Effect of Thoracic Gas Volume Changes on Body Composition Assessed by Air Displacement Plethysmography after Rapid Weight Loss and Regain in Elite Collegiate Wrestlers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Ethical Approval and Consent to Participate
2.2. Procedure
2.3. Anthropometric Data and Air Displacement Plethysmography
2.4. Total Body Water
2.5. Dual-Energy X-ray Absorptiometry
2.6. Four-Component Model of Body Composition
2.7. Energy and Macronutrient Intake
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berkovich, B.E.; Eliakim, A.; Nemet, D.; Stark, A.H.; Sinai, T. Rapid Weight Loss Among Adolescents Participating in Competitive Judo. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.J.; Nicholas, C. Extreme Rapid Weight Loss and Rapid Weight Gain Observed in UK Mixed Martial Arts Athletes Preparing for Competition. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Reale, R.; Cox, G.R.; Slater, G.; Burke, L.M. Regain in body mass after weigh-in is linked to success in real life judo competition. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Reljic, D.; Hassler, E.; Jost, J.; Friedmann-Bette, B. Rapid weight loss and the body fluid balance and hemoglobin mass of elite amateur boxers. J. Athl. Train. 2013, 48, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sagayama, H.; Yoshimura, E.; Yamada, Y.; Ichikawa, M.; Ebine, N.; Higaki, Y.; Kiyonaga, A.; Tanaka, H. Effects of rapid weight loss and regain on body composition and energy expenditure. Appl. Physiol. Nutr. Metab. 2014, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Khodaee, M.; Olewinski, L.; Shadgan, B.; Kiningham, R.R. Rapid Weight Loss in Sports with Weight Classes. Curr. Sports Med. Rep. 2015, 14, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ransone, J.; Hughes, B. Body-Weight Fluctuation in Collegiate Wrestlers: Implications of the National Collegiate Athletic Association Weight-Certification Program. J. Athl. Train. 2004, 39, 162–165. [Google Scholar]
- Dempster, P.; Aitkens, S. A new air displacement method for the determination of human body composition. Med. Sci. Sports Exerc. 1995, 27, 1692–1697. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Woolf, K.; Burke, L. Assessment of Nutrient Status in Athletes and the Need for Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 139–158. [Google Scholar] [CrossRef]
- McCrory, M.A.; Molé, P.A.; Gomez, T.D.; Dewey, K.G.; Bernauer, E.M. Body composition by air-displacement plethysmography by using predicted and measured thoracic gas volumes. J. Appl. Physiol. 1998, 84, 1475–1479. [Google Scholar] [CrossRef]
