Comparative Analysis of the Soluble Proteome and the Cytolytic Activity of Unbleached and Bleached Millepora complanata (“Fire Coral”) from the Mexican Caribbean
Abstract
:1. Introduction
2. Results
2.1. Sample Collection and Soluble Proteome Extraction
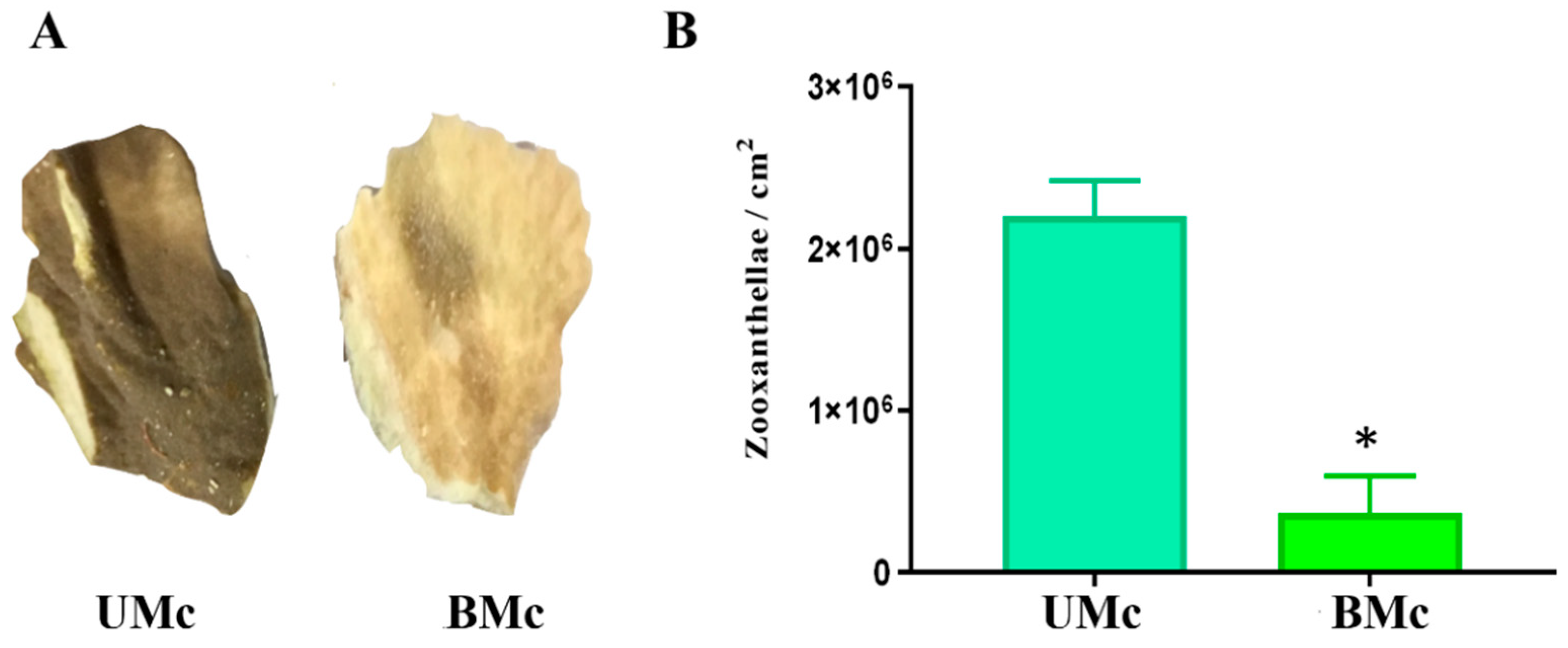
2.2. Determination of the Degree of Bleaching
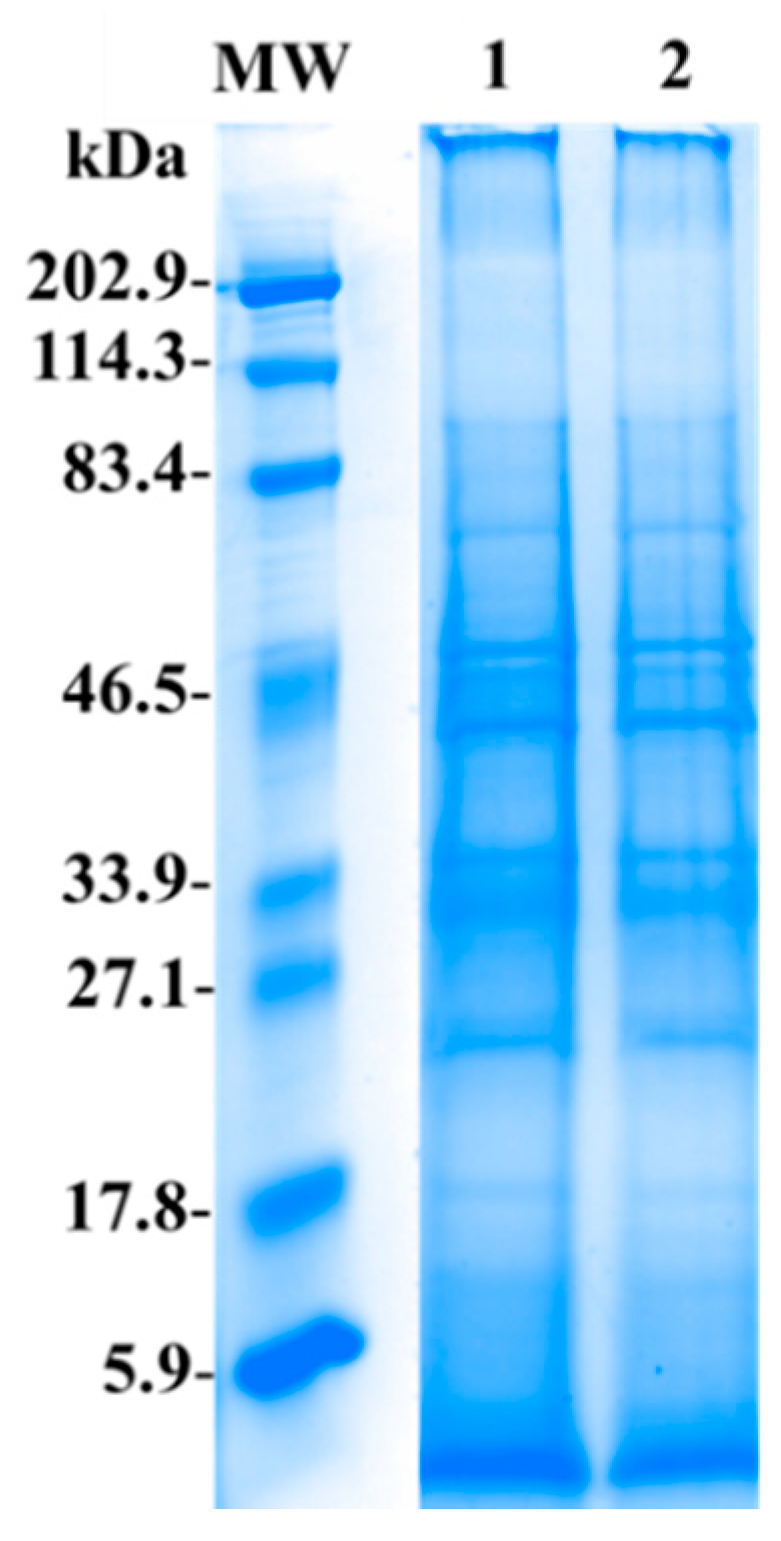
2.3. Electrophoresis SDS-PAGE
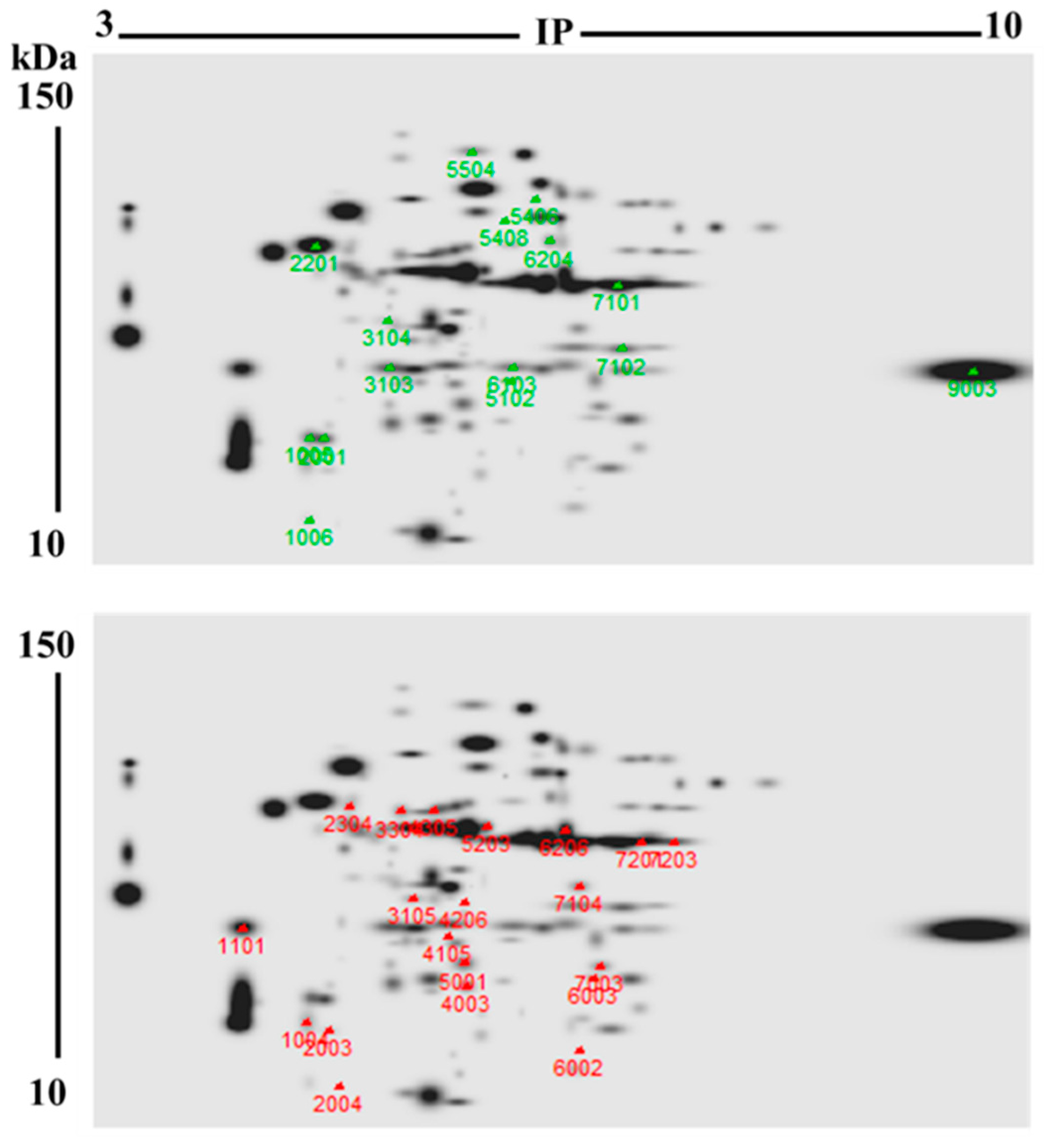
2.4. Two-Dimensional High-Resolution Gel Electrophoresis (2DE-PAGE)
2.5. Identification of Proteins Whose Levels Changed in Bleached M. complanata
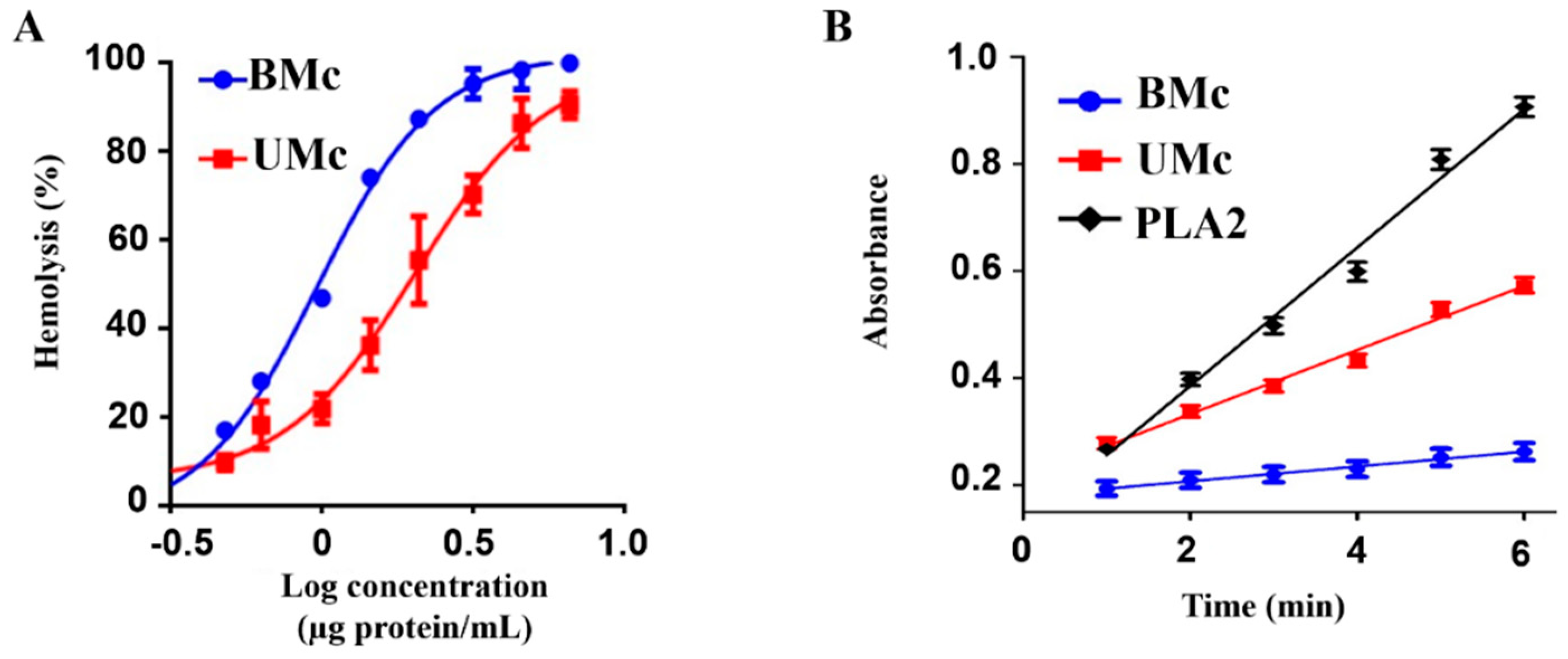
2.6. Effect of Elevated Sea Temperature on the Cytolytic Activity of Unbleached- and Bleached M. complanata-Soluble Proteomes
3. Discussion
3.1. Levels of Proteins Implicated in Key Cellular Processes Were Modified in Bleached M. complanata
3.2. Proteins That Showed Amino Acid Sequence Similarity to Toxins Showed Different Levels in Bleached M. complanata
4. Materials and Methods
4.1. Sample Collection
4.2. Determination of the Degree of Bleaching
4.3. Soluble Proteome Extraction from Bleached and Unbleached M. complanata
4.4. Electrophoresis SDS-PAGE
4.5. First-Dimension Step, Isoelectric Focusing (IEF) and Two-Dimensional High-Resolution Gel Electrophoresis (2DE-PAGE)
4.6. Image Analysis
4.7. Protein in-Gel Digestion, MALDI-TOF/TOF Mass Spectrometry, and Data Analysis
4.8. Effect of Thermal Stress on the Cytolytic and PLA2 Activities of M. complanata Soluble Proteome
4.8.1. Comparative Hemolytic Activity between the Soluble Proteomes from Unbleached and Bleached Specimens of M. complanata
4.8.2. Comparative PLA2 Activity between the Soluble Proteomes from Unbleached and Bleached Specimens of M. complanata
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thampi, V.A.; Anand, M.; Bauch, C.T. Socio-ecological dynamics of Caribbean coral reef ecosystems and conservation opinion propagation. Sci. Rep. 2018, 8, 2597. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Poloczanska, E.S.; Skirving, W.; Dove, S. Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 2017, 4, 158. [Google Scholar] [CrossRef]
- Lewis, J.B. The ecology of Millepora. Coral Reefs 1989, 8, 99–107. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fautin, D.G. Structural diversity, systematics, and evolution of cnidae. Toxicon 2009, 54, 1054–1064. [Google Scholar] [CrossRef]
- García-Arredondo, A.; Murillo-Esquivel, L.J.; Rojas, A.; Sanchez-Rodriguez, J. Characteristics of hemolytic activity induced by the aqueous extract of the Mexican fire coral Millepora complanata. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 49. [Google Scholar] [CrossRef]
- García-Arredondo, A.; Rojas-Molina, A.; Bah, M.; Ibarra-Alvarado, C.; Gallegos-Corona, M.A.; García-Servín, M. Systemic toxic effects induced by the aqueous extract of the fire coral Millepora complanata and partial purification of thermostable neurotoxins with lethal effects in mice. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 169, 55–64. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Fransolet, D.; Roberty, S.; Plumier, J.-C. Establishment of endosymbiosis: The case of cnidarians and Symbiodinium. J. Exp. Mar. Biol. Ecol. 2012, 420–421, 1–7. [Google Scholar] [CrossRef]
- Muller-Parker, G.; D’Elia, C.F.; Cook, C.B. Interactions Between Corals and Their Symbiotic Algae. In Coral Reefs in the Anthropocene; Birkeland, C., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 99–116. ISBN 978-94-017-7248-8. [Google Scholar]
- Venn, A.A.; Loram, J.E.; Douglas, A.E. Photosynthetic symbioses in animals. J. Exp. Bot. 2008, 59, 1069–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitt, W.K.; Brown, B.E.; Warner, M.E.; Dunne, R.P. Coral bleaching: Interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 2001, 20, 51–65. [Google Scholar] [CrossRef]
- Gleason, D.F.; Wellington, G.M. Ultraviolet radiation and coral bleaching. Nature 1993, 365, 836–838. [Google Scholar] [CrossRef]
- Glynn, P.W.; D’Croz, L. Experimental evidence for high temperature stress as the cause of El Niño-coincident coral mortality. Coral Reefs 1990, 8, 181–191. [Google Scholar] [CrossRef]
- Olguín-López, N.; Gutiérrez-Chávez, C.; Hérnández-Elizárraga, V.; Ibarra-Alvarado, C.; Rojas-Molina, A. Coral Reef Bleaching: An Ecological and Biological Overview. In Corals in a Changing World; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [Green Version]
- Douglas, A.E. Coral bleaching—How and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Eakin, C.M.; Lough, J.M.; Heron, S.F.; Liu, G. Climate Variability and Change: Monitoring Data and Evidence for Increased Coral Bleaching Stress. In Coral Bleaching; Ecological Studies; Springer: Cham, Switzerland, 2018; pp. 51–84. ISBN 978-3-319-75392-8. [Google Scholar]
