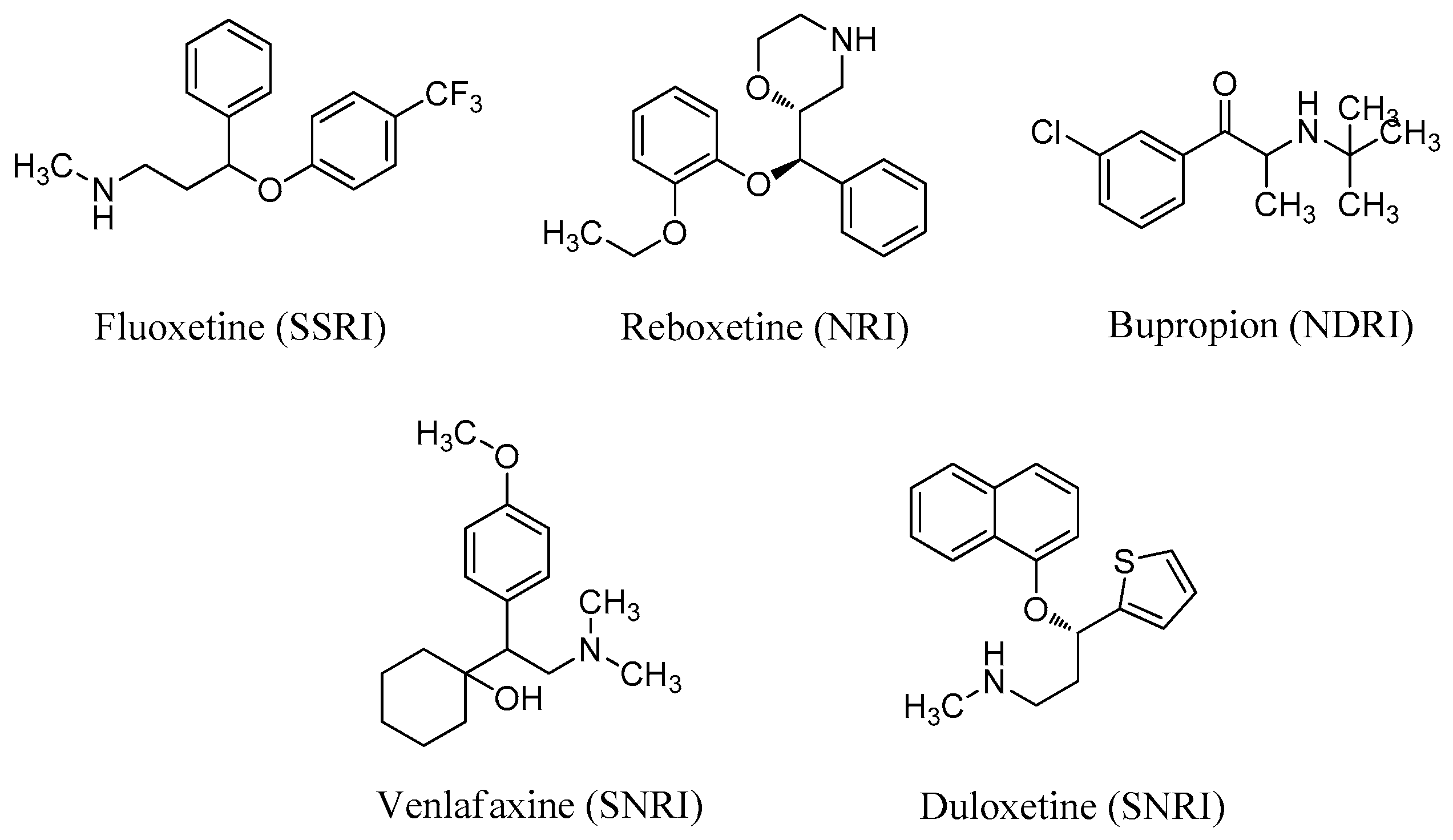
Preparation of (E)-N-((4-Methoxyphenyl)(piperazin-1-yl)methylene)-1-phenylmethanamine hydrochloride (I-1)
N-Benzyl-4-methoxybenzamide (0.50 g, 2.1 mmol) was dissolved in dry dichloroethane (10 mL), and the solution was heated to 50 °C for 30 min. PCl5 (0.44 g, 2.1 mmol) was added in one portion and the reaction was stirred at 90 °C for 2 h. After most of the solvent was removed in vacuo, the residue was dissolved in dry CH2Cl2 (10 mL) and was added dropwise to a solution of piperazine (0.54 g, 6 mmol) in dry CH2Cl2 (10 mL) at 0 °C. The reaction mixture was stirred at room temperature for 2 h, and then distilled in vacuo. Et2O (50 mL) and saturated aqueous Na2CO3 (30 mL) were added to the mixture. After the aqueous extract was separated, the organic extract was washed twice with saturated aqueous Na2CO3 and 10% aqueous NaOH, and then extracted with 1 mol/L HCl. The aqueous extract was washed twice with CH2Cl2 and Et2O, and was treated with 10% aqueous NaOH to increase the pH to 10. The aqueous extract was re-extracted with Et2O (50 mL), and the Et2O extract was dried over Na2SO4. After Et2O was removed under vacuum, the residue was dissolved in dry ethanol (5 mL), and an ethanolic solution of HCl was added