Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mulberry Leaf Polyphenol and Fiber Extracts
2.3. SD (Sprague-Dawley) Rats and Diet
2.4. Tissue Harvest
2.5. Cecal Microbiota
2.6. Metabolomics
2.7. Statistical Analysis
3. Results
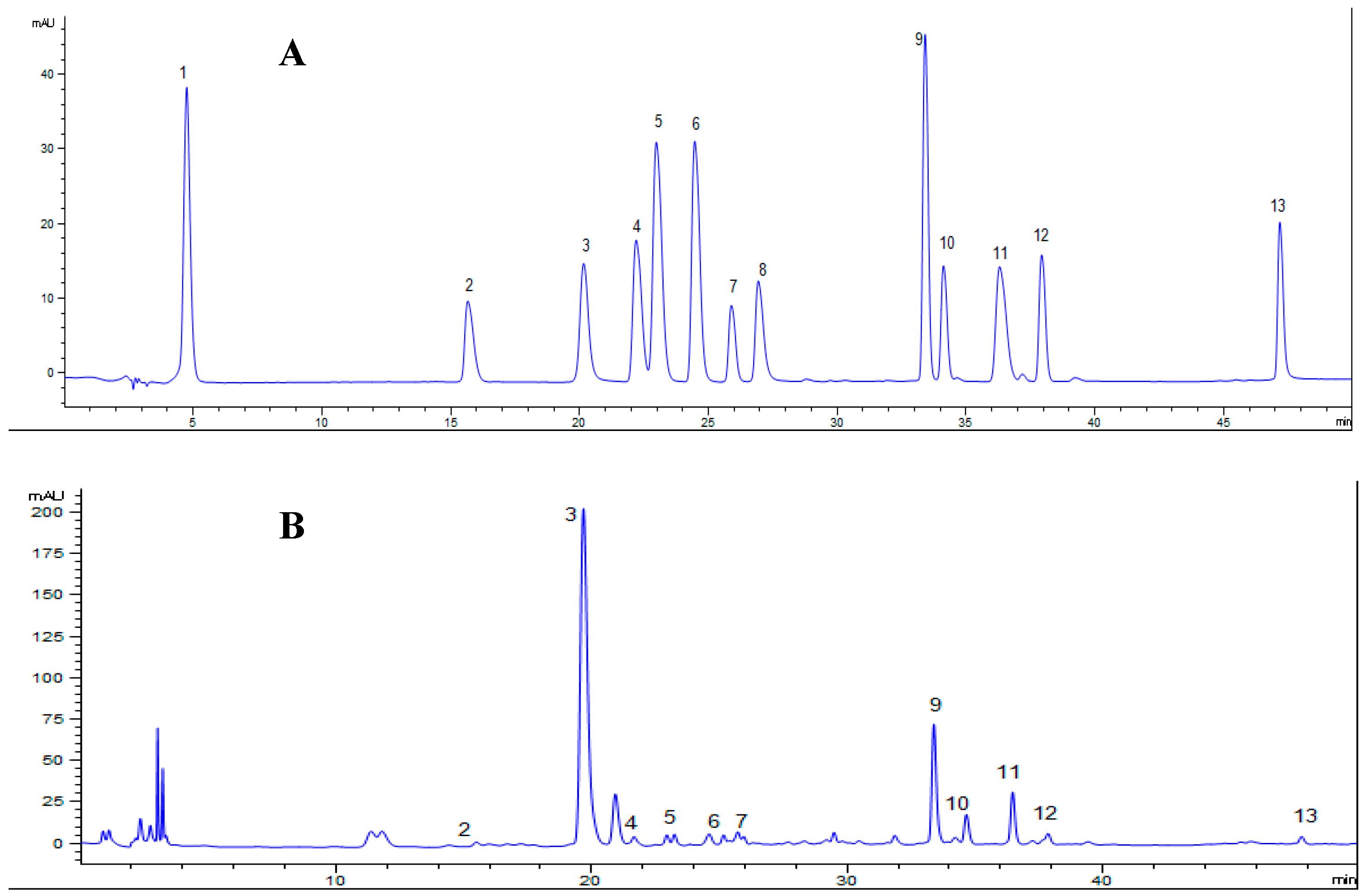
3.1. The Determination of the Main Polyphenols in MLPS Extract
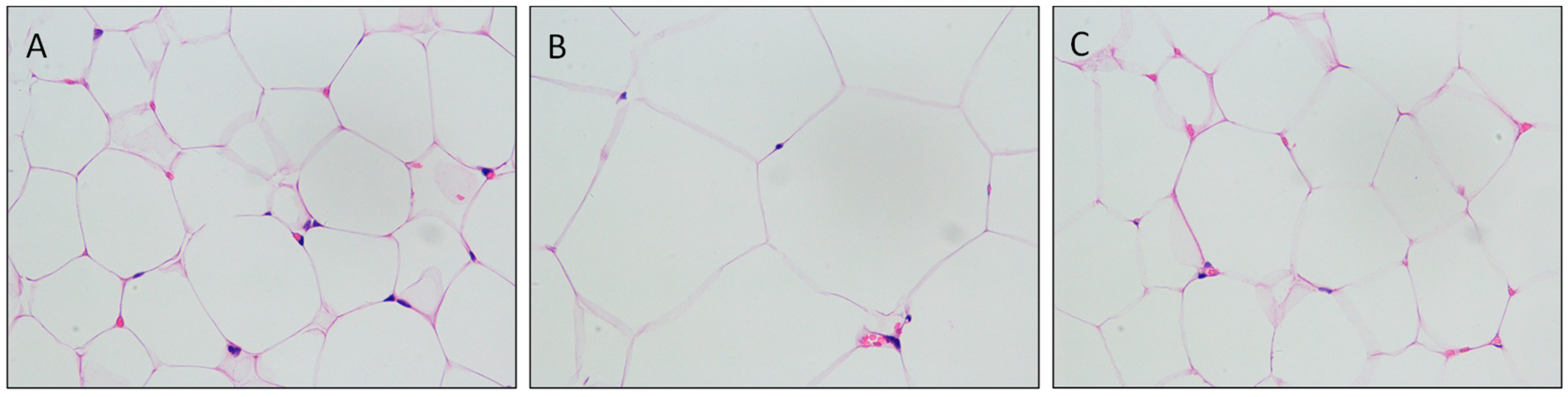
3.2. Establisment of the Diet Induced Obesity (DIO) Model
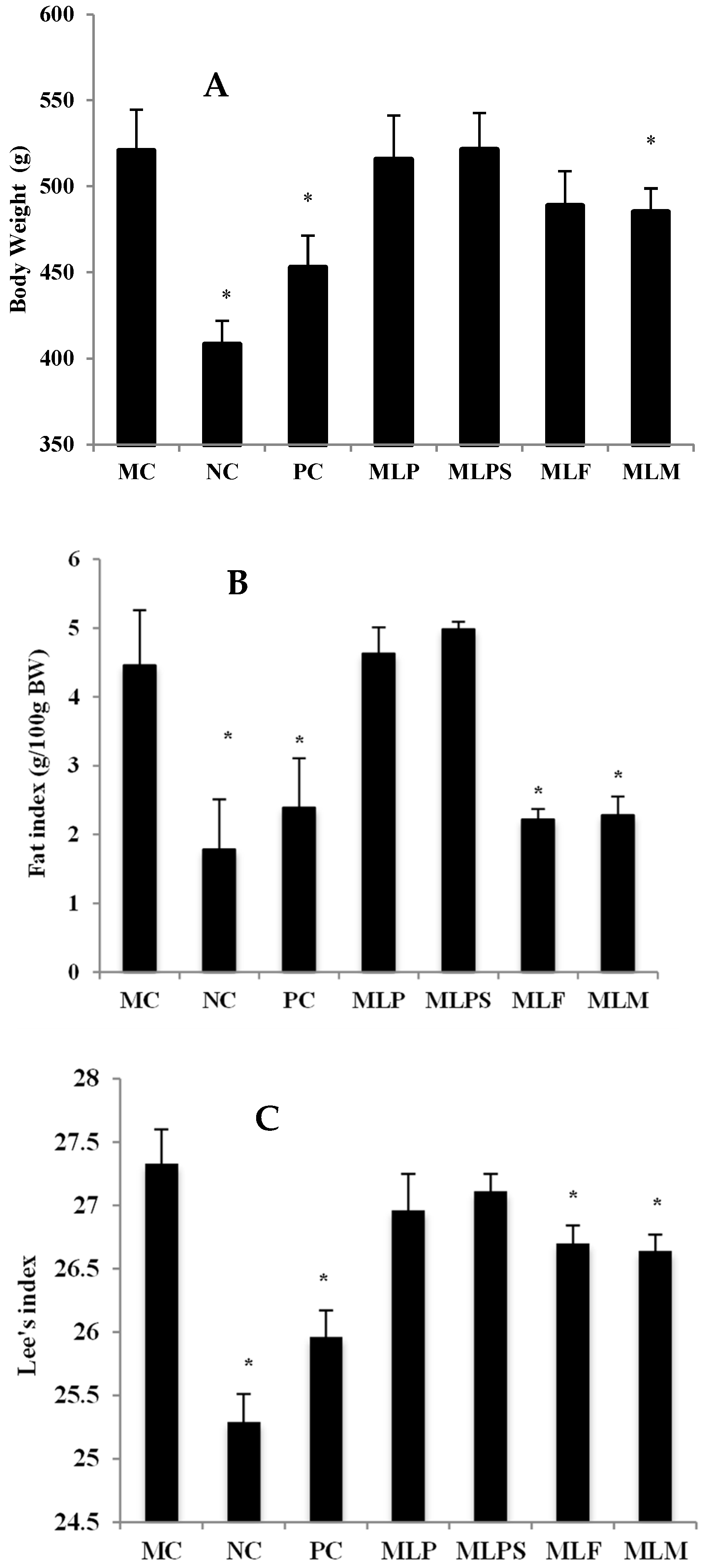
3.3. Mulberry Leaf Component Protected Rats from High Calorie Diet Induced Obesity
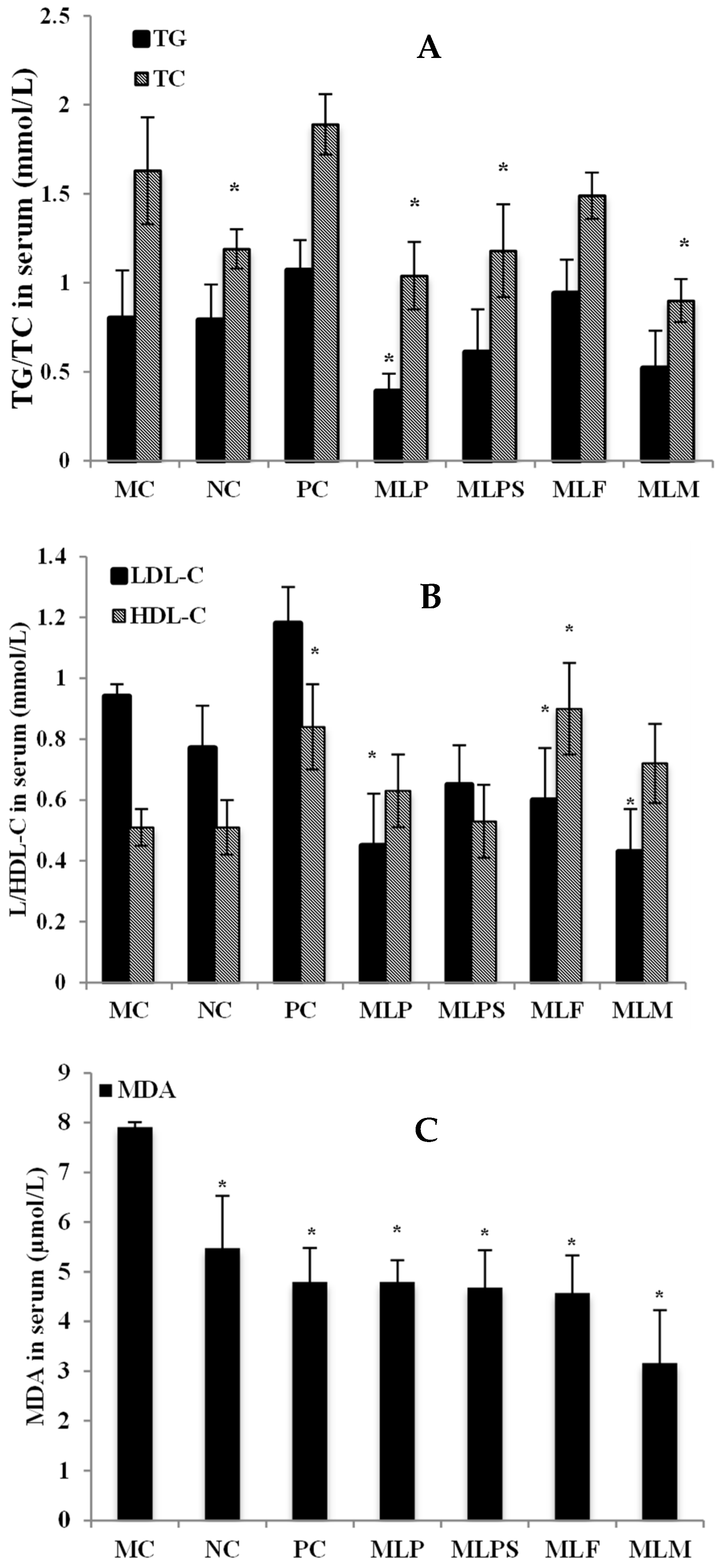
3.4. Mulberry Leaf Components Modulated Lipid Metabolism Disorder in DIO Serum
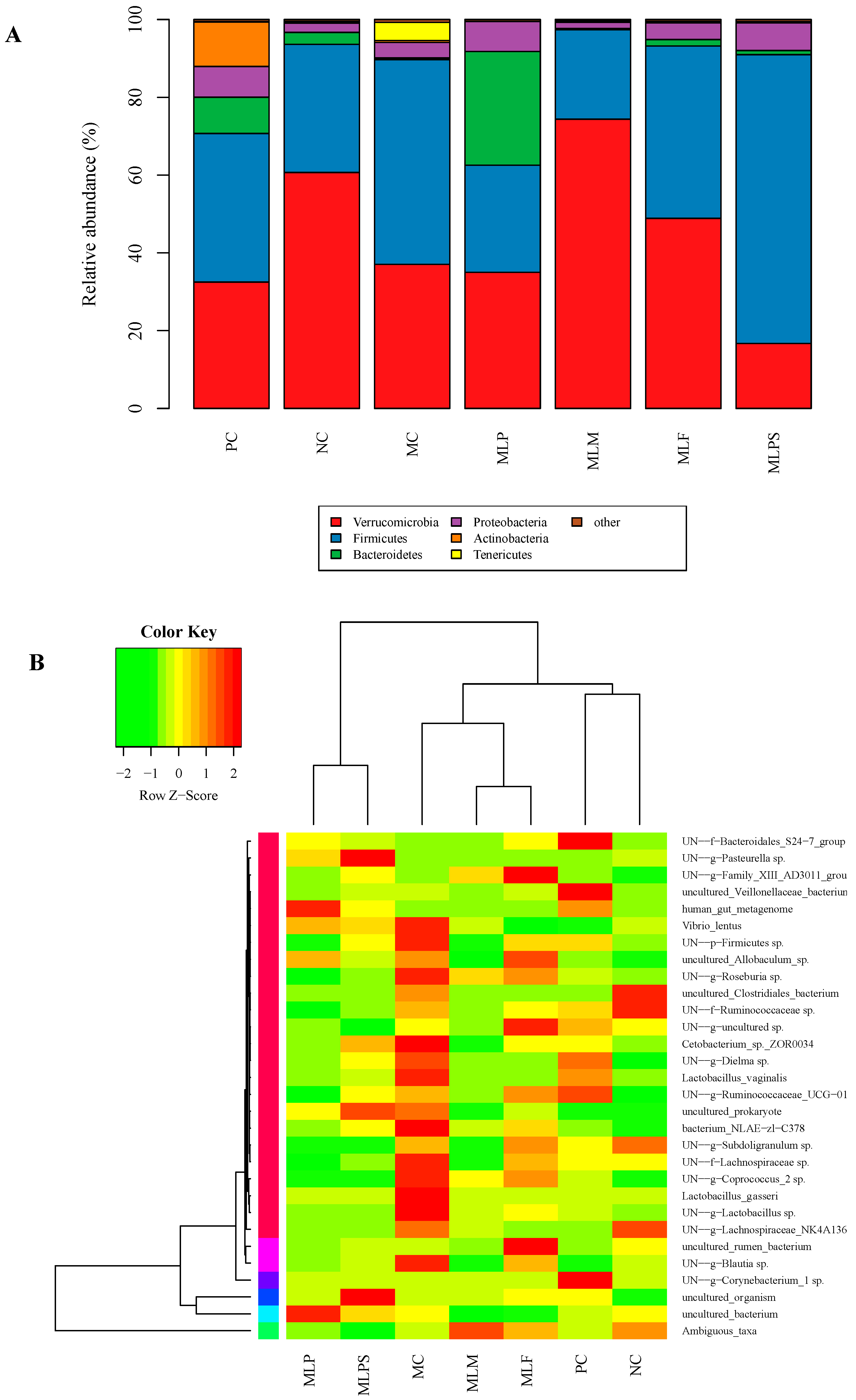
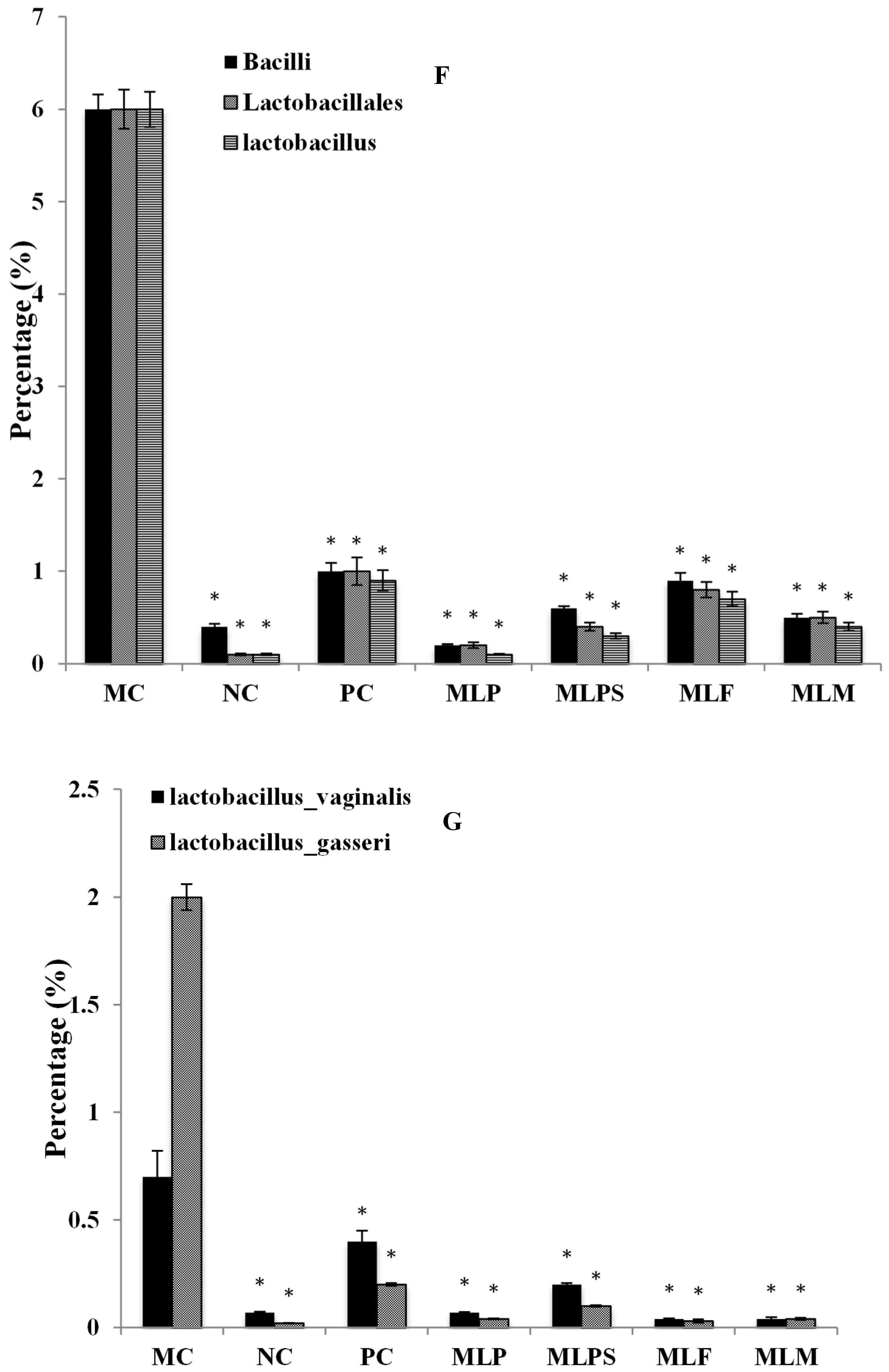
3.5. DIO Gut Environment is Altered by Mulberry Leaf Components Supplement
3.6. Urinary and Fecal Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E433. [Google Scholar] [CrossRef]
- Desprãs, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Sassi, F.; Devaux, M.; Cecchini, M.; Rusticelli, E. The obesity epidemic: Analysis of past and projected future trends in selected OECD countries. Oecd Health Work. Pap. 2009. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Gou, Y.K.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gu, Y.; Long, C.; Gao, L.; Ou, S.; Peng, X. Lean Rats Gained more Body Weight from a High-Fructooligosaccharide Diet. Food Funct. 2015, 6, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Palaurodriguez, M.; Tulipani, S.; Isabel, Q.M.; Urpisarda, M.; Tinahones, F.J.; Andreslacueva, C. Metabolomic insights into the intricate gut microbial-host interaction in the development of obesity and type 2 diabetes. Front. Microbiol. 2015, 6, 1151. [Google Scholar]