- Gates, R.D.; Baghdasarian, G.; Muscatine, L. Temperature Stress Causes Host Cell Detachment in Symbiotic Cnidarians: Implications for Coral Bleaching. Biol. Bull. 1992, 182, 324–332. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [Green Version]
- DeSalvo, M.K.; Voolstra, C.R.; Sunagawa, S.; Schwarz, J.A.; Stillman, J.H.; Coffroth, M.A.; Szmant, A.M.; Medina, M. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol. Ecol. 2008, 17, 3952–3971. [Google Scholar] [CrossRef]
- Downs, C.A.; Fauth, J.E.; Halas, J.C.; Dustan, P.; Bemiss, J.; Woodley, C.M. Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 2002, 33, 533–543. [Google Scholar] [CrossRef]
- Lesser, M.P.; Farrell, J.H. Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 2004, 23, 367–377. [Google Scholar] [CrossRef]
- Lilley, R.M.; Ralph, P.J.; Larkum, A.W.D. The determination of activity of the enzyme Rubisco in cell extracts of the dinoflagellate alga Symbiodinium sp. by manganese chemiluminescence and its response to short-term thermal stress of the alga. Plant Cell Environ. 2010, 33, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Weis, V.M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 2008, 211, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- DeSalvo, M.K.; Sunagawa, S.; Voolstra, C.R.; Medina, M. Transcriptomic responses to heat stress and bleaching in the elkhorn coral Acropora palmata. Mar. Ecol. Prog. Ser. 2010, 402, 97–113. [Google Scholar] [CrossRef]
- DeSalvo, M.K.; Estrada, A.; Sunagawa, S.; Medina, M. Transcriptomic responses to darkness stress point to common coral bleaching mechanisms. Coral Reefs 2012, 31, 215–228. [Google Scholar] [CrossRef]
- Desalvo, M.K.; Sunagawa, S.; Fisher, P.L.; Voolstra, C.R.; Iglesias-Prieto, R.; Medina, M. Coral host transcriptomic states are correlated with Symbiodinium genotypes. Mol. Ecol. 2010, 19, 1174–1186. [Google Scholar] [CrossRef]
- Pinzón, J.H.; Kamel, B.; Burge, C.A.; Harvell, C.D.; Medina, M.; Weil, E.; Mydlarz, L.D. Whole transcriptome analysis reveals changes in expression of immune-related genes during and after bleaching in a reef-building coral. R. Soc. Open Sci. 2015, 2, 140214. [Google Scholar] [CrossRef] [Green Version]
- Kenkel, C.D.; Meyer, E.; Matz, M.V. Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol. Ecol. 2013, 22, 4322–4334. [Google Scholar] [CrossRef]
- Maor-Landaw, K.; Levy, O. Gene expression profiles during short-term heat stress; branching vs. massive Scleractinian corals of the Red Sea. PeerJ 2016, 4, e1814. [Google Scholar] [CrossRef]
- Louis, Y.D.; Bhagooli, R.; Kenkel, C.D.; Baker, A.C.; Dyall, S.D. Gene expression biomarkers of heat stress in scleractinian corals: Promises and limitations. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenkel, C.D.; Aglyamova, G.; Alamaru, A.; Bhagooli, R.; Capper, R.; Cunning, R.; Haslun, J.A.; Hédouin, L.; Keshavmurthy, S.; Kuehl, K.A.; et al. Development of gene expression markers of acute heat-light stress in reef-building corals of the genus Porites. PLoS ONE 2011, 6, e26914. [Google Scholar] [CrossRef] [PubMed]
- Maor-Landaw, K.; Karako-Lampert, S.; Ben-Asher, H.W.; Goffredo, S.; Falini, G.; Dubinsky, Z.; Levy, O. Gene expression profiles during short-term heat stress in the red sea coral Stylophora pistillata. Glob. Chang. Biol. 2014, 20, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N.; Pernice, M.; Dove, S.; Dunn, S.; Hoegh-Guldberg, O. Gene expression profiles of cytosolic heat shock proteins Hsp70 and Hsp90 from symbiotic dinoflagellates in response to thermal stress: Possible implications for coral bleaching. Cell Stress Chaperones 2011, 16, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A.B.; Chen, Y.-J.; Lu, C.-Y.; Chen, C.-S. Exploring the environmental physiology of the Indo-Pacific Reef Coral Seriatopora hystrix with differential proteomics. Open J. Mar. Sci. 2018, 8, 223–252. [Google Scholar] [CrossRef]
- Ricaurte, M.; Schizas, N.V.; Ciborowski, P.; Boukli, N.M. Proteomic analysis of bleached and unbleached Acropora palmata, a threatened coral species of the Caribbean. Mar. Pollut. Bull. 2016, 107, 224–232. [Google Scholar] [CrossRef]
- Weston, A.J.; Dunlap, W.C.; Beltran, V.H.; Starcevic, A.; Hranueli, D.; Ward, M.; Long, P.F. Proteomics links the redox state to calcium signaling during bleaching of the scleractinian coral Acropora microphthalma on exposure to high solar irradiance and thermal stress. Mol. Cell. Proteom. 2015, 14, 585–595. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Chen, Y.-J.; Lu, C.-Y.; Chen, C.-S. The proteomic response of the reef coral Pocillopora acuta to experimentally elevated temperatures. PLoS ONE 2018, 13, e0192001. [Google Scholar] [CrossRef]