dropwise to adjust the pH to 2 to afford I-1 as a white powder (0.18 g, 25% yield). Mp: 195–197 °C. 1H-NMR (400 MHz, D2O): δ 3.37 (br, 2H, piperazinyl-H), 3.61 (br, 2H, piperazinyl-H), 3.66 (br, 2H,piperazinyl-H), 3.89 (s, 3H, -CH3), 4.13 (br, 2H, piperazinyl-H), 4.44 (s, 2H, -CH2-), 7.10–7.16 (m, 4H, ArH), 7.35–7.41 (m, 5H, ArH). HRMS calcd for C19H23N3O (M + H)+, 310.1914; found, 310.1912.
(E)-N-((4-Nitrophenyl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride (I-2): Yield 40%, Mp: 230–231 °C. 1H-NMR (400 MHz, D2O): δ 3.37 (br, 2H, piperazinyl-H), 3.61 (br, 2H, piperazinyl-H), 3.66 (br, 2H, piperazinyl-H), 4.13 (br, 2H, piperazinyl-H), 4.44 (s, 2H, -CH2-), 7.10–7.16 (m, 4H, ArH), 7.35–7.41 (m, 5H, ArH). HRMS calcd for C18H20N4O2 (M + H)+, 325.1659; found, 325.1654.
(E)-N-((2-Chlorophenyl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride (I-3): Yield 18%, Mp: 142–144 °C.1H-NMR (400 MHz, D2O): δ 3.40 (br, 2H, piperazinyl-H), 3.67 (br, 4H, piperazinyl-H), 4.24 (br, 2H, piperazinyl-H), 4.38 (d, 1H, J = 14.0 Hz, -CH′-), 4.46 (d, 1H, J = 14.0 Hz, -CH′′-), 7.11–7.12 (m, 2H, ArH), 7.37–7.38 (m, 2H, ArH), 7.48 (d, 1H, J = 7.6 Hz, ArH), 7.55–7.58 (m, 2H, ArH), 7.70–7.73 (m, 2H, ArH). HRMS calcd for C18H20ClN3 (M + H)+, 314.1419; found, 314.1421.
