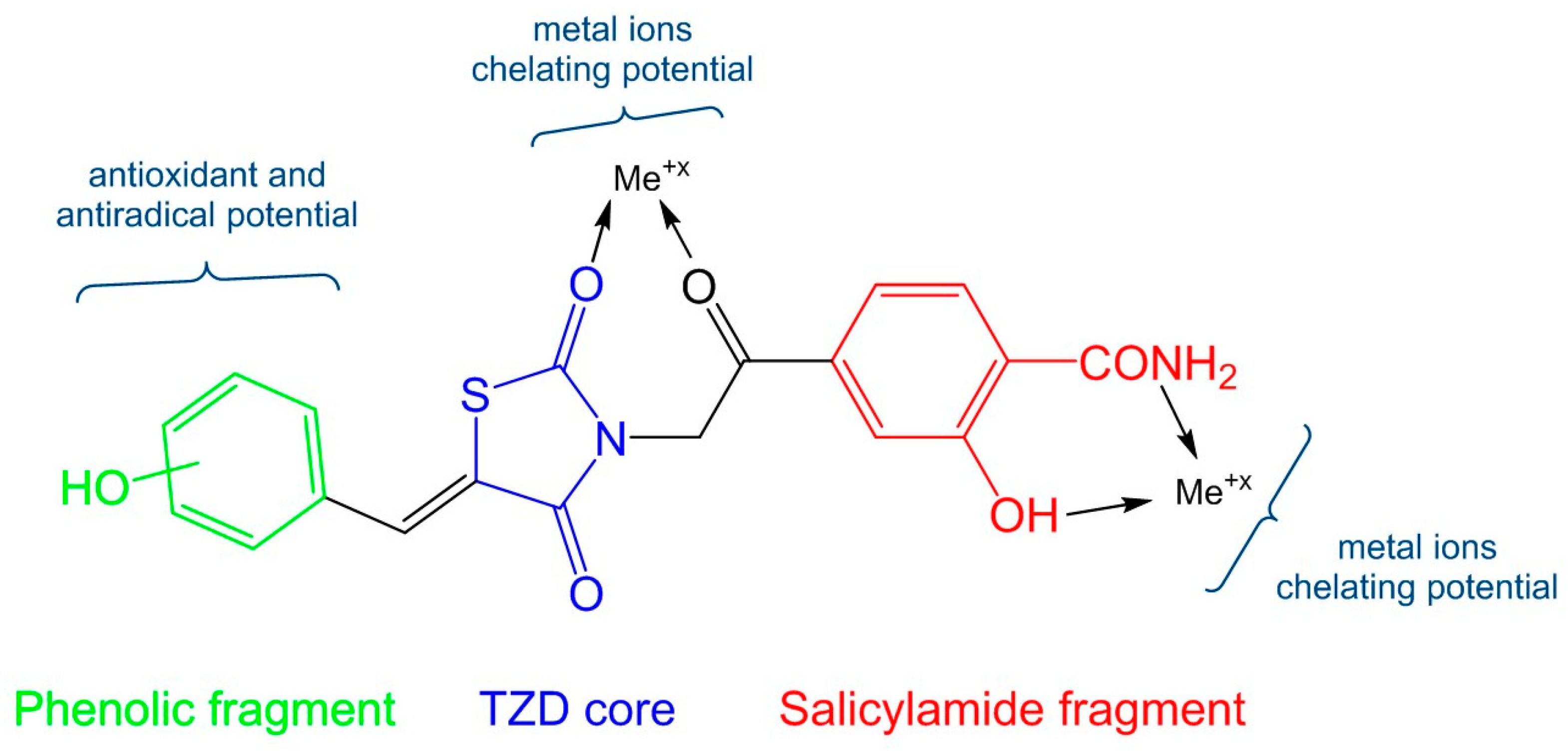
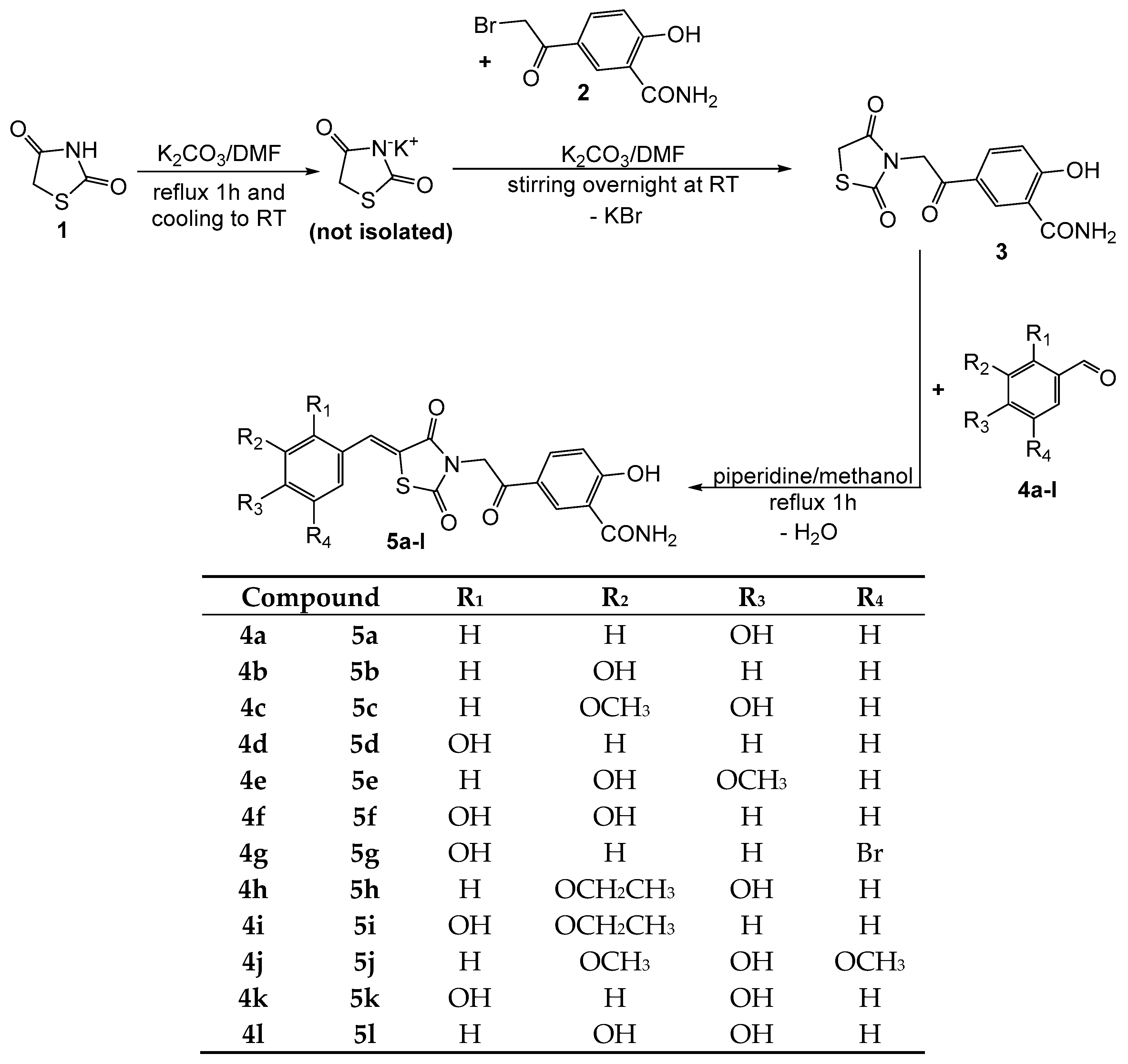All chemicals used for the synthesis, purification, analysis, and antioxidant assays, with appropriate grade purity, were purchased from local suppliers and were used as supplied. The melting points were measured using an MPM-H1 melting point device (Schorpp Gerätetechnik, Überlingen, Germany), based on the glass capillary method. The MS spectra of the compounds were recorded using an Agilent 1100 series device in positive ionization mode for intermediate compound 3 and in negative ionization mode for the final compounds 5a–l, connected to an Agilent Ion Trap SL mass spectrometer (70 eV) instrument (Agilent Technologies, Santa Clara, CA, USA). The IR spectra were recorded under vacuum, using a FT/IR 6100 spectrometer (Jasco, Cremella, Italy) in KBr pellets. The 1H-NMR and 13C-NMR spectra were recorded using an Avance NMR spectrometer (Bruker, Karlsruhe, Germany) in dimethyl sulfoxide-d6. Chemical shift values were reported in δ units, relative to tetramethylsilane as internal standard.