- Faith, C.W.S.; Walker, A.W.; Petra, L.; Julian, P.; Joan, V.; Douwina, B.; Duncan, S.H.; Flint, H.J. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016, 14, 3. [Google Scholar]
- Zhang, Z.; Peng, X.; Li, S.; Ning, Z.; Yong, W.; Hua, W. Isolation and Identification of Quercetin Degrading Bacteria from Human Fecal Microbes. PloS ONE 2014, 9, e90531. [Google Scholar] [CrossRef]
- Ann, J.Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef]
- Naowaboot, J.; Pannangpetch, P.; Kukongviriyapan, V.; Kongyingyoes, B.; Kukongviriyapan, U. Antihyperglycemic, Antioxidant and Antiglycation Activities of Mulberry Leaf Extract in Streptozotocin-Induced Chronic Diabetic Rats. Plant Foods Hum. Nutr. 2009, 64, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.K.; Choi, B.G.; Jung, M.J.; Wee, J.H.; Chung, K.H.; Kwon, O. Mulberry leaf water extract ameliorates insulin sensitivity in high fat or high sucrose diet induced overweight rats. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 612–618. [Google Scholar]
- Li, Q.; Dai, Y.; Zou, Y.; Liao, S.; Shen, W.; Hu, T.; Liu, F. Mulberry (Morus atropurpurea Roxb.) leaf polyphenols inhibits adipogenesis and lipogenesis-related gene expression in 3T3-L1 adipocytes. J. Food Biochem. 2018, 42, e12599. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metab. Clin. Exp. 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Bernardis, L.L.; Patterson, B.D. Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. J. Endocrinol. 1968, 40, 527–528. [Google Scholar] [CrossRef]
