Rapid Induction of Liver Regeneration for Major Hepatectomy (REBIRTH): A Randomized Controlled Trial of Portal Vein Embolisation versus ALPPS Assisted with Radiofrequency
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomisation
2.3. Procedures
2.4. Hepatic Resection
2.5. Measurement of Liver Volume
2.6. Outcomes
2.7. Failure of Treatment
- Disease progression (local, regional or systemic).
- Inadequate FLRV ≤25% for patients without preoperative chemotherapy, and ≤35% for those with preoperative chemotherapy.
2.8. Statistical Analysis
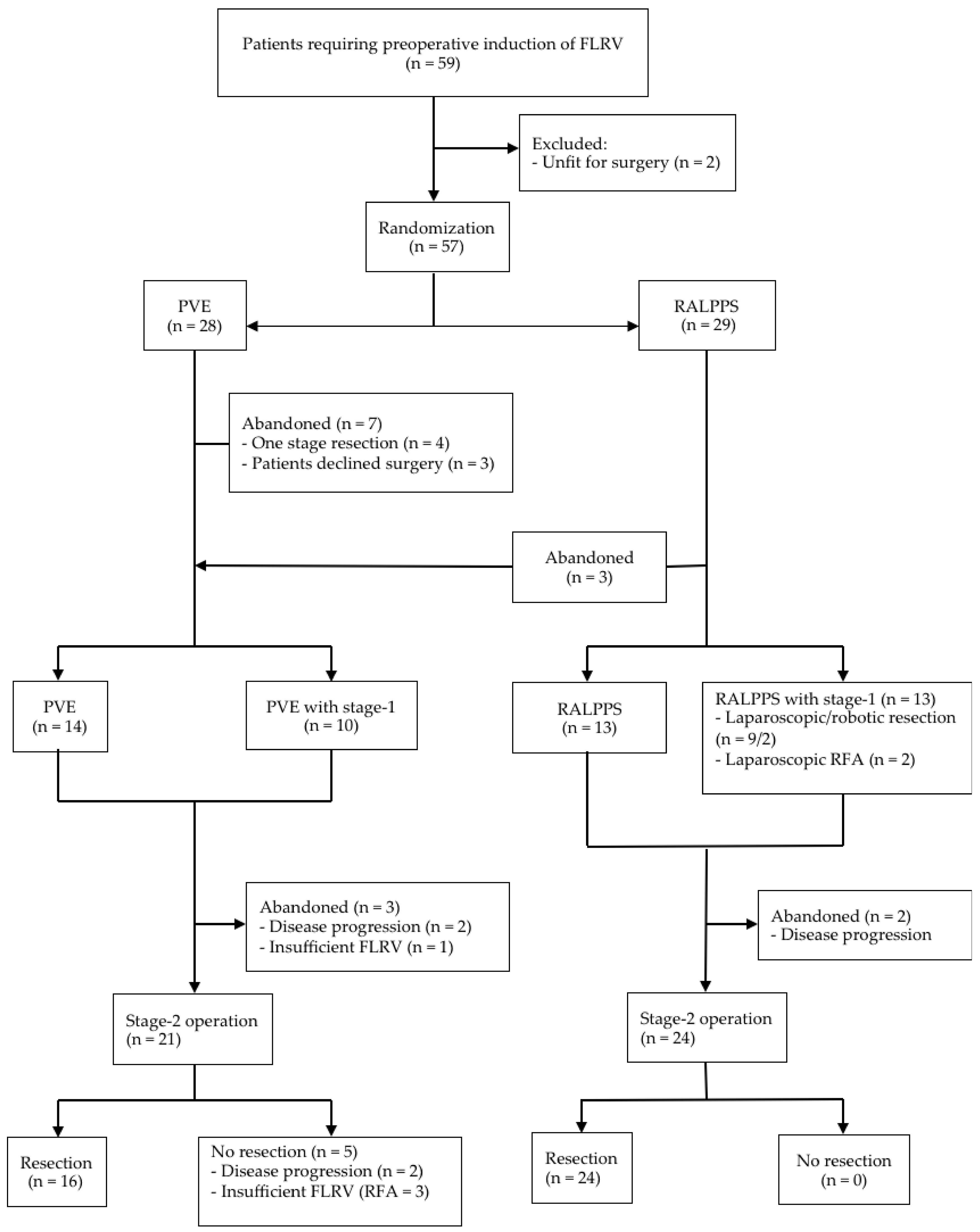
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adam, R.; Avisar, E.; Ariche, A.; Giachetti, S.; Azoulay, D.; Castaing, D.; Kunstlinger, F.; Levi, F.; Bismuth, F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann. Surg. Oncol. 2001, 8, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Kosuge, T.; Takayama, T.; Shimada, K.; Yamasaki, S.; Ozaki, H.; Yamaguchi, N.; Makuuchi, M. Recurrence of hepatocellular carcinoma after surgery. Br. J. Surg. 1996, 83, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Sadot, E.; Koerkamp, B.G.; Leal, J.N.; Shia, J.; Gonen, M.; Allen, P.J.; DeMatteo, R.P.; Kingham, T.P.; Kemeny, N.; Blumgart, L.H.; et al. MDResection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: Surgical technique or biologic surrogate? Ann. Surg. 2015, 262, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Tsim, N.; Healey, A.J.; Frampton, A.E.; Habib, N.A.; Bansi, D.S.; Wasan, H.; Cleator, S.J.; Stebbing, J.; Lowdell, C.P.; Jackson, J.E.; et al. Two-stage resection for bilobar colorectal liver metastases: R0 resection is the key. Ann. Surg. Oncol. 2011, 18, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, D.A.; Miller, R.; de Haas, R.J.; Bitsakou, G.; Vibert, E.; Veilhan, L.A.; Azoulay, D.; Bismuth, H.; Castaing, D.; Adam, R. Long-term results of two stage hepatectomy for irresectable colorectal cancer liver metastases. Ann. Surg. 2008, 248, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Pamecha, V.; Nedjat-Shokouhi, B.; Gurusamy, K.; Glantzounis, G.K.; Sharma, D.; Davidson, B.R. Prospective evaluation of two-stage hepatectomy combined with selective portal vein embolization and systemic chemotherapy for patients with unresectable bilobar colorectal liver metastases. Dig. Surg. 2008, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H.; Adam, R.; Levi, F.; Farabos, D.; Waechter, F.; Castaing, D.; Majno, P.; Engerran, L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann. Surg. 1996, 224, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Abulkhir, A.; Limongelli, P.; Healey, A.J.; Damrah, O.; Tait, P.; Jackson, J.; Habib, N.; Jiao, L.R. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann. Surg. 2008, 247, 49–57. [Google Scholar] [CrossRef] [PubMed]
- van Lienden, K.P.; van den Esschert, J.W.; de Graaf, W.; Bipat, S.; Lameris, J.S.; van Gulik, T.M.; van Delden, O.M. Portal vein embolization before liver resection: A systematic review. Cardiovasc. Intervent. Radiol. 2013, 36, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef] [PubMed]
