Treatment with, Resveratrol, a SIRT1 Activator, Prevents Zearalenone-Induced Lactic Acid Metabolism Disorder in Rat Sertoli Cells
Abstract
:1. Introduction
2. Results
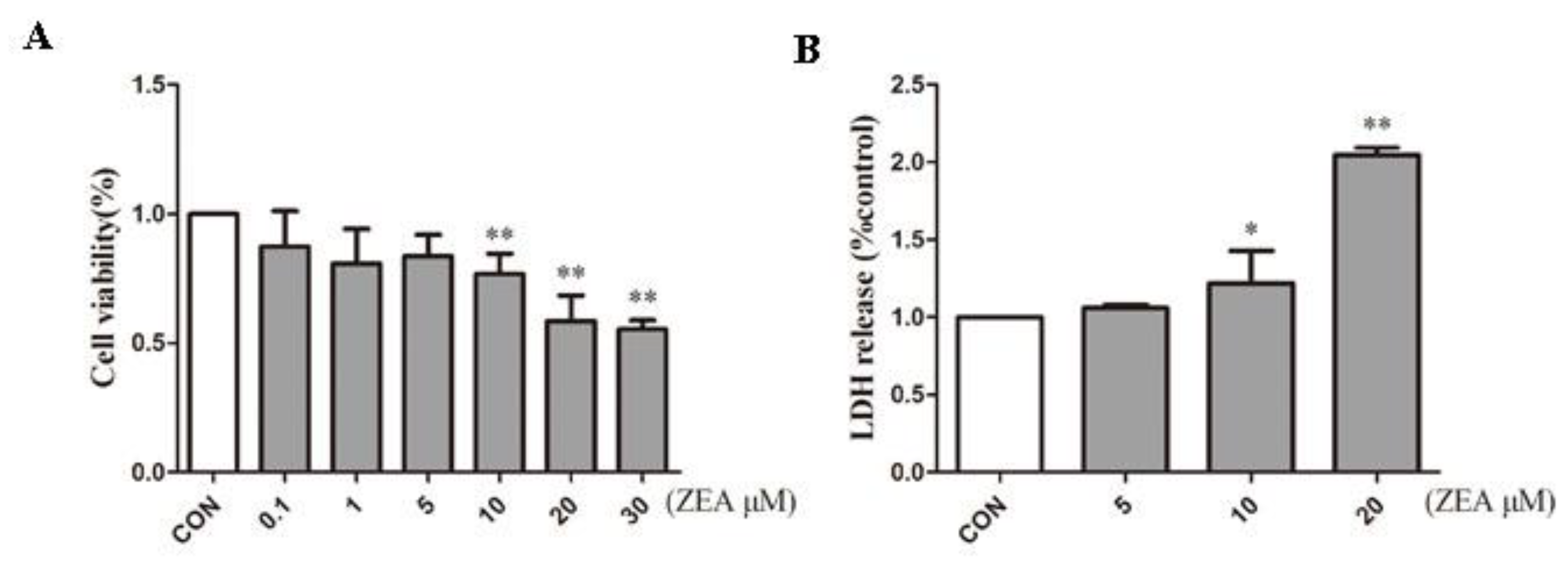
2.1. ZEA Inhibits Proliferation of SCs
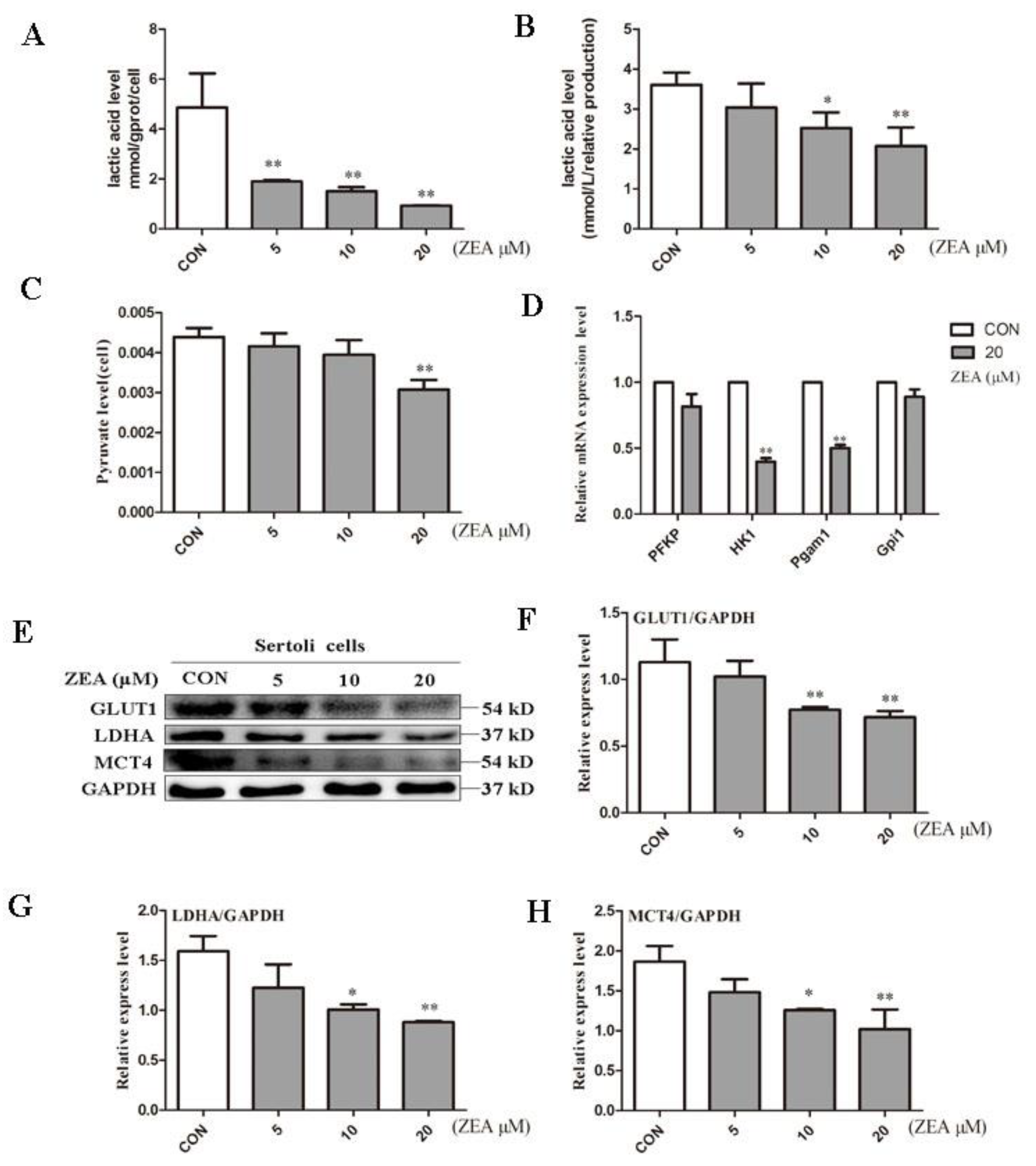
2.2. ZEA Decreased Lactic Acid Production
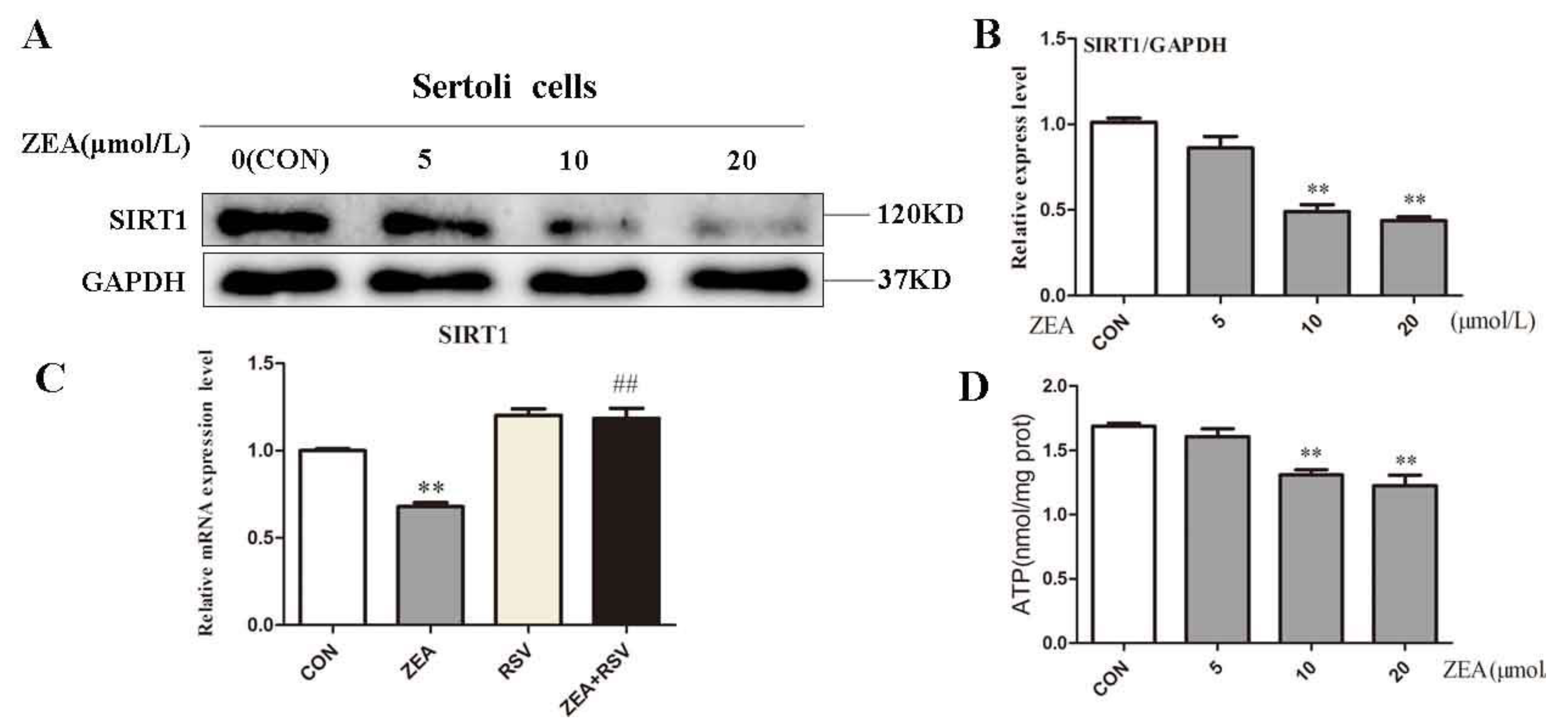
2.3. ZEA Inhibits Expression of SIRT1 and ATP Level
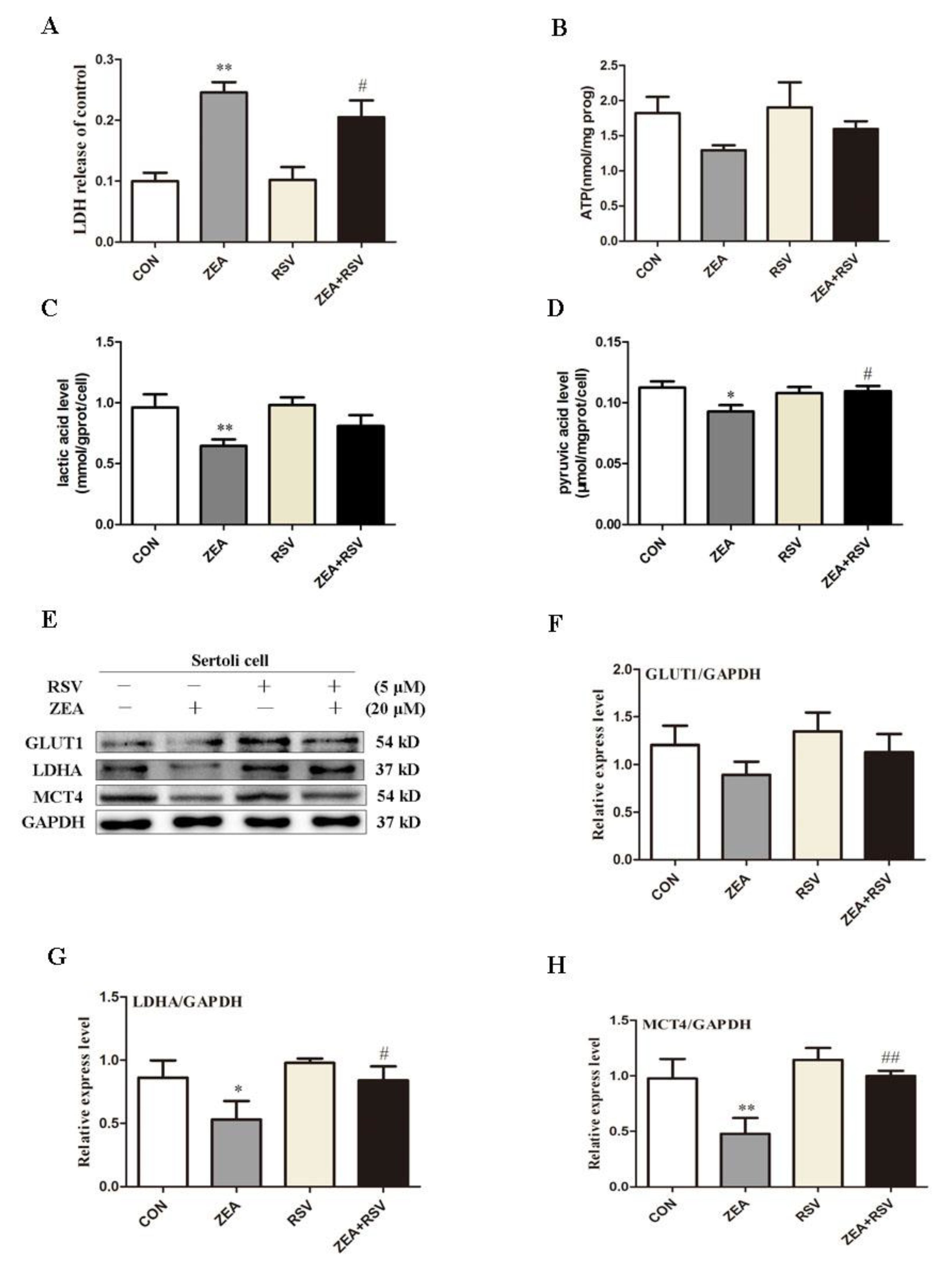
2.4. SIRT1 Activator RSV Treatment Partially Reverses ZEA-Induced Cytotoxicity and the Decline in Lactic Acid Production
3. Discussion
4. Materials and Methods
4.1. Reagents Chemicals and Antibodies
4.2. Cell Cultures
4.3. LDH (Lactate Dehydrogenase) Release Assay for Cytotoxicity
4.4. Lactic Acid Assay Kit to Detect Intracellular and Extracellular Lactate Levels
4.5. Pyruvate Assay Kit to Detect Intracellular Pyruvate Levels
4.6. Western Blotting Analysis
4.7. Total RNA Extraction and Quantitative Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCA | bicinchoninic acid |
| CCK-8 | cell counting kit-8 |
| DMSO | dimethyl sulfoxide |
| DMEM/F-12 | dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F-12) |
| ECL | electrochemiluminescence |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GLUT1 | glucose transporter 1 |
| HK1 | hexokinase 1 |
| LDH | lactate dehydrogenase |
| LD50 | lethal dose 50 |
| MCT4 | monocarboxylate transporters4 |
| M | mol/L |
| NAD | nicotinamide adenine dinucleotide |
| PFKP | phosphofructokinase |
| Pgam1 | phosphoglycerate mutase1 |
| PBS | phosphate buffer saline |
| RSV | Resveratrol |
| RIPA | radio immunoprecipitation assay |
| SCs | Sertoli cells |
| SIRT1 | silencing information regulator 1 |
| ZEA | zearalenone. |
References
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Mycotoxins in Cereals and Their Products-Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Goodman, T.; Scott, P.M.; Watanabe, H. Risk assessment of the mycotoxin zearalenone. Regul Toxicol Pharm. 1987, 7, 253–306. [Google Scholar] [CrossRef]