3.1.2. Synthesis of Compounds 5a–l
In a glass flask, 2 mmol (0.588 g) of compound 3 and 2 mmol of the appropriate aldehyde (compounds 4a–l) were mixed with 5 mL of methanol. Later, 4 mmol (0.32 g) of piperidine were added dropwise and the mixture was gently refluxed under condenser for one hour. The reaction mixture was then left to stay overnight at room temperature in order to remove by evaporation some methanol to get a more concentrated solution. The solution was mixed in a glass flask with ice and water, and a 10% hydrochloric acid solution was added dropwise until total precipitation of the desired product. The flask was left in a refrigerator for a few hours to favor the quantitative precipitation of the product. The precipitate was filtered under vacuum and crystalized twice from diethyl ether to get the pure final compounds.
(Z)-2-hydroxy-5-(2-(5-(4-hydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl) benzamide (5a): intense yellow solid; carbonization over 260 °C; yield = 56%; FT IR (KBr) νmax cm−1: 3546 (str O–H), 3419, 3179 (N–H amide), 2930 (CH2), 1738, 1686, 1671, 1655 (str C=O), 1614 (C=C), 1357 (bend OH); MS: m/z = 397.2 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 13.21 (br, 1H, OH), 10.36 (br, 1H, OH), 8.69 (s, 1H, Ar), 8.59 (br, 1H, NH), 8.09 (d, 1H, Ar), 8.01 (br, 1H, NH), 7.87 (s, 1H, –CH=), 7.58 (d, 2H, Ar), 7.05 (d, 1H, Ar), 6.89 (d, 2H, Ar), 5.19 (s, 2H, –CH2–); 13C-NMR (DMSO-d6, 125 MHz) δ: 187.99 (C=O), 170.82 (C=O), 167.26 (C=O), 166.84 (C=O), 166.80 (ArC–O), 159.99 (ArC–O), 134.62 (–CH=), 133.41, 133.06, 125.60, 129.96, 125.37, 118.92, 117.02, 114.57 (8 aromatic carbons), 116.51 (TZD C5), 45.93 (–CH2–).
(Z)-2-hydroxy-5-(2-(5-(3-hydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)benzamide (5b): yellow solid; carbonization over 260 °C; yield = 40%; FT IR (KBr) νmax cm−1: 3550 (str O–H), 3381, 3208 (N–H amide), 2928 (CH2), 1747, 1694, 1680, 1658 (str C=O), 1613 (C=C), 1357 (bend OH); MS: m/z = 397.2 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 13.98 (br, 1H, OH), 9.90 (br, 1H, OH), 8.75 (br, 1H, NH), 8.67 (s, 1H, Ar), 8.19 (br, 1H, NH), 8.08 (d, 1H, Ar), 7.92 (s, 1H, –CH=), 7.38 (t, 1H, Ar), 7.16 (d, 1H, Ar), 7.07–7.06 (m, 2H, Ar), 6.94 (d, 1H, Ar), 5.29 (s, 2H, –CH2–); 13C-NMR (DMSO-d6, 125 MHz) δ: 189.57 (C=O), 171.95 (C=O), 167.71 (C=O), 166.64 (C=O), 165.78 (ArC–O), 158.45 (ArC–O), 134.50, 134.29, 130.56, 125.29, 121.99, 121.20, 118.88, 118.68, 117.78, 114.67 (10 aromatic carbons), 131.00 (–CH=), 116.61 (TZD C5), 44.08 (–CH2–).
(Z)-2-hydroxy-5-(2-(5-(4-hydroxy-3-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)benzamide (5c): yellow solid; carbonization over 280 °C; yield = 31%; FT IR (KBr) νmax cm−1: 3545 (str O–H), 3374, 3234 (N–H amide), 2929 (CH2), 1745, 1680, 1663, 1655 (str C=O), 1616 (C=C), 1357 (bend OH), 1212, 1031 (str C–O–C asymm and symm); MS: m/z = 272.2 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 13.01 (br, 1H, OH), 10.29 (br, 1H, OH), 9.53 (br, 1H, NH), 8.71 (s, 1H, Ar), 8.66 (br, 1H, NH), 7.99 (d, 1H, Ar), 7.89 (d, 1H, –CH=), 7.61–7.59 (m, 2H, Ar), 7.01 (d, 1H, Ar), 6.95 (d, 1H, Ar), 5.18 (s, 2H, –CH2–), 3.71 (s, 3H, –CH3); 13C-NMR (DMSO-d6, 125 MHz) δ: 188.16 (C=O), 171.24 (C=O), 166.82 (C=O), 166.35 (C=O), 166.11 (ArC–O), 148.16 (ArC–O), 147.51 (ArC–O), 135.11, 130.51, 120.14, 126.94, 125.41, 119.47, 116.10, 115.29, 113.26 (9 aromatic carbons), 133.57 (–CH=), 116.64 (TZD C5), 57.43 (–CH3), 46.80 (–CH2–).