- Plasqui, G.; Soenen, S.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Measurement of longitudinal changes in body composition during weight loss and maintenance in overweight and obese subjects using air-displacement plethysmography in comparison with the deuterium dilution technique. Int. J. Obes. 2011, 35, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Minderico, C.S.; Silva, A.M.; Fields, D.A.; Branco, T.L.; Martins, S.S.; Teixeira, P.J.; Sardinha, L.B. Changes in thoracic gas volume with air-displacement plethysmography after a weight loss program in overweight and obese women. Eur. J. Clin. Nutr. 2008, 62, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kukidome, T.; Shirai, K.; Kubo, J.; Matsushima, Y.; Yanagisawa, O.; Homma, T.; Aizawa, K. MRI evaluation of body composition changes in wrestlers undergoing rapid weight loss. Br. J. Sports Med. 2008, 42, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Kukidome, T.; Aizawa, K.; Nakajima, K.; Masujima, A. The actual state of weight loss among the contestants for the All Japan Wrestling Championships. J. Jpn. Soc. Clin. Sports Med. 2006, 14, 325–332. (In Japanese) [Google Scholar]
- Fogelholm, G.M.; Koskinen, R.; Laakso, J.; Rankinen, T.; Ruokonen, I. Gradual and rapid weight loss: Effects on nutrition and performance in male athletes. Med. Sci. Sports Exerc. 1993, 25, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Hunter, G.R.; Goran, M.I. Validation of the BOD POD with hydrostatic weighing: Influence of body clothing. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar]
- Crapo, R.O.; Morris, A.H.; Clayton, P.D.; Nixon, C.R. Lung volumes in healthy nonsmoking adults. Bull. Eur. Physiopathol. Respir. 1982, 18, 419–425. [Google Scholar]
- Siri, W. Body composition from fluid spaces and density: Analysis of methods. In Techniques for Measuring Body Composition; Brozek, J., Henschel, A., Eds.; National Academy of Sciences, National Research Council: Washington, DC, USA, 1961; pp. 223–244. [Google Scholar]
- Shitara, K.; Hakamada, T.; Ohnishi, T.; Ikeda, T. Differences in body density and percent body fat found by different methods of evaluating body composition. Jpn. J. Phys. Fitness Sports Med. 2017, 66, 369–382. [Google Scholar] [CrossRef]
- Cole, T.J.; Coward, W.A. Precision and accuracy of doubly labeled water energy expenditure by multipoint and two-point methods. Am. J. Physiol. 1992, 263, E965–E973. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Schoeller, D.A.; Luke, A.H.; Shay, K.; Hnilicka, J.; Kushner, R.F. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am. J. Physiol. 1994, 267, E585–E590. [Google Scholar] [CrossRef] [PubMed]