- Xie, G.; Zheng, X.; Qi, X.; Cao, Y.; Chi, Y.; Su, M.; Ni, Y.; Qiu, Y.; Liu, Y.; Li, H. Metabonomic evaluation of melamine-induced acute renal toxicity in rats. J. Proteome Res. 2010, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Lee, S.O.; Kim, S.Y.; Yang, S.J.; Lim, Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp. Biol. Med. 2013, 238, 1160–1169. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, S.C.; Ou, T.T.; Chyau, C.C.; Chang, Y.C.; Wang, C.J. Mulberry leaf polyphenol extracts reduced hepatic lipid accumulation involving regulation of adenosine monophosphate activated protein kinase and lipogenic enzymes. J. Funct. Foods 2013, 5, 1620–1632. [Google Scholar] [CrossRef]
- Katsube, T.; Yamasaki, M.; Shiwaku, K.; Ishijima, T.; Matsumoto, I.; Abe, K.; Yamasaki, Y. Effect of flavonol glycoside in mulberry (Morus alba L.) leaf on glucose metabolism and oxidative stress in liver in diet-induced obese mice. J. Sci. Food Agric. 2010, 90, 2386–2392. [Google Scholar] [CrossRef]
- Woo, M.N.; Bok, S.H.; Lee, M.K.; Kim, H.J.; Jeon, S.M.; Do, G.M.; Shin, S.K.; Ha, T.Y.; Choi, M.S. Anti-obesity and hypolipidemic effects of a proprietary herb and fiber combination (S&S PWH) in rats fed high-fat diets. J. Med. Food 2008, 11, 169–178. [Google Scholar]
- Neyrinck, A.M.; Hée, V.F.V.; Piront, N.; Backer, F.D.; Toussaint, O.; Cani, P.D.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes 2012, 2, e28. [Google Scholar] [CrossRef]
- Eslinger, A.J.; Eller, L.K.; Reimer, R.A. Yellow pea fiber improves glycemia and reduces Clostridium leptum in diet-induced obese rats. Nutr. Res. 2014, 34, 714–722. [Google Scholar] [CrossRef]