- de Santibanes, E.; Clavien, P.A. Playing Play-Doh to prevent postoperative liver failure: The “ALPPS” approach. Ann. Surg. 2012, 255, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Schadde, E.; Ardiles, V.; Robles-Campos, R.; Malago, M.; Machado, M.; Hernandez-Alejandro, R.; Soubrane, O.; Schnitzbauer, A.A.; Raptis, D.; Tschuor, C.; et al. Early survival and safety of ALPPS: First report of the International ALPPS Registry. Ann. Surg. 2014, 260, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Olthof, P.B.; Schnitzbauer, A.A.; Schadde, E. The HPB controversy of the decade: 2007–2017—Ten years of ALPPS. Eur. J. Surg. Oncol. 2018, 44, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Sandström, P.; Rosok, B.I.; Sparrelid, E.; Larsen, P.N.; Larsson, A.L.; Lindell, G.; Schultz, N.A.; Bjornbeth, B.A.; Isaksson, B.; Rizell, M.; et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: Results from a scandinavian multicenter randomized controlled trial (LIGRO trial). Ann. Surg. 2018, 267, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.R. Percutaneous microwave ablation liver partition and portal vein embolization for rapid liver regeneration: A minimally invasive first step of ALPPS for hepatocellular carcinoma. Ann. Surg. 2016, 264, e3. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Schadde, E. Hypertrophy and liver function in ALPPS: Correlation with morbidity and mortality. Visc. Med. 2017, 33, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, M.J.; Sodergren, M.H.; Pucher, P.H.; Darzi, A.; Li, J.; Petrowsky, H.; Campos, R.R.; Serrablo, A.; Jiao, L.R. Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): Many routes to the summit. Surgery 2016, 159, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Robles, R.; Parrilla, P.; López-Conesa, A.; Brusain, R.; de la Peña, J.; Fuster, M.; Garcia-López, J.A.; Hernandez, E. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. BJS 2014, 101, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Petrowsky, H.; Györi, G.; de Oliveira, M.; Lesurtel, M.; Clavien, P.A. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann. Surg. 2015, 261, e90–e92. [Google Scholar] [CrossRef] [PubMed]
- Gall, T.M.; Sodergren, M.H.; Frampton, A.E.; Fan, R.; Spalding, D.F.; Habib, N.A.; Pai, M.; Jackson, J.E.; Tait, P.; Jiao, L.R. Radio-frequency-assisted liver partition with portal vein ligation (RALPP) for liver regeneration. Ann. Surg. 2015, 261, e45–e46. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Gringeri, E.; Feltracco, P.; Bassi, D.; D’Amico, F.E.; Polacco, M.; Boetto, R. Totally laparoscopic microwave ablation and portal vein ligation for staged hepatectomy: A new minimally invasive two-stage hepatectomy. Ann. Surg. Oncol. 2015, 22, 2787–2788. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.R. Percutaneous radiofrequency thermal ablation for liver tumours. Lancet 1999, 354, 427–428. [Google Scholar] [CrossRef]