- Traylor-Knowles, N. Heat stress compromises epithelial integrity in the coral, Acropora hyacinthus. PeerJ 2019, 7, e6510. [Google Scholar] [CrossRef]
- Stuhr, M.; Blank-Landeshammer, B.; Reymond, C.E.; Kollipara, L.; Sickmann, A.; Kucera, M.; Westphal, H. Disentangling thermal stress responses in a reef-calcifier and its photosymbionts by shotgun proteomics. Sci. Rep. 2018, 8, 3524. [Google Scholar] [CrossRef]
- Sogin, E.M.; Putnam, H.M.; Anderson, P.E.; Gates, R.D. Metabolomic signatures of increases in temperature and ocean acidification from the reef-building coral, Pocillopora damicornis. Metabolomics 2016, 12, 71. [Google Scholar] [CrossRef]
- Hillyer, K.E.; Dias, D.A.; Lutz, A.; Wilkinson, S.P.; Roessner, U.; Davy, S.K. Metabolite profiling of symbiont and host during thermal stress and bleaching in the coral Acropora aspera. Coral Reefs 2017, 36, 105–118. [Google Scholar] [CrossRef]
- Hillyer, K.E.; Dias, D.; Lutz, A.; Roessner, U.; Davy, S.K. 13C metabolomics reveals widespread change in carbon fate during coral bleaching. Metabolomics 2017, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, K.E.; Tumanov, S.; Villas-Bôas, S.; Davy, S.K. Metabolite profiling of symbiont and host during thermal stress and bleaching in a model cnidarian–dinoflagellate symbiosis. J. Exp. Biol. 2016, 219, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Olguín-López, N.; Hérnandez-Elizárraga, V.H.; Hernández-Matehuala, R.; Cruz-Hernández, A.; Guevara-González, R.; Caballero-Pérez, J.; Ibarra-Alvarado, C.; Rojas-Molina, A. Impact of El Niño-Southern Oscillation 2015-2016 on the soluble proteomic profile and cytolytic activity of Millepora alcicornis (“fire coral”) from the Mexican Caribbean. PeerJ 2019, 7, e6593. [Google Scholar] [CrossRef]
- Jacox, M.G.; Hazen, E.L.; Zaba, K.D.; Rudnick, D.L.; Edwards, C.A.; Moore, A.M.; Bograd, S.J. Impacts of the 2015–2016 El Niño on the California Current System: Early assessment and comparison to past events. Geophys. Res. Lett. 2016, 43, 7072–7080. [Google Scholar] [CrossRef]
- Varotsos, C.A.; Tzanis, C.G.; Sarlis, N.V. On the progress of the 2015–2016 El Niño event. Atmos. Chem. Phys. 2016, 16, 2007–2011. [Google Scholar] [CrossRef]
- Ampou, E.E.; Johan, O.; Menkes, C.E.; Niño, F.; Birol, F.; Ouillon, S.; Andréfouët, S. Coral mortality induced by the 2015–2016 El-Niño in Indonesia: The effect of rapid sea level fall. Biogeosciences 2017, 14, 817–826. [Google Scholar] [CrossRef]
- Heron, S.F.; Maynard, J.A.; van Hooidonk, R.; Eakin, C.M. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci. Rep. 2016, 6, 38402. [Google Scholar] [CrossRef]
- Marshall, P.A.; Baird, A.H. Bleaching of corals on the Great Barrier Reef: Differential susceptibilities among taxa. Coral Reefs 2000, 19, 155–163. [Google Scholar] [CrossRef]
- Strychar, K.B.; Coates, M.; Sammarco, P.W.; Piva, T.J.; Scott, P.T. Loss of Symbiodinium from bleached soft corals Sarcophyton ehrenbergi, Sinularia sp. and Xenia sp. J. Exp. Mar. Biol. Ecol. 2005, 320, 159–177. [Google Scholar] [CrossRef]
- Fitt, W.K.; Gates, R.D.; Hoegh-Guldberg, O.; Bythell, J.C.; Jatkar, A.; Grottoli, A.G.; Gomez, M.; Fisher, P.; Lajuenesse, T.C.; Pantos, O.; et al. Response of two species of Indo-Pacific corals, Porites cylindrica and Stylophora pistillata, to short-term thermal stress: The host does matter in determining the tolerance of corals to bleaching. J. Exp. Mar. Biol. Ecol. 2009, 373, 102–110. [Google Scholar] [CrossRef]
- Guzman, C.; Shinzato, C.; Lu, T.-M.; Conaco, C. Transcriptome analysis of the reef-building octocoral, Heliopora Coerulea. Sci. Rep. 2018, 8, 8397. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, A.T. Photoprotective physiological and biochemical responses of aquatic organisms. In UV Effects in Aquatic Organisms and Ecosystems; RSC Publishing: Cambrige, UK, 2003; pp. 329–356. [Google Scholar]
- Wagner, D.E.; Kramer, P.; van Woesik, R. Species composition, habitat, and water quality influence coral bleaching in southern Florida. Mar. Ecol. Prog. Ser. 2010, 408, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Dias, T.L.P.; Gondim, A.I. Bleaching in scleractinians, hydrocorals, and octocorals during thermal stress in a northeastern Brazilian reef. Mar. Biodivers. 2016, 46, 303–307. [Google Scholar] [CrossRef]
- Fitt, W.K.; Spero, H.J.; Halas, J.; White, M.W.; Porter, J.W. Recovery of the coral Montastrea annularis in the Florida Keys after the 1987 Caribbean “bleaching event.”. Coral Reefs 1993, 12, 57–64. [Google Scholar] [CrossRef]
- Rowan, R.; Knowlton, N.; Baker, A.; Jara, J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 1997, 388, 265–269. [Google Scholar] [CrossRef]