(E)-N-((3-Chlorophenyl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride (I-4): Yield 66%, Mp: 235–237 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (t, 2H, J = 5.4 Hz, piperazinyl-H), 3.58–3.66 (m, 4H, piperazinyl-H), 4.14 (t, 2H, J = 5.4 Hz, piperazinyl-H), 4.39 (d, 1H, J = 15.9 Hz, -CH′-), 4.45 (d, 1H, J = 15.9 Hz, -CH′′-), 7.07–7.11 (m, 2H, ArH), 7.35–7.38 (m, 4H, ArH), 7.45 (s, 1H, ArH), 7.56–7.61 (m, 1H, ArH), 7.73 (d, 1H, J = 7.2 Hz, ArH). HRMS calcd for C18H20ClN3 (M + H)+, 314.1419; found, 314.1411.
(E)-N-((4-Chlorophenyl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride (I-5): Yield 65%, Mp: 182–184 °C. 1H- NMR (400 MHz, D2O): δ 3.35 (br, 2H, piperazinyl-H), 3.64 (br, 4H, piperazinyl-H), 4.14 (br, 2H, piperazinyl-H), 4.42 (s, 2H, -CH2-), 7.11 (br, 2H, ArH), 7.35–7.42 (m, 5H, ArH), 7.63 (d, 2H, J = 8.4 Hz, ArH). HRMS calcd for C18H20ClN3 (M + H)+, 314.1419; found, 314.1414.
(E)-N-((4-Fluorophenyl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride (I-6): Yield 33%, Mp: 220–222 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (br, 2H, piperazinyl-H), 3.58 (br, 2H, piperazinyl-H), 3.64 (br, 2H, piperazinyl-H), 4.12 (br, 2H, piperazinyl-H), 4.43 (s, 2H, -CH2-), 7.11 (br, 2H, ArH), 7.32–7.37 (m, 5H, ArH), 7.46–7.50 (m, 2H, ArH). HRMS calcd for C18H20FN3 (M + H)+, 298.1714; found, 298.1710.
(E)-N-((2-Fluorophenyl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride (I-7): Yield 22%, Mp: 182–184 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (br, 2H, piperazinyl-H), 3.59 (br, 2H, piperazinyl-H), 3.71 (br, 2H, piperazinyl-H), 4.17 (br, 2H, piperazinyl-H), 4.42 (d, 1H, J = 15.6 Hz, -CH′′-), 4.49 (d, 1H, J = 15.6 Hz, -CH′′-), 7.08–7.10 (m, 2H, ArH), 7.34–7.39 (m, 4H, ArH), 7.43–7.45 (m, 2H, ArH), 7.72–7.80 (m, 1H, ArH). HRMS calcd for C18H20FN3 (M + H)+, 298.1714; found, 298.1706.
(E)-4-((Benzylimino)(piperazin-1-yl)methyl)benzonitrilehydrochloride (I-8): Yield 38%, Mp: 205–207 °C. 1H-NMR (400 MHz, D2O): δ 3.36 (br, 2H, piperazinyl-H), 3.61 (br, 4H, piperazinyl-H), 4.17 (br, 2H, piperazinyl-H), 4.40 (s, 2H, -CH2-), 7.04–7.08 (m, 2H, ArH), 7.34–7.36 (m, 3H, ArH), 7.60 (d, 2H, J = 8.4 Hz, ArH), 7.98 (d, 2H, J = 8.4 Hz, ArH). HRMS calcd for C19H20N4 (M + H)+, 305.1761; found, 305.1760.
(E)-3-((Benzylimino)(piperazin-1-yl)methyl)benzonitrilehydrochloride (I-9): Yield 60%, Mp: 190–192 °C. 1H-NMR (400 MHz, D2O): δ 3.35 (br, 2H, piperazinyl-H), 3.61 (br, 4H, piperazinyl-H), 4.18 (br, 2H, piperazinyl-H), 4.36 (d, 1H, J = 15.6 Hz, -CH′-), 4.44 (d, 1H, J = 15.6 Hz, -CH′′-), 7.04–7.06 (m, 2H, ArH), 7.31–7.41 (m, 3H, ArH), 7.72–7.81 (m, 3H, ArH), 8.07 (d, 1H, J = 6.9 Hz, ArH). HRMS calcd for C19H20N4 (M + H)+, 305.1761; found, 305.1758.