- Jiang, T.; Gao, X.; Wu, C.; Tian, F.; Lei, Q.; Bi, J.; Xie, B.; Wang, H.Y.; Chen, S.; Wang, X. Apple-Derived Pectin Modulates Gut Microbiota, Improves Gut Barrier Function, and Attenuates Metabolic Endotoxemia in Rats with Diet-Induced Obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. CRC Crit. Rev. Food Technol. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Blancas-Benítez, F.J.; Sáyago-Ayerdi, S.G. Polyphenols associated with dietary fibers in plant foods: Molecular interactions and bioaccessibility. Curr. Opin. Food Sci. 2017, 13, 84–88. [Google Scholar] [CrossRef]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179. [Google Scholar] [CrossRef]
- Zeng, H.; Ishaq, S.L.; Zhao, F.Q.; Wright, A.G. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J. Nutr. Biochem. 2016, 35, 30. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Dolara, P.; Luceri, C.; De, F.C.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res. 2005, 591, 237–246. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernándezquintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food. Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Lu, T.Y.; Tseng, Y.Y.; Pan, T.M. The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2006, 71, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Salaj, R.; Štofilová, J.; Šoltesová, A.; Hertelyová, Z.; Hijová, E.; Bertková, I.; Strojný, L.; Kružliak, P.; Bomba, A. The Effects of Two Lactobacillus plantarum Strains on Rat Lipid Metabolism Receiving a High Fat Diet. Scientific World J. 2013, 2013, 135142. [Google Scholar] [CrossRef] [PubMed]
- Botsford, J.L.; Demoss, R.D. Escherichia coli tryptophanase in the enteric environment. J. Bacteriol. 1972, 109, 74–80. [Google Scholar] [PubMed]
- Kikugawa, K.; Kato, T. Formation of a mutagenic diazoquinone by interaction of phenol with nitrite. Food Chem. Toxicol. 1988, 26, 209–214. [Google Scholar] [CrossRef]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228. [Google Scholar] [CrossRef]