- Nielsen, D.A.; Petrou, K.; Gates, R.D. Coral bleaching from a single cell perspective. ISME J. 2018, 12, 1558. [Google Scholar] [CrossRef]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl. Acad. Sci. USA 1999, 96, 8007–8012. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Lesser, M.P. Coral bleaching: Causes and mechanisms. In Coral Reefs: An Ecosystem in Transition; Springer: Dordrecht, The Netherlands, 2011; pp. 405–419. [Google Scholar]
- Lutz, A.; Raina, J.-B.; Motti, C.A.; Miller, D.J.; van Oppen, M.J.H. Host Coenzyme Q Redox State Is an Early Biomarker of Thermal Stress in the Coral Acropora millepora. PLoS ONE 2015, 10, e0139290. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.J.; Grottoli, A.G. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 2007, 52, 1874–1882. [Google Scholar] [CrossRef]
- Kemp, D.W.; Hernandez-Pech, X.; Iglesias-Prieto, R.; Fitt, W.K.; Schmidt, G.W. Community dynamics and physiology of Symbiodinium spp. before, during, and after a coral bleaching event. Limnol. Oceanogr. 2014, 59, 788–797. [Google Scholar] [CrossRef]
- Weis, V.M.; Levine, R.P. Differential protein profiles reflect the different lifestyles of symbiotic and aposymbiotic Anthopleura elegantissima, a sea anemone from temperate waters. J. Exp. Biol. 1996, 199, 883–892. [Google Scholar] [PubMed]
- Balasubramanian, P.G.; Beckmann, A.; Warnken, U.; Schnölzer, M.; Schüler, A.; Bornberg-Bauer, E.; Holstein, T.W.; Özbek, S. Proteome of Hydra Nematocyst. J. Biol. Chem. 2012, 287, 9672–9681. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. Pharmacologically distinct cardiovascular effects of box jellyfish (Chironex fleckeri) venom and a tentacle-only extract in rats. Toxicol. Lett. 2005, 155, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Crupi, R.; Rizzo, G.; Morabito, R.; Musci, G.; La Spada, G. The unusual toxicity and stability properties of crude venom from isolated nematocysts of Pelagia noctiluca (Cnidaria, Scyphozoa). Cell. Mol. Biol. 2007, 53, 994–1002. [Google Scholar]
- Wenger, Y.; Galliot, B. Punctuated emergences of genetic and phenotypic innovations in eumetazoan, bilaterian, euteleostome, and hominidae ancestors. Genome Biol. Evol. 2013, 5, 1949–1968. [Google Scholar] [CrossRef]
- Ji, H.; Wang, J.; Guo, J.; Li, Y.; Lian, S.; Guo, W.; Yang, H.; Kong, F.; Zhen, L.; Guo, L. Progress in the biological function of alpha-enolase. Anim. Nutr. 2016, 2, 12–17. [Google Scholar] [CrossRef]
- Leggat, W.; Seneca, F.; Wasmund, K.; Ukani, L.; Yellowlees, D.; Ainsworth, T.D. Differential Responses of the Coral Host and Their Algal Symbiont to Thermal Stress. PLoS ONE 2011, 6, e26687. [Google Scholar] [CrossRef]
- Davenport, R.C.; Bash, P.A.; Seaton, B.A.; Karplus, M.; Petsko, G.A.; Ringe, D. Structure of the triosephosphate isomerase-phosphoglycolohydroxamate complex: An analog of the intermediate on the reaction pathway. Biochemistry 1991, 30, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Godon, C.; Lagniel, G.; Lee, J.; Buhler, J.-M.; Kieffer, S.; Perrot, M.; Boucherie, H.; Toledano, M.B.; Labarre, J. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 22480–22489. [Google Scholar] [CrossRef] [PubMed]
- Nogae, I.; Johnston, M. Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase. Gene 1990, 96, 161–169. [Google Scholar] [CrossRef]
- Pollak, N.; Dölle, C.; Ziegler, M. The power to reduce: Pyridine nucleotides—Small molecules with a multitude of functions. Biochem. J. 2007, 402, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ralser, M.; Wamelink, M.M.; Kowald, A.; Gerisch, B.; Heeren, G.; Struys, E.A.; Klipp, E.; Jakobs, C.; Breitenbach, M.; Lehrach, H. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Sorokin, A.; Anderson, I.; Galleron, N.; Candelon, B.; Kapatral, V.; Bhattacharyya, A.; Reznik, G.; Mikhailova, N.; Lapidus, A.; et al. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 2003, 423, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiong, J.; Zhou, Z.; Huo, F.; Miao, W.; Ran, C.; Liu, Y.; Zhang, J.; Feng, J.; Wang, M.; et al. The Genome of the Myxosporean Thelohanellus kitauei Shows Adaptations to Nutrient Acquisition within Its Fish Host. Genome Biol. Evol. 2014, 6, 3182–3198. [Google Scholar] [CrossRef] [PubMed]
- Nesa, B.; Baird, A.H.; Harii, S.; Yakovleva, I.; Hidaka, M. Algal Symbionts Increase DNA Damage in Coral Planulae Exposed to Sunlight. Zool. Stud. 2012, 51, 12–17. [Google Scholar]