- Donati, M.; Basile, F.; Oldhafer, K.J. Present status and future perspectives of ALPPS (associating liver partition and portal vein ligation for staged hepatectomy). Future Oncol. 2015, 11, 2255–2258. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.R.; Hakim, D.N.; Gall, T.M.; Fajardo, A.; Pencavel, T.D.; Fan, R.; Sodergren, M.H. A totally laparoscopic associating liver partition and portal vein ligation for staged hepatectomy assisted with radiofrequency (radiofrequency assisted liver partition with portal vein ligation) for staged liver resection. Hepatobiliary Surg. Nutr. 2016, 5, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Dello, S.A.; van Dam, R.M.; Slangen, J.J.; van de Poll, M.C.; Bemelmans, M.H.; Greve, J.W.; Beets-Tan, R.G.; Wigmore, S.J.; Dejong, C.H. Liver volumetry plug and play: Do it yourself with ImageJ. World J. Surg. 2007, 31, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 149–713. [Google Scholar] [CrossRef] [PubMed]
- Guiu, B.; Chevallier, P.; Denys, A.; Delhom, E.; Pierredon-Foulongne, M.A.; Rouanet, P.; Fabre, J.M.; Quenet, F.; Herrero, A.; Panaro, F.; et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: The liver venous deprivation technique. Eur. Radiol. 2016, 26, 4259–4267. [Google Scholar] [CrossRef] [PubMed]
- de Santibañes, E.; Alvarez, F.A.; Ardiles, V. How to avoid postoperative liver failure: A novel method. World J. Surg. 2012, 36, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.A.; Ardiles, V.; Sanchez Claria, R.; Pekolj, J.; de Santibañes, E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): Tips and tricks. J. Gastrointest. Surg. 2013, 17, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Schadde, E.; Raptis, D.A.; Schnitzbauer, A.A.; Ardiles, V.; Tschuor, C.; Lesurtel, M.; Abdalla, E.K.; Hernandez-Alejandro, R.; Jovine, E.; Machado, M.; et al. Prediction of mortality after ALPPS Stage-1: An analysis of 320 patients from the international ALPPS registry. Ann. Surg. 2015, 262, 780–785. [Google Scholar] [CrossRef] [PubMed]
- de Santibañes, E.; Alvarez, F.A.; Ardiles, V.; Pekolj, J.; de Santibañes, M. Inverting the ALPPS paradigm by minimizing first stage impact: The Mini-ALPPS technique. Langenbecks Arch. Surg. 2016, 401, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, G.A.; Donati, M.; Fard-Aghaie, M.H.; Zeile, M.; Huber, T.M.; Stang, A.; Oldhafer, K.J. Did the International ALPPS meeting 2015 have an impact on daily practice? The Hamburg Barmbek experience of 58 cases. Visc. Med. 2017, 33, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Madoff, D.C.; Abdalla, E.K.; Palavecino, M.; Ribero, D.; Chun, Y.S.; Vauthey, J.N. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery 2008, 144, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, R.; Revilla-Nuin, B.; Martinez, C.M.; Bernabe-Garcia, A.; Baroja-Mazo, A.; Pascual-Parrilla, P. Associated liver partition and portal vein ligation (ALPPS) vs. selective portal vein ligation (PVL) for staged hepatectomy in a rat model. Similar regenerative response? PLoS ONE 2015, 10, e0144096. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.R.; Dokmak, S. Laparoscopic ALPPS innovation or innovation’s for sake of innovation? In Proceedings of the 25th International Congress of the European Association for Endoscopic Surgery (EAES), Frankfurt, Germany, 14–17 June 2017. [Google Scholar]