- Whiteley, H.R. Fermentation of amino acids by Micrococcus aerogenes. J. Bacteriol. 1957, 74, 324–330. [Google Scholar]
- Kasubuchi, M.H.S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- He, L.X.; Zhang, Z.F.; Sun, B.; Chen, Q.H.; Liu, R.; Ren, J.W.; Wang, J.B.; Li, Y. Sea cucumber (Codonopsis pilosula) oligopeptides: Immunomodulatory effects based on stimulating Th cells, cytokine secretion and antibody production. Food Funct. 2016, 7, 1208–1216. [Google Scholar] [CrossRef] [PubMed]


| Weeks | Maintain Diet Group (g) | High-Energy Diet Group (g) |
|---|---|---|
| 1 | 240.2 ± 20.7 | 244.6 ± 13.7 |
| 2 | 276.4 ± 29.8 | 300.6 ± 8.8 |
| 3 | 312.1 ± 17.3 | 346.7 ± 10.4 |
| 4 | 348.1 ± 18.8 | 381.4 ± 12.1 |
| 5 | 365.4 ± 12.9 | 420.3 ± 15.9 |
| 6 | 370.6 ± 13.1 | 442.8 ± 11.7 |
| MC | PC | MLP | MLPS | MLF | MLM | |
|---|---|---|---|---|---|---|
| Intake(g) | 17.80 ± 0.43 | 17.83 ± 1.44 | 17.37 ± 0.71 | 17.21 ± 1.17 | 16.12 ± 1.12 | 16.69 ± 1.03 |
| Utilization% | 11.05 ± 1.23 | 2.16 ± 0.76 | 8.87 ± 0.77 | 11.43 ± 1.54 | 7.04 ± 0.68 | 9.03 ± 0.97 |
| MLP | MLPS | MLF | MLM | |
|---|---|---|---|---|
| Body weight | × | × | × | ↓ |
| Fat index | × | × | ↓ | ↓ |
| Lee’s index | × | × | ↓ | ↓ |
| TG | ↓ | × | × | × |
| TC | ↓ | ↓ | × | ↓ |
| HDL-C | × | × | ↑ | × |
| LDL-C | ↓ | × | ↓ | ↓ |
| MDA | ↓ | ↓ | ↓ | ↓ |
| No. | Metabolites | The Relative Content | ||||||
|---|---|---|---|---|---|---|---|---|
| MC | NC | PC | MLP | MLPS | MLF | MLM | ||
| Tryptophan | ||||||||
| 1 | 2-Indolecarboxylic acid | 4.53 # | 1 | 3.14 * | 0.29 * | 0.19 * | 0.82 * | 0.52 * |