(Z)-2-hydroxy-5-(2-(5-(2-hydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)benzamide (5d): orange solid; mp = 225 °C; yield = 39%; FT IR (KBr) νmax cm−1: 3554 (str O–H), 3386, 3219 (N–H amide), 2928 (CH2), 1736, 1680, 1658, 1647 (str C=O), 1617 (C=C), 1359 (bend OH); MS: m/z = 397.1 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 12.98 (br, 1H, OH), 10.57 (br, 1H, OH), 8.91 (br, 1H, NH), 8.59 (s, 1H, Ar), 8.22 (br, 1H, NH), 8.18 (s, 1H, –CH=), 8.01 (d, 1H, Ar), 7.35–7.38 (m, 2H, Ar), 7.04 (d, 1H, Ar), 6.98–6.96 (m, 2H, Ar), 5.14 (s, 2H, –CH2–); 13C-NMR (DMSO-d6, 125 MHz) δ: 188.27 (C=O), 171.55 (C=O), 167.44 (C=O), 167.29 (ArC–O), 166.68 (C=O), 157.39 (ArC–O), 134.55, 130.01, 129.25, 129.01, 125.37, 121.52, 121.14, 120.96, 120.28, 116.23 (10 aromatic carbons), 132.87 (–CH=), 116.91 (TZD C5), 44.37 (–CH2–).
(Z)-2-hydroxy-5-(2-(5-(3-hydroxy-4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)benzamide (5e): pale yellow solid; mp = 216 °C; yield = 46%; FT IR (KBr) νmax cm−1: 3546 (str O–H), 3359, 3192 (N–H amide), 2935 (CH2), 1742, 1693, 1678, 1655 (str C=O), 1617 (C=C), 1334 (bend OH), 1243, 1029 (str C–O–C asymm and symm); MS: m/z = 427.4 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 13.14 (br, 1H, OH), 9.20 (br, 1H, OH), 8.99 (br, 1H, NH), 8.63 (s, 1H, Ar), 8.50 (br, 1H, NH), 7.96 (d, 1H, Ar), 7.91 (s, 1H, –CH=), 7.36 (d, 1H, Ar), 7.29 (s, 1H, Ar), 7.04 (d, 1H, Ar), 6.99 (d, 1H, Ar), 5.23 (s, 2H, –CH2–), 3.79 (s, 3H, –CH3); 13C-NMR (DMSO-d6, 125 MHz) δ: 188.91 (C=O), 170.89 (C=O), 166.22 (C=O), 165.61 (C=O), 165.36 (ArC–O), 146.89 (ArC–O), 146.19 (ArC–O), 135.09, 132.41, 130.26, 125.37, 120.64, 118.47, 116.76, 114.52, 112.96 (9 aromatic carbons), 132.12 (–CH=), 116.82 (TZD C5), 58.11 (–CH3), 45.56 (–CH2–).
(Z)-5-(2-(5-(2,3-dihydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)-2-hydroxy benzamide (5f): yellow solid; mp = 254 °C; yield = 61%; FT IR (KBr) νmax cm−1: 3540 (str O–H), 3365, 3164 (N–H amide), 2939 (CH2), 1733, 1678, 1671, 1648 (str C=O), 1609 (C=C), 1358 (bend OH); MS: m/z = 413.1 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 12.84 (br, 1H, OH), 9.95 (br, 2H, OH), 8.95 (br, 1H, NH), 8.59 (s, 1H, Ar), 8.45 (br, 1H, NH), 8.16 (s, 1H, –CH=), 8.02 (d, 1H, Ar), 7.28 (d, 1H, Ar), 7.04 (d, 1H, Ar), 6.71–6.73 (m, 2H, Ar), 5.16 (s, 2H, –CH2–); 13C-NMR (DMSO-d6, 125 MHz) δ: 189.16 (C=O), 170.98 (C=O), 166.87 (C=O), 165.89 (C=O), 165.38 (ArC–O), 150.23 (ArC–O), 146.19 (ArC–O), 133.98, 129.91, 125.39, 121.87, 121.80, 117.09, 117.01, 116.82, 116.70 (9 aromatic carbons), 132.81 (–CH=), 116.54 (TZD C5), 47.49 (–CH2–).