- Adam, R.; Imai, K.; Castro Benitez, C.; Allard, M.A.; Vibert, E.; Sa Cunha, A.; Cherqui, D.; Baba, H.; Castaing, D. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br. J. Surg. 2016, 103, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.Y.; Chok, K.; Dai, J.W.C.; Lo, C.M. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepatocellular carcinoma: Complete-ALPPS versus partial-ALPPS. Surgery 2017, 161, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Deal, R.; Frederiks, C.; Williams, L.; Olthof, P.B.; Dirscherl, K.; Keutgen, X.; Chan, E.; Deziel, D.; Hertl, M.; Schadde, E. Rapid liver hypertrophy after portal vein occlusion correlates with the degree of collateralization between lobes- a study in pigs. J. Gastrointest. Surg. 2018, 22, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.J.; Knudsen, A.R.; Jepsen, B.N.; Meier, M.; Gunnarsson, A.P.A.; Jensen, U.B.; Nyengaard, J.R.; Hamilton-Dutoit, S.; Mortensen, F.V. A new technique for accelerated liver regeneration: An experimental study in rats. Surgery 2017, 162, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, J.N.; Abdalla, E.K.; Doherty, D.A.; Gertsch, P.; Fenstermacher, M.J.; Loyer, E.M.; Le-rut, J.; Materne, R.; Wang, X.; Encarnacion, A.; et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; Oberlin, O.; Sergent, G.; Lebuffe, G.; Gambiez, L.; Ernst, O.; Pruvot, F.R. Remnant liver volume to body weight ratio > or = 0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J. Am. Coll. Surg. 2007, 204, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Truty, M.J.; Aloia, T.A.; Curley, S.A.; Zimmitti, G.; Huang, S.Y.; Mahvash, A.; Gupta, S.; Wallace, M.J.; Vauthey, J.N. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J. Am. Coll. Surg. 2013, 216, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Breguet, R.; Boudabbous, S.; Pupulim, L.F.; Becker, C.D.; Rubbia-Brandt, L.; Toso, C.; Ronot, M.; Terraz, S. Ethylene vinyl alcohol copolymer for occlusion of specific portal branches during preoperative portal vein embolisation with n-butyl-cyanoacrylate. Eur. Radiol. 2018, 28, 4810–4817. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, A.; Toor, S.S.; Rajan, D.K.; Mironov, O.; Kachura, J.R.; Cleary, S.P.; Smoot, R.; Tremblay St-Germain, A.; Tan, K. Comparison of clinical outcomes following glue versus polyvinyl alcohol portal vein embolization for hypertrophy of the future liver remnant prior to right hepatectomy. J. Vasc. Interv. Radiol. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Age ≥ 18 years Any patient requiring right or extended right hepatectomy with preoperative FLRV/TLV
Patient able to comply with protocol requirements and deemed fit for surgical resection Written informed consent |