| 2 | Indoleacetic acid | 0.40 # | 1 | 3.60 * | 0.63 * | 0.78 * | 0.57 * | 0.86 * |
| Tyrosine and phenylalanine | ||||||||
| 3 | Tyrosine | 1.55 # | 1 | 0.75 * | 0.93 * | 1.09 * | 1.60 | 1.31 |
| 4 | Shikimic acid | 2.67 # | 1 | 1.95 * | 1.36 * | 1.20 * | 0.74 * | 0.62 * |
| 5 | Thyronine | 0.20 # | 1 | 1.55 * | 0.47 * | 0.35 * | 0.47 * | 0.83 * |
| Histidine | ||||||||
| 6 | Urocanic acid | 1.07 | 1 | 2.33 * | 0.93 | 1.64 * | 1.47 * | 0.74 * |
| Cysteine/Methionine | ||||||||
| 7 | Pyroglutamic acid | 0.40 # | 1 | 4.33 * | 0.39 | 0.21 * | 0.84 * | 0.45 * |
| 8 | Homocysteine | 0.54 # | 1 | 3.74 * | 0.83* | 1.08 * | 0.58 | 0.62 * |
| 9 | Methionine | 1.53 # | 1 | 4.31 * | - | 1.04 * | 1.29 * | 0.95 * |
| Valine/leucine/isoleucine/lysine | ||||||||
| 10 | Glycine | 0.39 # | 1 | 1.32 * | 0.52 * | 0.49 * | 0.69 * | 0.62 * |
| Arginine/Proline | ||||||||
| 11 | 5-Aminopentanoic acid | 0.52 # | 1 | 0.30 * | 0.58 * | 0.74 * | 0.35 | 1.10 * |
| Purine/pyrimidine | ||||||||
| 12 | Methylguanine | 1.37 # | 1 | 5.33 * | 1.31 | 0.53 * | 1.14 * | 1.97 * |
| 13 | Deoxycytidine | 1.08 | 1 | 0.29 * | 0.91 | 2.77 * | 2.15 * | 0.63 * |
| Oligopeptides | ||||||||
| 14 | Aspartyl-glutamic acid | 0.37 # | 1 | 0.36 | 0.49 * | 0.40 | 0.80 * | 0.46 |
| 15 | Aspartyl-leucine | - | 1 | 1.13 | 0.17 * | 0.14 * | 0.43 * | 0.23 * |
| 16 | Leucyl-proline | 0.88 # | 1 | 0.11 * | 1.02 | 1.60 * | 2.53 * | 1.02 |
| 17 | Phenylalanyl-hydroxyproline | 0.02 # | 1 | 0.48 * | 0.21 * | 0.17 * | 1.10 * | 0.45 * |
| 18 | Prolyl-glycine | 1.39 # | 1 | 2.01 | - | 1.47 | - | - |
| Carbohydrate | ||||||||
| 19 | Arabitol | 0.46 # | 1 | 1.29 * | 0.78 * | 1.21 * | 0.85 * | 0.75 * |
| 20 | Arabinose | 0.40 # | 1 | 1.64 * | 1.06 * | 0.28 * | 1.11 * | 1.25 * |