(E)-1-Phenyl-N-(piperazin-1-yl(p-tolyl)methylene)methanaminehydrochloride (I-10): Yield 50%, Mp: 172–174 °C. 1H-NMR (400 MHz, D2O): δ 2.42 (s, 3H, -CH3), 3.34 (t, 2H, J = 5.4 Hz, J = 5.1 Hz, piperazinyl-H), 3.59 (t, 2H, J = 5.7 Hz, J = 5.1 Hz, piperazinyl-H), 3.64 (t, 2H, J = 5.7 Hz, piperazinyl-H), 4.12 (t, 2H, J = 6.0 Hz, piperazinyl-H), 4.42 (s, 2H, -CH2-), 7.09–7.12 (m, 2H, ArH), 7.30–7.37 (m, 5H, ArH), 7.45 (d, 2H, J = 8.1 Hz, ArH). HRMS calcd for C19H23N3 (M + H)+, 294.1965; found, 294.1962.
(E)-N-((2-Chloro-4-nitrophenyl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride (I-11): Yield 73%, Mp: 195–197 °C. 1H-NMR (400 MHz, D2O): δ 3.39 (br, 2H, piperazinyl-H), 3.66 (br, 4H, piperazinyl-H), 4.13 (br, 2H, piperazinyl-H), 4.35 (d, 1H, J = 15.6 Hz, -CH′-), 4.46 (d, 1H, J = 15.6 Hz, -CH′′-), 7.05 (d, 2H, J = 7.8 Hz, ArH), 7.31–7.38 (m, 3H, ArH), 7.71 (d, 1H, J = 8.7 Hz, ArH), 8.35 (d, 1H, J = 8.4 Hz, ArH), 8.57 (s, 1H, J = 8.7 Hz, ArH). HRMS calcd for C18H19ClN4O2 (M + H)+, 359.1269; found, 359.1277.
(E)-1-(4-Chlorophenyl)-N-((4-nitrophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-12): Yield 22%, Mp: 172–174 °C. 1H-NMR (400 MHz, D2O): δ 3.35 (br, 2H, piperazinyl-H), 3.65 (br, 4H, piperazinyl-H), 4.18 (br, 2H, piperazinyl-H), 4.39 (s, 2H, -CH2-), 7.01 (d, 2H, J = 8.4 Hz, ArH), 7.34 (d, 2H, J = 8.4 Hz, ArH), 7.67 (d, 2H, J = 8.7 Hz, ArH), 8.42 (d, 2H, J = 8.7 Hz, ArH). HRMS calcd for C18H19ClN4O2 (M + H)+, 359.1269; found, 359.1271.
(E)-1-(4-Chlorophenyl)-N-((2-chlorophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-13): Yield 56%, Mp: 181–183 °C. 1H-NMR (400 MHz, D2O): δ 3.36 (br, 2H, piperazinyl-H), 3.61 (br, 4H, piperazinyl-H), 4.14 (br, 2H, piperazinyl-H), 4.34 (d, 1H, J = 15.6 Hz, -CH′-), 4.42 (d, 1H, J = 15.6 Hz, -CH′′-), 7.01 (d, 2H, J = 8.4 Hz, ArH), 7.33 (d, 2H, J = 8.4 Hz, ArH), 7.42 (d, 1H, J = 7.8 Hz, ArH), 7.55 (t, 1H, J = 7.5 Hz, ArH), 7.64–7.72 (m, 2H, ArH). HRMS calcd for C18H19Cl2N3 (M + H)+, 348.1029; found, 348.1032.