- Sagayama, H.; Yamada, Y.; Racine, N.M.; Shriver, T.C.; Schoeller, D.A.; Group, D.L.W.S. Dilution space ratio of 2H and 18O of doubly labeled water method in humans. J. Appl. Physiol. 2016, 120, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Matias, C.N.; Santos, D.A.; Rocha, P.M.; Minderico, C.S.; Thomas, D.; Heymsfield, S.B.; Sardinha, L.B. Do dynamic fat and fat-free mass changes follow theoretical driven rules in athletes? Med. Sci. Sports Exerc. 2017, 49, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Shen, W.; Withers, R.T.; Heymsfield, S.B. Multicomponent Molecular Level Models of Body Composition Analysis; Human Kinetics: Champaign, IL, USA, 1996; pp. 163–176. [Google Scholar]
- Sagayama, H.; Kondo, E.; Shiose, K.; Yamada, Y.; Motonaga, K.; Ouchi, S.; Kamei, A.; Osawa, T.; Nakajima, K.; Takahashi, H.; et al. Energy requirement assessment and water turnover in Japanese college wrestlers using the doubly labeled water method. J. Nutr. Sci. Vitaminol. 2017, 63, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Brozek, J.; Grande, F.; Anderson, J.T.; Keys, A. Densitometric analysis of body composition: Revision of some quantitative assumptions. Ann. N. Y. Acad. Sci. 1963, 110, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Kossel, E.; Goele, K.; Later, W.; Hitze, B.; Settler, U.; Heller, M.; Gluer, C.C.; Heymsfield, S.B.; Muller, M.J. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am. J. Clin. Nutr. 2009, 90, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Kelley, D.E.; Thornton, J.; Boxt, L.; Pi-Sunyer, X.; Lipkin, E.; Nyenwe, E.; Janumala, I.; Heshka, S. Changes in skeletal muscle and organ size after a weight-loss intervention in overweight and obese type 2 diabetic patients. Am. J. Clin. Nutr. 2017, 105, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gils Contreras, A.; Bonada Sanjaume, A.; Montero Jaime, M.; Rabassa Soler, A.; Sabench Pereferrer, F.; Molina Lopez, A.; Becerra Tomas, N.; Del Castillo Dejardin, D.; Salas-Salvado, J. Effects of Two Preoperatory Weight Loss Diets on Hepatic Volume, Metabolic Parameters, and Surgical Complications in Morbid Obese Bariatric Surgery Candidates: A Randomized Clinical Trial. Obes. Surg. 2018, 28, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, T.B.; Moerland, P.D.; Frijters, R.; Sokolovic, A.; Labruyere, W.T.; Vermeulen, J.L.; Ver Loren van Themaat, E.; Breit, T.M.; Wittink, F.R.; van Kampen, A.H.; et al. Interorgan coordination of the murine adaptive response to fasting. J. Biol. Chem. 2011, 286, 16332–16343. [Google Scholar] [CrossRef]