| Exclusion Criteria | Inability to give informed consent Pregnancy WHO performance status 3 or 4 New York Heart Association Classification Grade III or IV |
| PVE | RALPPS | p Value | |
|---|---|---|---|
| (n = 24) | (n = 26) | ||
| Patient and Tumour Characteristics | |||
| Age (mean ± SD, in years) | 64.3 ± 8.9 | 62.4 ± 10.2 | 0.49 |
| Male (%) | 12 (50.0) | 15 (57.7) | 0.78 |
| Type of tumor (%) | 0.06 | ||
| CRLM | 19 (79.2) | 20 (76.9) | |
| ICC | 4 (16.7) | 0 (0) | |
| HCC | 0 (0) | 1 (3.8) | |
| Others * | 1 (4.2) | 5 (19.2) | |
| Bilobar liver disease (%) | 9 (37.5) | 13 (50.0) | 0.06 |
| Synchronous metastases (%) | 11 (45.8) | 9 (34.6) | 0.57 |
| Number of metastases (median, range) | 2 (0–11) | 3 (1–10) | 0.18 |
| Size of largest metastasis (median, range in mm) | 43 (15–108) | 39 (12–150) | 0.53 |
| Primary tumour in situ (%) | 1 (4.2) | 3 (11.5) | 0.61 |
| Neoadjuvant Chemotherapy Data | |||
| Neoadjuvant chemotherapy (%) ** | 20 (83.3) | 22 (84.6) | 0.99 |
| FOLFOX | 7 | 7 | |
| FOLFIRI | 5 | 3 | |
| FOLFOX + Ab | 1 | 2 | |
| FOLFIRI + Ab | 4 | 6 | |
| FOLFIRI + aflibercept | 1 | 1 | |
| Capecitabine | 1 | 0 | |
| Oxaliplatin with capecitabine | 1 | 1 | |
| POMB-ACE | 0 | 1 | |
| Paclitaxel + cisplatin | 0 | 1 | |
| Number of cycles | |||
| <10 | 1 | 2 | 0.97 |
| ≥10 | 18 | 18 | 0.99 |
| Details of PVE and RALPPS | |||
| PVE/RALPPS without stage 1 | 14 (58.3) | 13 (50.0) | 0.89 |
| PVE/RALPPS with stage 1 | |||
| Tumorectomy (lap †/robotic) | 9/1 | 9/2 | |
| RFA (lap/robotic) | 0/0 | 2/0 | |
| Length of operation (median, range in mins) | 90 (60–180) | 115 (60–225) | 0.88 |
| Blood loss (median, range in mls) | 300 (10–450) | 310 (20–480) | 0.88 |
| Perioperative blood transfusion & (%) | 0 | 1 (3.8) | 0.33 |
| Post procedural complications (%) | 5 (20.1) | 6 (23.0) | 0.20 |
| Dindo 1 | 3 | 3 | |
| Dindo 2 | 2 | 2 | |
| Dindo 3b | 0 | 1 | |
| Length of stay (median, range in days) | 2 (1–13) | 3 (2–17) | 0.06 |
| Details of RALPPS (n = 29, %) | |||
| Laparoscopic | n/a | 24 (82.8) | |
| Robotic | n/a | 2 (6.9) | |
| Abandoned | n/a | 3 (10.3) | |
| Details of Stage-2 Operation | |||
| Type of operation | |||
| Right hepatectomy (open/lap/robotic) | 8 (7/1/0) | 18 (14/3/1) | |
| Extended right hepatectomy (open/lap/robotic) | 5 (5/0/0) | 5 (4/1/0) | |
| Right hepatectomy with wedge resection/RFA (open/lap/robotic) | 3 (3/0/0) | 1 (0/1/0) | |
| RFA | 3 | 0 | |
| Abandoned intraoperatively | 2 | 0 | |
| Length of operation (median, range in mins) | 180 (100–420) | 180 (110–390) | 0.87 |
| Blood loss (median, range in mls) | 500 (50–2850) | 300 (50–3200) | 0.30 |
| Perioperative blood transfusion & (%) | 6 (25.0) | 10 (38.5) | 0.18 |
| Postoperative complications (%) | 14 (66.7) | 14 (53.8) | 0.75 |
| Dindo 1 | 4 (19.0) | 0 | |
| Dindo 2 | 9 (42.9) | 9 (34.6) | |
| Dindo 3a | 0 | 1 (3.8) | |
| Dindo 3b | 1 (4.8) | 0 | |
| Dindo 4a | 0 | 2 (7.7) | |
| Dindo 4b | 0 | 1 (3.8) | |
| Dindo 5 | 0 | 1 (3.8) | |
| 90 day mortality (%) | 0 (0) | 1 (3.8) | 0.99 |
| Length of stay (median, range in days) | 7 (5–27) | 8 (4–32) | 0.25 |
| Resection Margin (%) | |||