| Others | ||||||||
| 21 | Glycylprolyl-hydroxyproline | 0.41 # | 1 | 2.57 * | 0.63 * | 1.32 * | 1.41 * | 1.22 * |
| 22 | Ophthalmic acid | - | 1 | 3.80 * | 0.39 * | 0.57 * | 0.61 * | 0.50 * |
| No. | Metabolites | The Relative Content | ||||||
|---|---|---|---|---|---|---|---|---|
| MC | NC | PC | MLP | MLPS | MLF | MLM | ||
| Tryptophan | ||||||||
| 1 | Tryptophan | 1.78 # | 1 | 0.76 * | 2.59 * | 0.76 * | 3.47 * | 1.10 * |
| 2 | 2-Indolecarboxylic acid | 1.10 | 1 | 0.57 * | 0.39 * | 0.88 * | 1.16 | 0.88 * |
| 3 | Nicotinic acid | 32.70 # | 1 | 0.41 * | 6.59 * | 1.56 * | 20.89 * | 14.10 * |
| Tyrosine and phenylalanine | ||||||||
| 4 | Tyrosine | 2.09 # | 1 | 3.08 * | 2.89 * | 0.33 * | 2.93 * | 1.33 * |
| 5 | 2-Phenylacetamide | 1.92 # | 1 | 5.30 * | 0.96 | 0.36 * | 2.75 * | 1.13 |
| 6 | Phenyl-acetaldehyde | 2.24 # | 1 | 16.50 * | - | 2.69 * | 2.47 | - |
| 7 | Phenylacetic acid | 4.52 # | 1 | 26.82 * | - | 4.24 | 5.19 * | 2.71 * |
| Histidine | ||||||||
| 8 | Urocanic acid | 0.41 # | 1 | 0.11 * | 0.53 * | 0.31 * | 1.85 * | - |
| 9 | Histidine | 6.01 # | 1 | 4.12 * | 1.04 * | - | - | 3.59 * |
| 10 | Methylimidazoleacetic acid | 4.71 # | 1 | 2.75 * | 0.97 * | 1.74 * | 1.43 * | 0.83 * |
| Cysteine/ Methionine | ||||||||
| 11 | 2-Oxo-4-methylthiobutyric acid | 0.39 # | 1 | 2.26 * | 1.40 * | 2.25 * | 1.79 * | 0.77 * |
| Valine/leucine/isoleucine/lysine | ||||||||
| 12 | Isoleucine | 2.23 # | 1 | 1.29 * | 1.84 * | 0.98 * | 1.94 * | 1.36 * |
| Arginine/Proline | ||||||||
| 13 | Creatinine | 0.06 # | 1 | 4.53 * | 2.76 * | - | 0.02 | 1.21 * |
| 14 | Glutamic acid | 0.91 | 1 | 14.89 * | - | 5.38 * | 5.19 * | 0.65 * |
| Purine/pyrimidine | ||||||||