- Tai, S.; Tsurumi, Y.; Yokota, Y.; Masuhara, M.; Okamura, K. Effects of rapid or slow body mass reduction on body composition in adult rats. J. Clin. Biochem. Nutr. 2009, 45, 185–192. [Google Scholar] [CrossRef]
- Tai, S.; Yokota, Y.; Tsurumi, Y.; Hasegawa, H.; Masuhara, M.; Okamura, K. Effects of short-term refeeding after rapid or slow body mass reduction on body composition in adult rats. Obes. Res. Clin. Pract. 2010, 4, e191–e199. [Google Scholar] [CrossRef] [PubMed]
- Decombaz, J.; Jentjens, R.; Ith, M.; Scheurer, E.; Buehler, T.; Jeukendrup, A.; Boesch, C. Fructose and galactose enhance postexercise human liver glycogen synthesis. Med. Sci. Sports Exerc. 2011, 43, 1964–1971. [Google Scholar] [PubMed]
- Ekelund, M.; Kristensson, E.; Ekelund, M.; Ekblad, E. Total Parenteral Nutrition Causes Circumferential Intestinal Atrophy, Remodeling of the Intestinal Wall, and Redistribution of Eosinophils in the Rat Gastrointestinal Tract. Dig. Dis. Sci. 2007, 52, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Niinikoski, H.; Stoll, B.; Guan, X.; Kansagra, K.; Lambert, B.D.; Stephens, J.; Hartmann, B.; Holst, J.J.; Burrin, D.G. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J. Nutr. 2004, 134, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.E.; Ahonen, E.; Nousiainen, U. Differential effects of sauna-, diuretic-, and exercise-induced hypohydration. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.; Rutt, R.; Weltman, A. Physiological effects of a weight loss regimen practiced by college wrestlers. Med. Sci. Sports Exerc. 1990, 22, 229–234. [Google Scholar] [PubMed]
- Fields, D.A.; Higgins, P.B.; Hunter, G.R. Assessment of body composition by air-displacement plethysmography: Influence of body temperature and moisture. Dyn. Med. 2004, 3, 3. [Google Scholar] [CrossRef]
- Kondo, E.; Sagayama, H.; Yamada, Y.; Shiose, K.; Osawa, T.; Motonaga, K.; Ouchi, S.; Kamei, A.; Nakajima, K.; Higaki, Y.; et al. Energy Deficit Required for Rapid Weight Loss in Elite Collegiate Wrestlers. Nutrients 2018, 10, 536. [Google Scholar] [CrossRef]
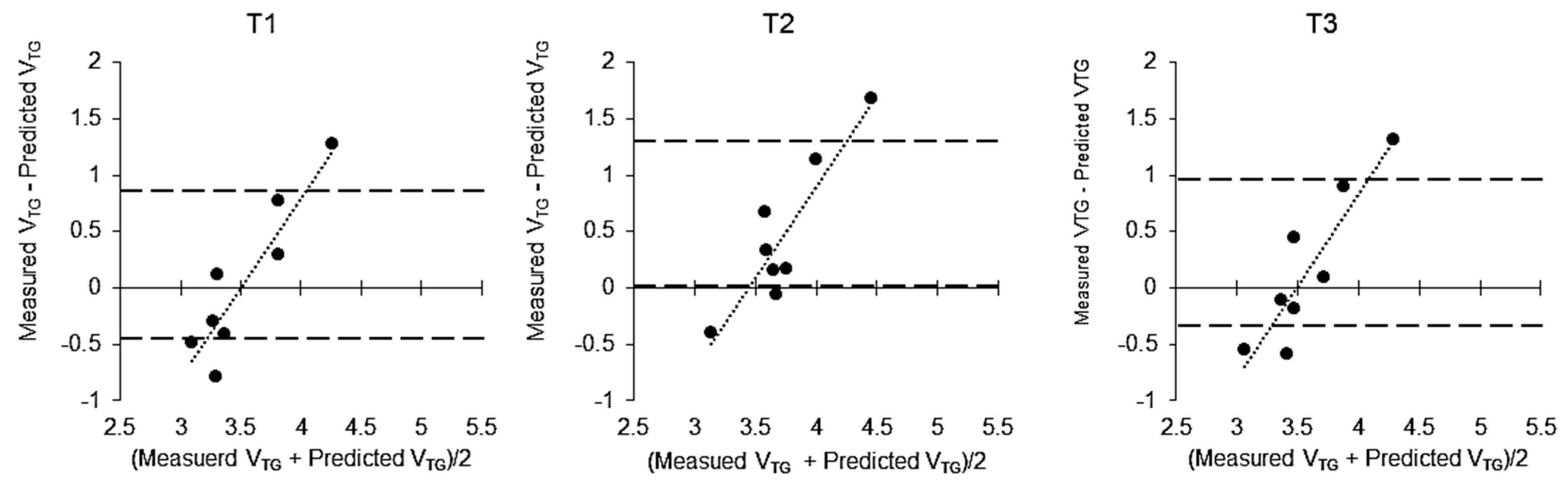
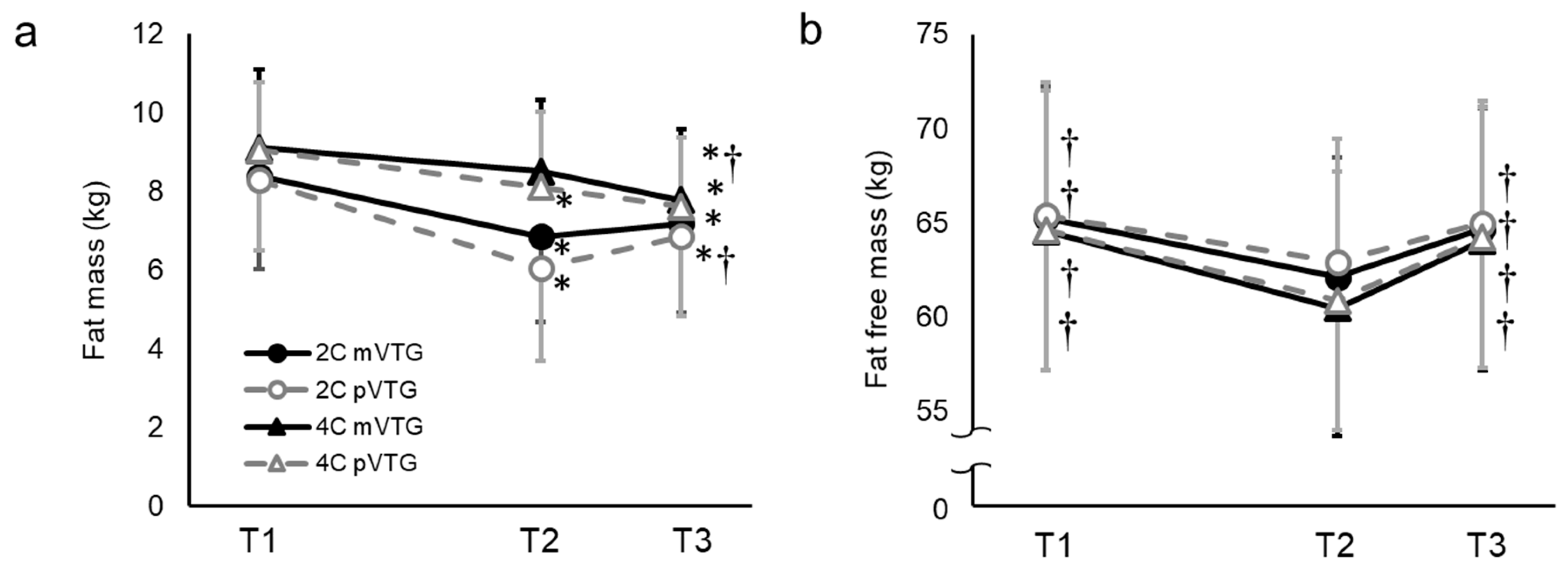


| Variable | Unit | Baseline (Per Day) | RWL Period (53 h) | WR Period (13 h) |
|---|---|---|---|---|
| Food weight | (g) | 3686 ± 1615 | 3234 ± 1591 | 4669 ± 1051 |
| Energy | (MJ) | 14.76 ± 3.47 | 9.90 ± 4.95 | 12.10 ± 1.23 |
| (kcal) | 3528 ± 829 | 2366 ± 1184 | 2891 ± 295 | |
| Protein | (g) | 125 ± 30 | 85 ± 41 | 64 ± 8 |
| (g/kg) | 1.7 ± 0.4 | 1.2 ± 0.5 | 0.9 ± 0.0 | |
| (%) | 14.2 ± 0.9 | 14.6 ± 2.8 | 8.8 ± 0.2 | |
| Fat | (g) | 110 ± 24 | 34 ± 20 | 60 ± 9 |
| (%) | 28.9 ± 5.3 | 28.4 ± 9.3 | 18.5 ± 1.1 | |
| Carbohydrate | (g) | 509± 149 | 340 ± 170 | 524 ± 45 |