(E)-1-(4-Chlorophenyl)-N-((3-chlorophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-14): Yield 53%, Mp: 218–220 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (br, 2H, piperazinyl-H), 3.63 (br, 4H, piperazinyl-H), 4.14 (br, 2H, piperazinyl-H), 4.35 (d, 1H, J = 15.9 Hz, -CH′-), 4.35 (d, 1H, J = 15.9 Hz, -CH′′-), 7.03 (d, 2H, J = 8.7 Hz, ArH), 7.33–7.37 (m, 3H, ArH), 7.40 (s, 1H, ArH), 7.55–7.61 (m, 1H, ArH), 7.72 (d, 1H, J = 7.2 Hz, ArH). HRMS calcd for C18H19Cl2N3 (M + H)+, 348.1029; found, 348.1027.
(E)-1-(4-Chlorophenyl)-N-((4-chlorophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-15): Yield 36%, Mp: 157–159 °C. 1H-NMR (400 MHz, D2O): δ 3.33 (br, 2H, piperazinyl-H), 3.62 (br,4H, piperazinyl-H), 4.13 (br, 2H, piperazinyl-H), 4.40 (s, 2H, -CH2-), 7.03 (d, 2H, J = 8.4 Hz, ArH), 7.35 (d, 2H, J = 8.1 Hz, ArH), 7.37 (d, 2H, J = 8.1 Hz, ArH), 7.63 (d, 2H, J = 8.4 Hz, ArH). HRMS calcd for C18H19Cl2N3 (M + H)+, 348.1029; found, 348.1027.
(E)-1-(4-Chlorophenyl)-N-((4-fluorophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-16): Yield 20%, Mp: 165–167 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (br, 2H, piperazinyl-H), 3.62 (br, 4H, piperazinyl-H), 4.13 (br, 2H, piperazinyl-H), 4.41 (s, 2H, -CH2-), 7.05 (d, 2H, J = 8.1 Hz, ArH), 7.31–7.37 (m, 4H, ArH), 7.42–7.47 (m, 2H, ArH). HRMS calcd for C18H19ClFN3 (M + H)+, 332.1324; found, 332.1323.
(E)-1-(4-Chlorophenyl)-N-((2-fluorophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-17): Yield 21%, Mp: 215–217 °C. 1H-NMR (400 MHz, D2O): δ 3.26 (br, 2H, piperazinyl-H), 3.52 (br, 2H, piperazinyl-H), 3.70 (br, 2H, piperazinyl-H), 4.18 (br, 2H, piperazinyl-H), 4.41 (d, 1H, J = 15.6 Hz, -CH′-), 4.47 (d, 1H, J = 15.6 Hz, -CH′′-), 7.02 (d, 2H, J = 8.4 Hz, ArH), 7.32–7.38 (m, 3H, ArH), 7.41–7.43 (m, 2H, ArH), 7.72–7.79 (m, 1H, ArH). HRMS calcd for C18H19ClFN3 (M + H)+, 332.1324; found, 332.1321.
(E)-1-(4-Chlorophenyl)-N-((4-cyanophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-18): Yield 39%, Mp: 180–182 °C. 1H-NMR (400 MHz, D2O): δ 3.35 (br, 2H, piperazinyl-H), 3.62 (br, 4H, piperazinyl-H), 4.16 (br, 2H, piperazinyl-H), 4.37 (s, 2H, -CH2-), 7.01 (d, 2H, J = 8.4 Hz, ArH), 7.34 (d, 2H, J = 8.4 Hz, ArH), 7.58 (d, 2H, J = 8.7 Hz, ArH), 7.97 (d, 2H, J = 8.7 Hz, ArH). HRMS calcd for C19H19ClN4 (M + H)+, 339.1371; found, 339.1368.