| 15 | Methylguanine | 0.58 # | 1 | 1.38 * | 2.23 * | 0.68 * | 2.09 * | 1.13 * |
| 16 | Xanthine | 0.28 # | 1 | 0.94 * | 0.33 * | 0.67 * | 0.59 * | 0.51 * |
| 17 | Adenine | 0.03 # | 1 | 1.13 * | 0.04 | - | - | 0.46 * |
| Carbohydrate | ||||||||
| 18 | Threonic acid | 3.85 # | 1 | 3.91 | 1.59 * | 7.85 * | 6.89 * | 1.80 * |
| SCFAs | ||||||||
| 19 | Acetate | 1.20 | 1 | 1.5 * | 0.78 * | 1.23 * | 0.87 * | 0.96 |
| 20 | propionate | 0.50 # | 1 | 0.61 * | 0.71 * | 0.55 | 0.63 * | 0.67 * |
| 21 | butyrate | 0.37 # | 1 | 0.62 * | 0.48 * | 0.26 | 0.72 * | 0.81 * |
| Others | ||||||||
| 22 | Argininic acid | 1.95 # | 1 | 0.96 * | - | 9.71 * | 16.87 * | 0.85 * |
| 23 | D-1-Piperideine-2-carboxylic acid | 5.04 # | 1 | 2.51 * | 1.41 * | 1.46 * | 2.23 * | - |
| 24 | Ophthalmic acid | 1 | - | 0.39 * | 0.57 * | 1.31 * | 1.99 * | - |
| 25 | Phenylalanyl-hydroxyproline | 1.61 # | 1 | 9.56 * | 6.69 * | 0.60 * | 2.31 * | 2.23 * |
| 26 | Carnitine | - | 1 | 5.36 * | 0.67 * | 0.74 * | 1.00 | 0.69 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, F.; Liu, J.; Liao, S.; Zou, Y. Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites. Nutrients 2019, 11, 1017. https://doi.org/10.3390/nu11051017
Li Q, Liu F, Liu J, Liao S, Zou Y. Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites. Nutrients. 2019; 11(5):1017. https://doi.org/10.3390/nu11051017
Chicago/Turabian StyleLi, Qian, Fan Liu, Jun Liu, Sentai Liao, and Yuxiao Zou. 2019. "Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites" Nutrients 11, no. 5: 1017. https://doi.org/10.3390/nu11051017
APA StyleLi, Q., Liu, F., Liu, J., Liao, S., & Zou, Y. (2019). Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites. Nutrients, 11(5), 1017. https://doi.org/10.3390/nu11051017