- Long, M.; Chen, X.; Wang, N.; Wang, M.; Pan, J.; Tong, J.; Li, P.; Yang, S.; He, J. Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines. Molecules 2018, 23, 1508. [Google Scholar] [CrossRef] [PubMed]
- Benzoni, E.; Minervini, F.; Giannoccaro, A.; Fornelli, F.; Vigo, D.; Visconti, A. Influence of in vitro exposure to mycotoxin zearalenone and its derivatives on swine sperm quality. Reprod. Toxicol. 2008, 25, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tsakmakidis, I.; Lymberopoulos, A.; Vainas, E.; Boscos, C.; Kyriakis, S.; Alexopoulos, C. Study on the in vitro effect of zearalenone and alpha-zearalenol on boar sperm-zona pellucida interaction by hemizona assay application. J. Appl. Toxicol. 2010, 27, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Gu, J.; Yuan, Y.; Liu, X.; Zheng, W.; Huang, Q.; Liu, Z.; Bian, J. Zearalenone inhibits testosterone biosynthesis in mouse Leydig cells via the crosstalk of estrogen receptor signaling and orphan nuclear receptor Nur77 expression. Toxicology in Vitro 2014, 28, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.; Dong, S.; Chen, X.; Zhang, Y.; He, J. Characterization of semen quality, testicular marker enzyme activities and gene expression changes in the blood testis barrier of Kunming mice following acute exposure to zearalenone. Environ. Sci. Pollut. Res. 2017, 24, 27235–27243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Pan, S.; Wang, G.; Wang, Y.J.; Liu, Q.; Gu, J.; Yuan, Y.; Liu, X.Z.; Liu, Z.P.; Bian, J.C. Zearalenone impairs the male reproductive system functions via inducing structural and functional alterations of sertoli cells. Env. Toxicol Pharm. 2016, 42, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Crisã3Stomo, L.; Alves, M.G.; Gorga, A.; Sousa, M.; Riera, M.F.; Galardo, M.N.; Meroni, S.B.; Oliveira, P.F. Molecular Mechanisms and Signaling Pathways Involved in the Nutritional Support of Spermatogenesis by Sertoli Cells. Methods Mol Biol 2018, 1748, 129–155. [Google Scholar]