- Zheng, B.; Han, M.; Bernier, M.; Wen, J. Nuclear actin and actin-binding proteins in the regulation of transcription and gene expression. FEBS J. 2009, 276, 2669–2685. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Bermudez, A.; Miller, D.J.; Sprungala, S. The neuronal calcium sensor protein Acrocalcin: A potential target of calmodulin regulation during development in the coral Acropora millepora. PLoS ONE 2012, 7, e51689. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Ganot, P.; Furla, P.; Sabourault, C. The transcriptomic response to thermal stress is immediate, transient and potentiated by ultraviolet radiation in the sea anemone Anemonia viridis. Mol. Ecol. 2012, 21, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Mueller, E.; Phillips, S.; Fauth, J.E.; Woodley, C.M. A Molecular Biomarker System for Assessing the Health of Coral (Montastraea faveolata) During Heat Stress. Mar. Biotechnol. 2000, 2, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.L.; King, C.M. Induction of 70-kD heat shock protein in scleractinian corals by elevated temperature: Significance for coral bleaching. Mol. Mar. Biol. Biotechnol. 1995, 4, 36–42. [Google Scholar] [PubMed]
- Brown, B.E.; Downs, C.A.; Dunne, R.P.; Gibb, S.W. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Prog. Ser. 2002, 242, 119–129. [Google Scholar] [CrossRef]
- Fang, L.; Huang, S.; Lin, K. High temperature induces the synthesis of heat-shock proteins and the elevation of intracellular calcium in the coral Acropora grandis. Coral Reefs 1997, 16, 127–131. [Google Scholar] [CrossRef]
- Barshis, D.J.; Ladner, J.T.; Oliver, T.A.; Seneca, F.O.; Traylor-Knowles, N.; Palumbi, S.R. Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. USA 2013, 110, 1387–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, C.A.; McDougall, K.E.; Woodley, C.M.; Fauth, J.E.; Richmond, R.H.; Kushmaro, A.; Gibb, S.W.; Loya, Y.; Ostrander, G.K.; Kramarsky-Winter, E. Heat-Stress and Light-Stress Induce Different Cellular Pathologies in the Symbiotic Dinoflagellate during Coral Bleaching. PLoS ONE 2013, 8, e77173. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Fabbri, E.; Goffredo, S.; Airi, V.; Franzellitti, S. Physiological plasticity related to zonation affects hsp70 expression in the reef-building coral Pocillopora verrucosa. PLoS ONE 2017, 12, e0171456. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.-L.; Sabourault, C.; Richier, S.; Allemand, D.; Furla, P. Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic. Biol. Med. 2007, 42, 236–246. [Google Scholar] [CrossRef]
- Griffin, S.P.; Bhagooli, R.; Weil, E. Evaluation of thermal acclimation capacity in corals with different thermal histories based on catalase concentrations and antioxidant potentials. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Fujimura, H.; Arakaki, T.; Oomori, T. Activities of antioxidant enzymes (SOD and CAT) in the coral Galaxea fascicularis against increased hydrogen peroxide concentrations in seawater. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; pp. 7–11. [Google Scholar]
- Krueger, T.; Hawkins, T.D.; Becker, S.; Pontasch, S.; Dove, S.; Hoegh-Guldberg, O.; Leggat, W.; Fisher, P.L.; Davy, S.K. Differential coral bleaching—Contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 190, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, C.D.; Sheridan, C.; Leal, M.C.; Bhagooli, R.; Castillo, K.D.; Kurata, N.; McGinty, E.; Goulet, T.L.; Matz, M.V. Diagnostic gene expression biomarkers of coral thermal stress. Mol. Ecol. Resour. 2014, 14, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Bieri, T.; Onishi, M.; Xiang, T.; Grossman, A.R.; Pringle, J.R. Relative contributions of various cellular mechanisms to loss of algae during cnidarian bleaching. PLoS ONE 2016, 11, e0152693. [Google Scholar] [CrossRef] [PubMed]
- Picco, A.; Irastorza-Azcarate, I.; Specht, T.; Böke, D.; Pazos, I.; Rivier-Cordey, A.-S.; Devos, D.P.; Kaksonen, M.; Gallego, O. The in vivo architecture of the exocyst provides structural basis for exocytosis. Cell 2017, 168, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Sampayo, E.M.; Ridgway, T.; Bongaerts, P.; Hoegh-Guldberg, O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl. Acad. Sci. USA 2008, 105, 10444–10449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, T.D.; Heron, S.F.; Ortiz, J.C.; Mumby, P.J.; Grech, A.; Ogawa, D.; Eakin, C.M.; Leggat, W. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 2016, 352, 338–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salih, A.; Cox, G.; Szymczak, R.; Coles, S.L.; Baird, A.H.; Dunstan, A.; Cocco, G.; Mills, J.; Larkum, A. The role of host-based color and fluorescent pigments in photoprotection and in reducing bleaching stress in corals. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004; pp. 746–756. [Google Scholar]
- Coles, S.L.; Brown, B.E. Coral bleaching—Capacity for acclimatization and adaptation. Adv. Mar. Biol. 2003, 46, 183–223. [Google Scholar]