| R0 | 11 (68.7) | 18 (75.0) | 0.87 |
| R1 | 5 (31.2) | 6 (25.0) | 0.71 |
| R2 | 0 | 0 | |
| PVE | RALPPS | p Value | |
|---|---|---|---|
| (n = 24) | (n = 26) | ||
| (no chemo (4); chemo (20)) | (no chemo (4); chemo (n = 22)) | ||
| Time from First-Stage Operation to Second CT | 35 (21–75) | 20 (14–36) | <0.001 |
| (Median in days and range) | |||
| Pre-Intervention FLRV | |||
| (Mean ± SD) | |||
| no chemo | 23.7 ± 1.1 | 23.1 ± 1.2 | 0.74 |
| chemo | 33.1 ± 1.5 | 33.8 ± 1.1 | 0.2 |
| Post-Intervention FLRV | |||
| (Mean ± SD) | |||
| no chemo | 28.5 ± 9.4 | 44.6 ± 5.6 | 0.04 |
| chemo | 40.4 ± 6.6 | 59.4 ± 4.3 | <0.001 |
| Increase FLRV Post-Intervention (%) | 18.4 ± 9.8 | 80.7 ± 13.7 | <0.001 |
| RALPPS | ALPPS 15 | |
|---|---|---|
| (n = 26) | (n = 48) | |
| Stage 1 | ||
| Type of operation | ||
| Open | 0 | 48 |
| Laparoscopic/Robotic | 24/2 | 0/0 |
| Length of operation (median, range, mins) | 115 (60–225) | NA |
| Length of stay (median, range, days) | 3 (2–17) | NA |
| Morbidity | 23.0 | NA |
| Mortality | 0 | NA |
| FLRV Increase (Mean ± SD, %) | 80.7 ± 13.7 | 68.0 ± 38.0 |
| Time from Stage 1 to Stage 2 (Mean ± SD, days) | 20.0 ± 5.6 | 11.0 ± 11.0 |
| Stage 2 | ||
| Complications grade ≥ 3b (%) | 15.3 | 11.0 |
| 30 (90) day mortality (%) | 3.8 (0) | 9.1(0) |
| Total length of stay (Mean ± SD, days) | 15.3 ± 9.7 | 23.0 ± 17.0 |
| Resection Rates (%) | 92.3 | 92.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.R.; Fajardo Puerta, A.B.; Gall, T.M.H.; Sodergren, M.H.; Frampton, A.E.; Pencavel, T.; Nagendran, M.; Habib, N.A.; Darzi, A.; Pai, M.; et al. Rapid Induction of Liver Regeneration for Major Hepatectomy (REBIRTH): A Randomized Controlled Trial of Portal Vein Embolisation versus ALPPS Assisted with Radiofrequency. Cancers 2019, 11, 302. https://doi.org/10.3390/cancers11030302
Jiao LR, Fajardo Puerta AB, Gall TMH, Sodergren MH, Frampton AE, Pencavel T, Nagendran M, Habib NA, Darzi A, Pai M, et al. Rapid Induction of Liver Regeneration for Major Hepatectomy (REBIRTH): A Randomized Controlled Trial of Portal Vein Embolisation versus ALPPS Assisted with Radiofrequency. Cancers. 2019; 11(3):302. https://doi.org/10.3390/cancers11030302
Chicago/Turabian StyleJiao, Long R., Ana B. Fajardo Puerta, Tamara M.H. Gall, Mikael H. Sodergren, Adam E. Frampton, Tim Pencavel, Myura Nagendran, Nagy A. Habib, Ara Darzi, Madhava Pai, and et al. 2019. "Rapid Induction of Liver Regeneration for Major Hepatectomy (REBIRTH): A Randomized Controlled Trial of Portal Vein Embolisation versus ALPPS Assisted with Radiofrequency" Cancers 11, no. 3: 302. https://doi.org/10.3390/cancers11030302
APA StyleJiao, L. R., Fajardo Puerta, A. B., Gall, T. M. H., Sodergren, M. H., Frampton, A. E., Pencavel, T., Nagendran, M., Habib, N. A., Darzi, A., Pai, M., Thomas, R., & Tait, P. (2019). Rapid Induction of Liver Regeneration for Major Hepatectomy (REBIRTH): A Randomized Controlled Trial of Portal Vein Embolisation versus ALPPS Assisted with Radiofrequency. Cancers, 11(3), 302. https://doi.org/10.3390/cancers11030302