- Caminos, J.E.; Nogueiras, R.; Gaytã, n. F.; Pineda, R.; Gonzà lez, C.R.; Barreiro, M.L.; Castaã±O, J.P.; Malagã3N, M.M.; Pinilla, L.; Toppari, J. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology 2008, 149, 3390–3402. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Sem. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueiras, R.; Barreiro, M.L.; Caminos, J.E.; Gaytán, F.; Suominen, J.S.; Navarro, V.M.; Casanueva, F.F.; Aguilar, E.; Toppari, J.; Diéguez, C. Novel expression of resistin in rat testis: Functional role and regulation by nutritional status and hormonal factors. J. Cell Sci. 2004, 117, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Boussouar, F.; Benahmed, M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. Tem. 2004, 15, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg Effect Revisited—Lesson from the Sertoli Cell. Med. Res. Rev. 2015, 35, 126–151. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Hall, P.F. Metabolism of round spermatids from rats: Lactate as the preferred substrate. Biol. Reprod. 1982, 26, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Rato, L.; Rui, A.C.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. Cmls 2013, 70, 777. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Martins, A.D.; Rato, L.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Bba - Mol. Basis Dis. 2013, 1832, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Chen, G.; Wang, L.; Kong, J.; Pan, J.; Xi, Y.; Shen, F.; Huang, Z. Triptolide-Induced Mitochondrial Damage Dysregulates Fatty Acid Metabolism in Mouse Sertoli Cells. Toxicol. Lett. 2018, 291, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Tang, B.L. Sirtuins’ modulation of autophagy. J. Cell. Physiol. 2013, 228, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Li, X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai) 2013, 45, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Agostina, G.; Gustavo, M.R.; Mariana, R.; Eliana, H.P.; María, D.C.C.; Selva, B.C.; María, F.R.; María, N.G.; Silvina, B.M. Effect of resveratrol on Sertoli cell proliferation. J. Cell. Biochem. 2018. [Google Scholar]