(E)-1-(4-Chlorophenyl)-N-((3-cyanophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-19): Yield 26%, Mp: 156–158 °C. 1H-NMR (400 MHz, D2O): δ 3.21 (br, 2H, piperazinyl-H), 3.49 (br,4H, piperazinyl-H), 4.03 (br, 2H, piperazinyl-H), 4.21 (d, 1H, J = 15.9 Hz, -CH′-), 4.29 (d, 1H, J = 15.9 Hz, -CH′′-), 6.85 (d, 2H, J = 8.7 Hz, ArH), 7.21 (d, 2H, J = 8.7 Hz, ArH), 7.56–7.67 (m, 3H, ArH), 7.93 (d, 1H, J = 7.8 Hz, ArH). HRMS calcd for C19H19ClN4 (M + H)+, 339.1371; found, 339.1366.
(E)-1-(4-Chlorophenyl)-N-(piperazin-1-yl(p-tolyl)methylene)methanaminehydrochloride (I-20): Yield 57%, Mp: 183–185 °C. 1H-NMR (400 MHz, D2O): δ 2.41 (s, 3H, -CH3), 3.32 (br, 2H, piperazinyl-H), 3.58 (br, 2H, piperazinyl-H), 3.63 (br, 2H, piperazinyl-H), 4.11 (br, 2H, piperazinyl-H), 4.40 (s, 2H, -CH2-), 7.04 (d, 2H, J = 8.1 Hz, ArH), 7.27 (d, 2H, J = 8.4 Hz, ArH), 7.35 (d, 2H, J = 8.4 Hz, ArH), 7.43 (d, 2H, J = 8.1 Hz, ArH). HRMS calcd for C19H22ClN3 (M + H)+, 328.1575; found, 328.1574.
(E)-1-(4-Methoxyphenyl)-N-((4-nitrophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (I-21): Yield 15%, Mp: 210–212 °C. 1H-NMR (400 MHz, D2O): δ 3.33 (br, 2H, piperazinyl-H), 3.59 (br, 4H, piperazinyl-H), 3.81 (s, 3H, -OCH3), 4.15 (br, 2H, piperazinyl-H), 4.35 (s, 2H, -CH2-), 6.90 (d, 2H, J = 8.7 Hz, ArH), 6.97 (d, 2H, J = 8.7 Hz, ArH), 7.66 (d, 2H, J = 9.3 Hz, ArH), 8.42 (d, 2H, J = 9.3 Hz, ArH). HRMS calcd for C19H22N4O3 (M + H)+, 355.1765; found, 355.1766.
(E)-1-(Naphthalen-1-yl)-N-(phenyl(piperazin-1-yl)methylene)methanaminehydrochloride (II-1): Yield 34%, Mp: 187–189 °C. 1H-NMR (400 MHz, D2O): δ 3.33 (t, 2H, J = 5.4 Hz, piperazinyl-H), 3.54–3.68 (m, 4H, piperazinyl-H), 4.12 (t, 2H, J = 5.4 Hz, piperazinyl-H), 4.82 (s, 2H, -CH2-), 7.22 (d, 1H, J = 7.2 Hz, ArH), 7.40–7.44 (m, 3H, ArH), 7.51–7.57 (m, 4H, ArH), 7.60–7.68 (m, 2H, ArH), 7.87 (d, 1H, J = 8.1 Hz, ArH), 7.87 (d, 1H, J = 9.0 Hz, ArH). HRMS calcd for C22H23N3 (M + H)+, 330.1965; found, 330.1962.
(E)-1-(Naphthalen-1-yl)-N-((4-nitrophenyl)(piperazin-1-yl)methylene)methanaminehydrochloride (II-2): Yield 11%, Mp: 202–204 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (br, 2H, piperazinyl-H), 3.60 (br, 4H, piperazinyl-H), 4.21 (br, 2H, piperazinyl-H), 4.84 (s, 2H, -CH2-), 7.20 (d, 1H, J = 6.9 Hz, ArH), 7.38–7.43 (m, 1H, ArH), 7.46–7.53 (m, 2H, ArH), 7.56–7.61 (m, 3H, ArH), 7.84–7.89 (m, 2H, ArH), 8.17 (d, 2H, J = 9.0 Hz, ArH). HRMS calcd for C22H22N4O2 (M + H)+, 375.1816; found, 375.1814.