(Z)-5-(2-(5-(5-bromo-2-hydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)-2-hydroxybenzamide (5g): yellow solid; mp = 235 °C; yield = 76%; FT IR (KBr) νmax cm−1: 3543 (str O–H), 3419, 3192 (N–H amide), 2926 (CH2), 1729, 1670, 1660, 1648 (str C=O), 1615 (C=C), 1356 (bend OH), 620 (str C-Br); MS: m/z = 475.7 (M − 1) with bromine isotopic pattern; 1H-NMR (DMSO-d6, 500 MHz) δ: 12.71 (br, 1H, OH), 10.59 (br, 1H, OH), 9.43 (br, 1H, NH), 9.01 (br, 1H, NH), 8.66 (s, 1H, Ar), 8.10–8.09 (m, 2H, Ar, –CH=), 7.90 (s, 1H, Ar), 7.14 (d, 1H, Ar), 7.04 (d, 1H, Ar), 6.90 (d, 1H, Ar), 5.19 (s, 2H, Ar); 13C-NMR (DMSO-d6, 125 MHz) δ: 188.61 (C=O), 171.09 (C=O), 167.41 (ArC–O), 166.80 (C=O), 166.17 (C=O), 157.87 (ArC–O), 134.26, 130.99, 130.29, 129.83, 125.37, 121.63, 119.54, 118.99, 118.02, 115.22 (10 aromatic carbons), 132.96 (–CH=), 116.73 (TZD C5), 46.49 (–CH2–).
(Z)-5-(2-(5-(3-ethoxy-4-hydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)-2-hydroxybenzamide (5h): yellow solid; carbonization over 290 °C; yield = 65%; FT IR (KBr) νmax cm−1: 3547 (str O–H), 3418, 3237 (N–H amide), 2928 (CH2), 1740, 1680, 1671, 1661 (str C=O), 1615 (C=C), 1360 (bend OH), 1244, 1032 (str C–O–C asymm and symm); MS: m/z = 441.2 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 12.54 (br, 1H, OH), 9.96 (br, 1H, OH), 9.01 (br, 1H, NH), 8.64 (s, 1H, Ar), 8.50 (br, 1H, NH), 7.94 (d, 1H, Ar), 7.85 (s, 1H, –CH=), 7.38–7.35 (m, 2H, Ar), 7.05 (d, 1H, Ar), 6.94 (d, 1H, Ar), 5.22 (s, 2H, –CH2–), 4.10 (q, 2H, –CH2–), 1.53 (t, 3H, –CH3); 13C-NMR (DMSO-d6, 125 MHz) δ: 188.53 (C=O), 171.24 (C=O), 167.26 (C=O), 166.84 (C=O), 165.43 (ArC–O), 149.51 (ArC–O), 146.01 (ArC–O), 134.43, 129.86, 129.47, 125.30, 123.42, 120.73, 116.21, 115.92, 114.47 (9 aromatic carbons), 133.84 (–CH=), 116.62 (TZD C5), 65.01 (–CH2–), 44.55 (–CH2–), 16.26 (–CH3).
(Z)-5-(2-(5-(3-ethoxy-2-hydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)-2-hydroxybenzamide (5i): yellow solid; mp = 227°C; yield=71%; FT IR (KBr) νmax cm−1: 3545 (str O–H), 3417, 3241 (N–H amide), 2941 (CH2), 1738, 1678, 1656, 1649 (str C=O), 1615 (C=C), 1354 (bend OH), 1236, 1027 (str C–O–C asymm and symm); MS: m/z = 441.2 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 12.66 (br, 1H, OH), 10.26 (br, 1H, OH), 9.66 (br, 1H, NH), 8.57 (s, 1H, Ar), 8.22 (s, 1H, –CH=), 7.86 (d, 1H, Ar), 7.52 (br, 1H, NH), 7.03 (d, 1H, Ar), 7.12 (d, 1H, Ar), 6.94 (t, 1H, Ar), 6.70 (d, 1H, Ar), 5.14 (s, 2H, –CH2–), 4.12 (q, 2H, –CH2–), 1.38 (t, 3H, –CH3); 13C-NMR (DMSO-d6, 125 MHz) δ: 187.89 (C=O), 170.65 (C=O), 167.90 (C=O), 167.13 (ArC–O), 166.07 (C=O), 147.77 (ArC–O), 147.73 (ArC–O), 133.12, 132.04, 129.46, 125.29, 120.67, 120.27, 120.15, 116.88, 115.88 (9 aromatic carbons), 132.01 (–CH=), 120.89 (TZD C5), 64.85 (–CH2–), 47.55 (–CH2–), 15.05 (–CH3).