- Liu, C.; Song, Z.; Wang, L.; Yu, H.; Liu, W.; Shang, Y.; Xu, Z.; Zhao, H.; Gao, F.; Wen, J. Sirt1 regulates acrosome biogenesis by modulating autophagic flux during spermiogenesis in mice. Development 2017, 144, 441–451. [Google Scholar] [CrossRef]
- Seifert, E.L.; Caron, A.Z.; Morin, K.; Coulombe, J.; He, X.H.; Jardine, K.; Dewardarch, D.; Boekelheide, K.; Harper, M.E.; Mcburney, M.W. SirT1 catalytic activity is required for male fertility and metabolic homeostasis in mice. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Abuamero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and Ophthalmic Diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Siva, L.; Wood, J.G.; Zipkin, R.E.; Phuong, C.; Anne, K.; Li-Li, Z. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Yin, X.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Resveratrol-Induced White Adipose Tissue Browning in Obese Mice by Remodeling Fecal Microbiota. Molecules 2018, 23, 3356. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Carlos, L.; Wilhelm, H.; Gygi, S.P.; Spiegelman, B.M.; Pere, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Taher, M.; Leen, W.G.; Wevers, R.A.; Willemsen, M.A. Lactate and its many faces. Eur. J. Paediatr. Neurol. 2016, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Manerba, M.; Ianni, L.D.; Govoni, M.; Roberti, M.; Recanatini, M.; Stefano, G.D. Lactate dehydrogenase inhibitors can reverse inflammation induced changes in colon cancer cells. Eur. J. Pharm. Sci. 2017, 96, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Brauchi, S.; Rauch, M.C.; Alfaro, I.E.; Cea, C.; Concha, I.I.; Benos, D.J.; Reyes, J.G. Kinetics, molecular basis, and differentiation of L-lactate transport in spermatogenic cells. Ajp Cell Physiol. 2005, 288, C523–C534. [Google Scholar] [CrossRef] [PubMed]