| (g/kg) | 6.9 ± 1.9 | 4.6 ± 2.1 | 7.1 ± 0.3 | |
| (%) | 56.9 ± 5.5 | 57.0 ± 11.4 | 72.7 ± 1.3 |
| Variables | T1 | T2 | T3 | Change | ||
|---|---|---|---|---|---|---|
| T1–T2 | T2–T3 | |||||
| Age | 20.4 ± 0.5 | ± | ± | |||
| Height (cm) | 169.7 ± 3.5 | ± | ± | |||
| BM (kg) | 73.7 ± 8.0 | 69.0 ± 7.7 * | 71.8 ± 7.7 *# | −4.7 ± 0.5 | 2.9 ± 0.3 | |
| TBW (kg) | 46.8 ± 5.6 | 43.5 ± 5.2 * | 46.6 ± 5.3 *# | −3.4 ± 0.6 | 3.1 ± 0.6 | |
| Mo (kg) | 3.2 ± 0.3 | 3.2 ± 0.3 * | 3.2 ± 0.3 * | 0.0 ± 0.0 | 0.0 ±0.0 | |
| VTG (L) | mVTG | 3.56 ± 0.72 | 3.96 ± 0.70 * | 3.67 ± 0.69 # | 0.36 ± 0.31 | −0.29 ± 0.15 † |
| pVTG | 3.51 ± 0.16 | 3.51 ± 0.16 | 3.51 ± 0.17 | 0.00 ± 0.01 a | 0.00 ± 0.01 a | |
| BV (L) | mVTG | 68.67 ± 7.53 | 64.09 ± 7.26 * | 66.76 ± 7.32 *# | −4.58 ± 0.43 | 2.66 ± 0.36 † |
| pVTG | 68.65 ± 7.48 | 63.91 ± 7.22 * | 66.69 ± 7.30 *# | −4.74 ± 0.46 a | 2.78 ± 0.35 †a | |
| Db (g/cm3) | mVTG | 1.073 ± 0.006 | 1.076 ± 0.006 * | 1.076 ± 0.006 * | 0.004 ± 0.002 | 0.000 ± 0.002 † |
| pVTG | 1.073 ± 0.005 | 1.080 ± 0.007 * | 1.077 ± 0.006 *# | 0.006 ± 0.003 a | −0.002 ± 0.002 †a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, E.; Shiose, K.; Yamada, Y.; Osawa, T.; Sagayama, H.; Motonaga, K.; Ouchi, S.; Kamei, A.; Nakajima, K.; Takahashi, H.; et al. Effect of Thoracic Gas Volume Changes on Body Composition Assessed by Air Displacement Plethysmography after Rapid Weight Loss and Regain in Elite Collegiate Wrestlers. Sports 2019, 7, 48. https://doi.org/10.3390/sports7020048
Kondo E, Shiose K, Yamada Y, Osawa T, Sagayama H, Motonaga K, Ouchi S, Kamei A, Nakajima K, Takahashi H, et al. Effect of Thoracic Gas Volume Changes on Body Composition Assessed by Air Displacement Plethysmography after Rapid Weight Loss and Regain in Elite Collegiate Wrestlers. Sports. 2019; 7(2):48. https://doi.org/10.3390/sports7020048
Chicago/Turabian StyleKondo, Emi, Keisuke Shiose, Yosuke Yamada, Takuya Osawa, Hiroyuki Sagayama, Keiko Motonaga, Shiori Ouchi, Akiko Kamei, Kohei Nakajima, Hideyuki Takahashi, and et al. 2019. "Effect of Thoracic Gas Volume Changes on Body Composition Assessed by Air Displacement Plethysmography after Rapid Weight Loss and Regain in Elite Collegiate Wrestlers" Sports 7, no. 2: 48. https://doi.org/10.3390/sports7020048
APA StyleKondo, E., Shiose, K., Yamada, Y., Osawa, T., Sagayama, H., Motonaga, K., Ouchi, S., Kamei, A., Nakajima, K., Takahashi, H., & Okamura, K. (2019). Effect of Thoracic Gas Volume Changes on Body Composition Assessed by Air Displacement Plethysmography after Rapid Weight Loss and Regain in Elite Collegiate Wrestlers. Sports, 7(2), 48. https://doi.org/10.3390/sports7020048