(Z)-2-hydroxy-5-(2-(5-(4-hydroxy-3,5-dimethoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)benzamide (5j): yellow solid; mp = 302 °C; yield = 64%; FT IR (KBr) νmax cm−1: 3546 (str O–H), 3423, 3203 (N–H amide), 2932 (CH2), 1743, 1682, 1667, 1648 (str C=O), 1617 (C=C), 1360 (bend OH), 1243, 1027 (str C–O–C asymm and symm); MS: m/z = 457.3 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 14.00 (br, 1H, OH), 9.44 (br, 1H, OH), 8.81 (br, 1H, NH), 8.75 (br, 1H, NH), 8.71 (s, 1H, Ar), 8.07 (d, 1H, Ar), 7.93 (s, 1H, –CH=), 7.07 (d, 1H, Ar), 6.99 (s, 2H, Ar), 5.29 (s, 2H, –CH2–), 3.85 (s, 6H, –CH3); 13C-NMR (DMSO-d6, 125 MHz) δ: 189.69 (C=O), 171.91 (C=O), 167.69 (ArC–O), 166.59 (C=O), 165.84 (C=O), 148.78 (ArC–O), 139.58 (ArC–O), 135.27, 130.60, 125.34, 123.56, 118.76, 114.70, 108.80 (7 aromatic carbons), 134.24 (–CH=), 117.42 (TZD C5), 56.62 (–CH3), 48.08 (–CH2–).
(Z)-5-(2-(5-(2,4-dihydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)-2-hydroxybenzamide (5k): yellow mustard solid; carbonization over 250 °C; yield = 68%; FT IR (KBr) νmax cm−1: 3544 (str O–H), 3420, 3220 (N–H amide), 2938 (CH2), 1729, 1680, 1661, 1647 (str C=O), 1618 (C=C), 1370 (bend OH); MS: m/z = 413.0 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 13.53 (br, 1H, OH), 10.40 (br, 2H, OH), 8.83 (br, 1H, NH), 8.68 (s, 1H, Ar), 8.42 (br, 1H, NH), 8.14 (s, 1H, –CH=), 7.99 (d, 1H, Ar), 7.54 (d, 1H, Ar), 7.04 (d, 1H, Ar), 6.65 (d, 1H, Ar), 6.34 (s, 1H, Ar), 5.19 (s, 2H, –CH2–); 13C-NMR (DMSO-d6, 125 MHz) δ: 188.73 (C=O), 171.50 (C=O), 166.65 (C=O), 166.21 (ArC–O), 165.82 (C=O), 161.66 (ArC–O), 156.81 (ArC–O), 135.15, 131.14, 130.22, 125.31, 121.47, 119.84, 116.19, 111.82, 104.15 (9 aromatic carbons), 133.31 (–CH=), 116.80 (TZD C5), 47.56 (–CH2–).
(Z)-5-(2-(5-(3,4-dihydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)-2-hydroxybenzamide (5l): dark orange solid; carbonization over 260 °C; yield = 76%; FT IR (KBr) νmax cm−1: 3543 (str O–H), 3357, 3264 (N–H amide), 2936 (CH2), 1736, 1679, 1662, 1646 (str C=O), 1618 (C=C), 1363 (bend OH); MS: m/z = 413.0 (M − 1); 1H-NMR (DMSO-d6, 500 MHz) δ: 13.41 (br, 1H, OH), 9.81 (br, 2H, OH), 9.78 (br, 1H, NH), 9.24 (br, 1H, NH), 8.67 (s, 1H, Ar), 8.06 (d, 1H, Ar), 7.84 (s, 1H, –CH=), 7.21 (d, 1H, Ar), 7.05–7.03 (m, 2H, Ar), 6.85 (d, 1H, Ar), 5.17 (s, 2H, –CH2–); 13C-NMR (DMSO-d6, 125 MHz) δ: 187.93 (C=O), 170.99 (C=O), 166.89 (C=O), 166.60 (C=O), 166.26 (ArC–O), 146.16 (ArC–O), 146.98 (ArC–O), 133.84, 130.05, 128.55, 127.84, 125.39, 120.56, 116.52, 115.29, 114.29 (9 aromatic carbons), 133.61 (–CH=), 116.90 (TZD C5), 45.99 (–CH2–).