- Scialli, A.R. The sertoli cell: Russell LD, Griswold MD, eds. Clearwater, Florida: Cache River Press; 1993. 801 pages, $137.50. Reprod. Toxicol. 1995, 9, 211–213. [Google Scholar]
- Russell, L.D. Sertoli-germ cell interrelations: A review. Gamete Res. 1980, 3, 179–202. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Jiao, R.; Peng, Z.; Li, Y.; Bai, W. Toxic effects of zearalenone on gametogenesis and embryonic development: A molecular point of review. Food Chem. Toxicol. 2018, 119, 24–30. [Google Scholar] [CrossRef]
- Luís, R.; Alves, M.G.; Sílvia, S.; Rui, A.C.; Cavaco, J.E.; Oliveira, P.F. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci. Rep. 2012, 32, 61–69. [Google Scholar]
- Alves, M.G.; Neuhaus-Oliveira, A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Exposure to 2,4-dichlorophenoxyacetic acid alters glucose metabolism in immature rat Sertoli cells. Reprod. Toxicol. 2013, 38, 81–88. [Google Scholar] [CrossRef]
- Yu, K.; Deng, S.L.; Sun, T.C.; Li, Y.Y.; Liu, Y.X. Melatonin Regulates the Synthesis of Steroid Hormones on Male Reproduction: A Review. Molecules 2018, 23, 447. [Google Scholar] [CrossRef]
- Adibnia, E.; Razi, M.; Malekinejad, H. Zearalenone and 17 β-estradiol induced damages in male rats reproduction potential; evidence for ERα and ERβ receptors expression and steroidogenesis. Toxicon 2016, 120, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Finkgremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar]
- Rato, L.; Duarte, A.I.; Tomás, G.D.; Santos, M.S.; Moreira, P.I.; Socorro, S.; Cavaco, J.E.; Alves, M.G.; Oliveira, P.F. Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim. Et Biophys. Acta 2014, 1837, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.L.; Wang, B.J.; Wang, L.; Shan, Y.P.; Zou, H.; Song, R.L.; Wang, T.; Gu, J.H.; Yuan, Y.; Liu, X.Z. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Beauvieux, M.C.; Stephant, A.; Gin, H.; Serhan, N.; Couzigou, P.; Gallis, J.L. Resveratrol mainly stimulates the glycolytic ATP synthesis flux and not the mitochondrial one: A saturation transfer NMR study in perfused and isolated rat liver. Pharmacol. Res. 2013, 78, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Lagouge, M.; Canto, C.; Strehle, A.; Houten, S.M.; Milne, J.C.; Lambert, P.D.; Mataki, C.; Elliott, P.J.; Auwerx, J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008, 8, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, B.; Si, M.; Hui, Z.; Song, R.; Gu, J.; Yan, Y.; Liu, X.; Zhu, G.; Bai, J. Zearalenone altered the cytoskeletal structure via ER stress- autophagy- oxidative stress pathway in mouse TM4 Sertoli cells. Sci. Rep. 2018, 8, 3320. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Zheng, W.L.; Feng, N.N.; Wang, T.; Zou, H. The Effects of Autophagy and PI3K/AKT/m-TOR Signaling Pathway on the Cell-Cycle Arrest of Rats Primary Sertoli Cells Induced by Zearalenone. Toxins 2018, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.Q.; Zhang, G.Y.; Li, W.Z. Resveratrol attenuates the toxicity of zearalenone toward human embryonic kidney 293 cells. Mod. Food Sci. Technol. 2016, 32, 10. [Google Scholar]
- Rowlands, B.D.; Lau, C.L.; Ryall, J.G.; Thomas, D.S.; Klugmann, M.; Beart, P.M.; Rae, C.D. Silent information regulator 1 modulator resveratrol increases brain lactate production and inhibits mitochondrial metabolism, whereas SRT1720 increases oxidative metabolism. J. Neurosci. Res. 2015, 93, 1147–1156. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells – Immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, K.; Aito, H.; Aalto, K.; Pentikäinen, V.; Dunkel, L. Lactate inhibits germ cell apoptosis in the human testis. Mol. Hum. Reprod. 2002, 8, 109. [Google Scholar] [CrossRef]