- Palumbi, S.R.; Barshis, D.J.; Traylor-Knowles, N.; Bay, R.A. Mechanisms of reef coral resistance to future climate change. Science 2014, 344, 895–898. [Google Scholar] [CrossRef]
- Grottoli, A.G.; Warner, M.E.; Levas, S.J.; Aschaffenburg, M.D.; Schoepf, V.; McGinley, M.; Baumann, J.; Matsui, Y. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Chang. Biol. 2014, 20, 3823–3833. [Google Scholar] [CrossRef]
- Levas, S.; Grottoli, A.G.; Schoepf, V.; Aschaffenburg, M.; Baumann, J.; Bauer, J.E.; Warner, M.E. Can heterotrophic uptake of dissolved organic carbon and zooplankton mitigate carbon budget deficits in annually bleached corals? Coral Reefs 2016, 35, 495–506. [Google Scholar] [CrossRef]
- Tchernov, D.; Kvitt, H.; Haramaty, L.; Bibby, T.S.; Gorbunov, M.Y.; Rosenfeld, H.; Falkowski, P.G. Apoptosis and the selective survival of host animals following thermal bleaching in zooxanthellate corals. Proc. Natl. Acad. Sci. USA 2011, 108, 9905–9909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houlbrèque, F.; Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 2009, 84, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Yellowlees, D.; Rees, T.A.V.; Leggat, W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 2008, 31, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Aichelman, H.E.; Townsend, J.E.; Courtney, T.A.; Baumann, J.H.; Davies, S.W.; Castillo, K.D. Heterotrophy mitigates the response of the temperate coral Oculina arbuscula to temperature stress. Ecol. Evol. 2016, 6, 6758–6769. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.; Gori, A.; Maguer, J.F.; Hoogenboom, M.; Ferrier-Pagès, C. Heterotrophy promotes the re-establishment of photosynthate translocation in a symbiotic coral after heat stress. Sci. Rep. 2016, 6, 38112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzat, L.; Towle, E.; Irisson, J.-O.; Langdon, C.; Ferrier-Pagès, C. The relationship between heterotrophic feeding and inorganic nutrient availability in the scleractinian coral T. reniformis under a short-term temperature increase. Limnol. Oceanogr. 2016, 61, 89–102. [Google Scholar] [CrossRef]
- Takama, O.; Fernandez-Silva, I.; López, C.; Reimer, J.D. Molecular phylogeny demonstrates the need for taxonomic reconsideration of species diversity of the hydrocoral genus Millepora (Cnidaria: Hydrozoa) in the Pacific. Zool. Sci. 2018, 35, 123–134. [Google Scholar] [CrossRef]
- Kawashima, Y.; Nagai, H.; Ishida, M.; Nagashima, Y.; Shiomi, K. Primary structure of echotoxin 2, an actinoporin-like hemolytic toxin from the salivary gland of the marine gastropod Monoplex echo. Toxicon 2003, 42, 491–497. [Google Scholar] [CrossRef]
- Il’ina, A.P.; Monastyrnaya, M.M.; Isaeva, M.P.; Guzev, K.V.; Rasskazov, V.A.; Kozlovskaya, E.P. Primary structures of actinoporins from sea anemone Oulactis orientalis. Russ. J. Bioorg. Chem. 2005, 31, 320–324. [Google Scholar] [CrossRef]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Anderluh, G. Pore-forming toxins in Cnidaria. Semin. Cell Dev. Biol. 2017, 72, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Macrander, J.; Daly, M. Evolution of the cytolytic pore-forming proteins (Actinoporins) in sea anemones. Toxins 2016, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, H.; Paz, M.; Sher, D. The chemical armament of reef-building corals: Inter-and intra-specific variation and the identification of an unusual actinoporin in Stylophora pistilata. Sci. Rep. 2018, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, G.; Sepčić, K.; Turk, T.; Maček, P. Cytolytic proteins from cnidarians—An overview. Acta Chim. Slov. 2011, 58, 724–729. [Google Scholar] [PubMed]
- Glasser, E.; Rachamim, T.; Aharonovich, D.; Sher, D. Hydra actinoporin-like toxin-1, an unusual hemolysin from the nematocyst venom of Hydra magnipapillata which belongs to an extended gene family. Toxicon 2014, 91, 103–113. [Google Scholar] [CrossRef]
- Pungerčar, J.; Anderluh, G.; Maček, P.; Franc, G.; Štrukelj, B. Sequence analysis of the cDNA encoding the precursor of equinatoxin V, a newly discovered hemolysin from the sea anemone Actinia equine. Biochim. Biophys. Acta (BBA) 1997, 1341, 105–107. [Google Scholar] [CrossRef]
- Anderluh, G.; Razpotnik, A.; Podlesek, Z.; Maček, P.; Separovic, F.; Norton, R.S. Interaction of the Eukaryotic Pore-forming Cytolysin Equinatoxin II with Model Membranes: 19F NMR Studies. J. Mol. Biol. 2005, 347, 27–39. [Google Scholar] [CrossRef]
- Razpotnik, A.; Križaj, I.; Kem, W.R.; Maček, P.; Turk, T. A new cytolytic protein from the sea anemone Urticina crassicornis that binds to cholesterol-and sphingomyelin-rich membranes. Toxicon 2009, 53, 762–769. [Google Scholar] [CrossRef]
