere with the Nutraceutical Potential of Bread Supplemented with Green Coffee Beans. Antioxidants. 2019; 8(7):228. https://doi.org/10.3390/antiox8070228
Chicago/Turabian StyleGawlik-Dziki, Urszula, Marcin Luty, Dariusz Dziki, Michał Świeca, Katarzyna Piwowarczyk, Urszula Złotek, and Jarosław Czyż. 2019. "Cytoprotective Compounds Interfere with the Nutraceutical Potential of Bread Supplemented with Green Coffee Beans" Antioxidants 8, no. 7: 228. https://doi.org/10.3390/antiox8070228
APA StyleGawlik-Dziki, U., Luty, M., Dziki, D., Świeca, M., Piwowarczyk, K., Złotek, U., & Czyż, J. (2019). Cytoprotective Compounds Interfere with the Nutraceutical Potential of Bread Supplemented with Green Coffee Beans. Antioxidants, 8(7), 228. https://doi.org/10.3390/antiox8070228