- Courtens, J.L.; Ploen, L. Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol. Reprod. 1999, 61, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Son, H.Y.; Cho, S.W.; Ha, C.S.; Kang, B.H. Zearalenone induces male germ cell apoptosis in rats. Toxicol. Lett. 2003, 138, 185–192. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds are not available from authors. |
| Primers | Primer Sequence | Product Length (bp) |
|---|---|---|
| PFKP | Sence:5’- TCAAACTCTCCGAGAACCGTG -3’ | 160 |
| Antisense: 5’- TGTCCAAGAATGGTGACCCG -3’ | ||
| HK1 | Sence: 5’- CATTGTCTCCTGCATCTCCGA -3’ | 64 |
| Antisense: 5’- ATTCCGCAATCTAGGCTCGTC -3’ | ||
| Pgam1 | Sence: 5’- TCGTCATGGCTGCCTACAAG -3’ | 156 |
| Antisense:5’-CATAGCCAGCATCTCGCAAC -3’ | ||
| Gpi1 | Sence: 5’-CCTGTCTACGAACACGGACAA -3’ | 127 |
| Antisense: 5’- ATGCAGTGCGATGGAGAGTC -3’ | ||
| SIRT1 | Sence: 5’- GACGACGAGGGCGAGGAG -3’ | 363 |
| Antisense: 5’- ACAGGAGGTTGTCTCGGTAGC -3’ | ||
| GAPDH | Sence: 5’- GATGACATCAAGAAGGTGGTGA -3’ | 1802 |
| Antisense: 5’- TTATACCGATGTCGTTGTCCCA -3’ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, P.; Feng, N.; Zheng, W.; Zheng, H.; Zou, H.; Yuan, Y.; Liu, X.; Liu, Z.; Gu, J.; Bian, J. Treatment with, Resveratrol, a SIRT1 Activator, Prevents Zearalenone-Induced Lactic Acid Metabolism Disorder in Rat Sertoli Cells. Molecules 2019, 24, 2474. https://doi.org/10.3390/molecules24132474
Cai P, Feng N, Zheng W, Zheng H, Zou H, Yuan Y, Liu X, Liu Z, Gu J, Bian J. Treatment with, Resveratrol, a SIRT1 Activator, Prevents Zearalenone-Induced Lactic Acid Metabolism Disorder in Rat Sertoli Cells. Molecules. 2019; 24(13):2474. https://doi.org/10.3390/molecules24132474
Chicago/Turabian StyleCai, Peirong, Nannan Feng, Wanglong Zheng, Hao Zheng, Hui Zou, Yan Yuan, Xuezhong Liu, Zongping Liu, Jianhong Gu, and Jianchun Bian. 2019. "Treatment with, Resveratrol, a SIRT1 Activator, Prevents Zearalenone-Induced Lactic Acid Metabolism Disorder in Rat Sertoli Cells" Molecules 24, no. 13: 2474. https://doi.org/10.3390/molecules24132474