- Rojko, N.; Dalla Serra, M.; Maček, P.; Anderluh, G. Pore formation by actinoporins, cytolysins from sea anemones. Biochim. Biophys. Acta Biomembr. 2016, 1858, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Malovrh, P.; Barlić, A.; Podlesek, Z.; Maćek, P.; Menestrina, G.; Anderluh, G. Structure–function studies of tryptophan mutants of equinatoxin II, a sea anemone pore-forming protein. Biochem. J. 2000, 346, 223–232. [Google Scholar] [PubMed]
- Hong, Q.; Gutiérrez-Aguirre, I.; Barlič, A.; Malovrh, P.; Kristan, K.; Podlesek, Z.; Maček, P.; Turk, D.; González-Mañas, J.M.; Lakey, J.H.; et al. Two-step Membrane Binding by Equinatoxin II, a Pore-forming Toxin from the Sea Anemone, Involves an Exposed Aromatic Cluster and a Flexible Helix. J. Biol. Chem. 2002, 277, 41916–41924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameirika; Sha, H.X.; Hwang, J.S. Identification of a target protein of Hydra actinoporin-like toxin-1 (HALT-1) using GST affinity purification and SILAC-based quantitative proteomics. Toxicon 2017, 133, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Kwon, Y.C.; Shin, K.; Yoon, W.D.; Han, C.H.; Yum, S.; Kim, E. cDNA and gene structures of two phospholipase A2 isoforms, acidic PLA2 PA4 and PLA2 PA3A/PA3B/PA5, in Nemopilema nomurai jellyfish venom. Toxicon 2016, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Choristoneura fumiferana acidic calcium-independent phospholipase A2-like protein mRNA, complete cds. 2005. Available online: https://www.ncbi.nlm.nih.gov/nuccore/DQ238110.1 (accessed on 30 June 2005).
- Talvinen, K.A.; Nevalainen, T.J. Cloning of a novel phospholipase A2 from the cnidarian Adamsia carciniopados. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 571–578. [Google Scholar] [CrossRef]
- Razpotnik, A.; Križaj, I.; Šribar, J.; Kordiš, D.; Maček, P.; Frangež, R.; Kem, W.R.; Turk, T. A new phospholipase A2 isolated from the sea anemone Urticina crassicornis—Its primary structure and phylogenetic classification. FEBS J. 2010, 277, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.; Marcussi, S.; Marchi-Salvador, D.P.; Silva, F.P., Jr.; Fuly, A.L.; Stábeli, R.G.; da Silva, S.L.; González, J.; del Monte, A.; Soares, A.M. Enzymatic and structural characterization of a basic phospholipase A2 from the sea anemone Condylactis gigantea. Biochimie 2010, 92, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.D.; Alves, R.S.; Martins, A.M.C.; Barbosa, P.S.F.; Evangelista, J.S.A.M.; Evangelista, J.J.F.; Ximenes, R.M.; Toyama, M.H.; Toyama, D.O.; Souza, A.J.F.; et al. Purification and characterization of the biological effects of phospholipase A2 from sea anemone Bunodosoma caissarum. Toxicon 2009, 54, 413–420. [Google Scholar] [CrossRef]
- Landucci, E.C.T.; Dias, Q.C.; Marangoni, F.A.; Vilca-Quispe, A.; Valeriano-Zapana, J.A.; Torres-Huaco, F.D.; Martins-de-Souza, D.; Marangoni, S.; Ponce-Soto, L.A. Purification and inflammatory edema induced by two PLA2 (Anch TX-I and Anch TX-II) from sea anemone Anthothoe chilensis (Actiniaria: Sagartiidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 161, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Peuravuori, H.J.; Quinn, R.J.; Llewellyn, L.E.; Benzie, J.A.H.; Fenner, P.J.; Winkel, K.D. Phospholipase A2 in Cnidaria. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 731–735. [Google Scholar] [CrossRef] [PubMed]
- García-Arredondo, A.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Iglesias-Prieto, R. Effects of bleaching on the pharmacological and toxicological activities elicited by the aqueous extracts prepared from two “fire corals” collected in the Mexican Caribbean. J. Exp. Mar. Biol. Ecol. 2011, 396, 171–176. [Google Scholar] [CrossRef]
- Trevisan-Silva, D.; Gremski, L.H.; Chaim, O.M.; da Silveira, R.B.; Meissner, G.O.; Mangili, O.C.; Barbaro, K.C.; Gremski, W.; Veiga, S.S.; Senff-Ribeiro, A. Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles). Biochimie 2010, 92, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, L.; Gremski, W.; Veiga, S.S.; Elias, M.C.Q.; Graner, E.; Mangili, O.C.; Brentani, R.R. Detection and characterization of metalloproteinases with gelatinolytic, fibronectinolytic and fibrinogenolytic activities in brown spider (Loxosceles intermedia) venom. Toxicon 1998, 36, 1039–1051. [Google Scholar] [CrossRef]
- Da Silveira, R.B.; Wille, A.C.; Chaim, O.M.; Appel, M.H.; Silva, D.T.; Franco, C.R.; Toma, L.; Mangili, O.C.; Gremski, W.; Dietrich, C.P.; et al. Identification, cloning, expression and functional characterization of an astacin-like metalloprotease toxin from Loxosceles intermedia (brown spider) venom. Biochem. J. 2007, 406, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-L.; Gröger, H.; Schmid, V.; Spring, J. A toxin homology domain in an astacin-like metalloproteinase of the jellyfish Podocoryne carnea with a dual role in digestion and development. Dev. Gene Evol. 1998, 208, 259–266. [Google Scholar] [CrossRef]
- Weston, A.J.; Chung, R.; Dunlap, W.C.; Morandini, A.C.; Marques, A.C.; Moura-da-Silva, A.M.; Ward, M.; Padilla, G.; da Silva, L.F.; Andreakis, N.; et al. Proteomic characterisation of toxins isolated from nematocysts of the South Atlantic jellyfish Olindias Sambaquiensis. Toxicon 2013, 71, 11–17. [Google Scholar] [CrossRef]
- Moran, Y.; Praher, D.; Schlesinger, A.; Ayalon, A.; Tal, Y.; Technau, U. Analysis of Soluble Protein Contents from the Nematocysts of a Model Sea Anemone Sheds Light on Venom Evolution. Mar. Biotechnol. 2013, 15, 329–339. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish venomics and venom gland transcriptomics analysis of Stomolophus meleagris to reveal the toxins associated with sting. J. Proteom. 2014, 106, 17–29. [Google Scholar] [CrossRef]
- Lee, H.; Jung, E.; Kang, C.; Yoon, W.D.; Kim, J.-S.; Kim, E. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef]
- Hoepner, C.M.; Abbott, C.A.; Burke da Silva, K. The ecological importance of toxicity: Sea anemones maintain toxic defence when bleached. Toxins 2019, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Matehuala, R.; Rojas-Molina, A.; Vuelvas-Solórzano, A.A.; Garcia-Arredondo, A.; Alvarado, C.I.; Olguín-López, N.; Aguilar, M. Cytolytic and systemic toxic effects induced by the aqueous extract of the fire coral Millepora alcicornis collected in the Mexican Caribbean and detection of two types of cytolisins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Spot | Protein | Accession Number | MW/pI a | P b | Log2-fold Change |
|---|---|---|---|---|---|
| Toxins | |||||
| 2001 | Acidic PLA2 PA4 | A0A1D8GZE6_9CNID | 16.0/4.7 | ↑ | 4.4 |
| 2201 | Echotoxin-2 | ACTP2_MONPT | 39.7/4.6 | ↑ | 3.1 |
| 3103 | DELTA-actitoxin-Oor1b | ACTPG_OULOR | 22.805.1 | ↑ | 5.6 |
| 7101 | PREDICTED: Astacin-like metalloprotease toxin 5 | XP_002162822.1 | 33.1/7.0 | ↑ | 2.2 |
| 5001 | Acidic calcium-independent phospholipase A2-like protein | Q307P2_CHOFU | 19.1/5.8 | ↓ | 0.3 |
| Primary metabolism | |||||
| 5406 | Alpha enolase | T2MHB9_HYDVU | 53.2/6.3 | ↑ | 2.8 |
| 7104 | PREDICTED: triosephosphate isomerase | XP_0021676111 | 27.6/6.7 | ↓ | 0.4 |
| DNA repair | |||||
| 3104 | UV DNA endonuclease | UVSE_BACCR | 28.5/5.1 | ↑ | 3.0 |
| 6002 | DNA endonuclease repair XPF | A0A0C2MR15_THEKT | 12.5/6.7 | ↓ | 0.04 |
| Cytoskeleton Component | |||||
| 5408 | Actin | ACT_HYDVU | 47.0/6.1 | ↓ | 0.3 |
| Signaling protein | |||||
| 1101 | Calmodulin | T2MET0_HYDVU | 22.7/4.7 | ↓ | 0.4 |
| Stress response | |||||
| 5504 | HSP70 | HSP70_HYDVU | 70.0/5.9 | ↑ | 2.6 |
| Homeostasis redox | |||||
| 6103 | Peroxiredoxin-6 | T2MGB9_HYDVU | 22.8/6.2 | ↑ | 4.7 |
| Exocytosis protein | |||||
| 7103 | PREDICTED: exocyst complex component 4-like protein | XP_004208568.1 | 33.2/7.5 | ↑ | 2.2 |
| Unknown | |||||
| 6206 | Hypothetical protein NEMVEDRAFT_v1g45829 | A7TDG2_NEMVE | 34.7/6.6 | ↓ | 0.3 |
| Extracts | Soluble Protein Content a | Hemolytic Activity b | PLA2 Activity c |
|---|---|---|---|
| UMc | 31.04 ± 1.30 | 2.07 ± 0.35 | 124.70 ± 1.98 |
| BMc | 22.02 ± 0.70 | 0.96 ± 0.08 * | 29.13 ± 1.26 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Elizárraga, V.H.; Olguín-López, N.; Hernández-Matehuala, R.; Ocharán-Mercado, A.; Cruz-Hernández, A.; Guevara-González, R.G.; Caballero-Pérez, J.; Ibarra-Alvarado, C.; Sánchez-Rodríguez, J.; Rojas-Molina, A. Comparative Analysis of the Soluble Proteome and the Cytolytic Activity of Unbleached and Bleached Millepora complanata (“Fire Coral”) from the Mexican Caribbean. Mar. Drugs 2019, 17, 393. https://doi.org/10.3390/md17070393
Hernández-Elizárraga VH, Olguín-López N, Hernández-Matehuala R, Ocharán-Mercado A, Cruz-Hernández A, Guevara-González RG, Caballero-Pérez J, Ibarra-Alvarado C, Sánchez-Rodríguez J, Rojas-Molina A. Comparative Analysis of the Soluble Proteome and the Cytolytic Activity of Unbleached and Bleached Millepora complanata (“Fire Coral”) from the Mexican Caribbean. Marine Drugs. 2019; 17(7):393. https://doi.org/10.3390/md17070393
Chicago/Turabian StyleHernández-Elizárraga, Víctor Hugo, Norma Olguín-López, Rosalina Hernández-Matehuala, Andrea Ocharán-Mercado, Andrés Cruz-Hernández, Ramón Gerardo Guevara-González, Juan Caballero-Pérez, César Ibarra-Alvarado, Judith Sánchez-Rodríguez, and Alejandra Rojas-Molina. 2019. "Comparative Analysis of the Soluble Proteome and the Cytolytic Activity of Unbleached and Bleached Millepora complanata (“Fire Coral”) from the Mexican Caribbean" Marine Drugs 17, no. 7: 393. https://doi.org/10.3390/md17070393