(E)-N-((4-Fluorophenyl)(piperazin-1-yl)methylene)-1-(naphthalen-1-yl)methanaminehydrochloride (II-3): Yield 24%, Mp: 193–195 °C. 1H-NMR (400 MHz, D2O): δ 3.36 (br, 2H, piperazinyl-H), 3.58 (br,2H, piperazinyl-H), 3.65 (t, 2H, J = 4.2 Hz, J = 5.7 Hz, piperazinyl-H), 4.12 (t, 2H, J = 5.1 Hz, J = 5.4 Hz, piperazinyl-H), 4.87 (s, 2H, -CH2-), 7.22–7.28 (m, 3H, ArH), 7.41–7.48 (m, 3H, ArH), 7.52–7.60 (m, 2H, ArH), 7.65–7.68 (m, 1H, ArH), 7.91 (d, 1H, J = 8.4 Hz, ArH), 7.95–7.98 (m, 1H, ArH). HRMS calcd for C22H22FN3 (M + H)+, 348.1871; found, 348.1873.
(E)-N-((2-Fluorophenyl)(piperazin-1-yl)methylene)-1-(naphthalen-1-yl)methanaminehydrochloride (II-4): Yield 41%, Mp: 148–150 °C. 1H-NMR (400 MHz, D2O): δ 3.33 (br, 2H, piperazinyl-H), 3.56 (br,2H, piperazinyl-H), 3.67 (br, 2H, piperazinyl-H), 4.12–4.21 (m, 2H, piperazinyl-H), 4.92 (s, 2H, -CH2-), 7.12 (br, 1H, ArH), 7.23–7.29 (m, 1H, ArH), 7.33–7.46 (m, 3H, ArH), 7.56 (br, 2H, ArH), 7.64–7.72 (m, 2H, ArH), 7.88–8.05 (m, 2H, ArH). HRMS calcd for C22H22FN3 (M + H)+, 348.1871; found, 348.1868.
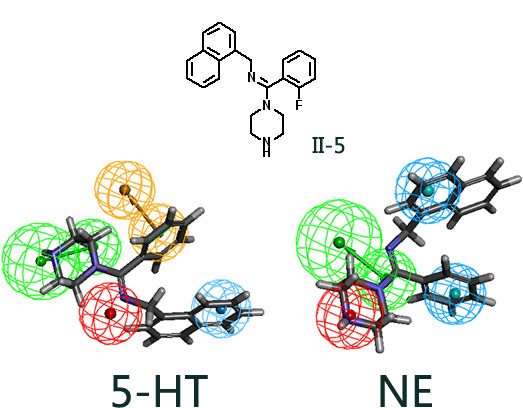
(E)-N-((2-Chlorophenyl)(piperazin-1-yl)methylene)-1-(naphthalen-1-yl)methanaminehydrochloride (II-5): Yield 58%, Mp: 165–167 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (br, 2H, piperazinyl-H), 3.63 (br, 4H, piperazinyl-H), 4.15 (br, 2H, piperazinyl-H), 4.89 (s, 2H, -CH2-), 7.07 (d, 1H, J = 6.6 Hz, ArH), 7.37–7.49 (m, 3H, ArH), 7.55–7.65 (m, 4H, ArH), 7.78–7.81 (m, 1H, ArH), 7.91 (d, 1H, J = 8.7 Hz, ArH), 7.98–8.04 (m, 1H, ArH). HRMS calcd for C22H22ClN3 (M + H)+, 364.1575; found, 364.1574.
(E)-N-((3-Chlorophenyl)(piperazin-1-yl)methylene)-1-(naphthalen-1-yl)methanaminehydrochloride (II-6): Yield 34%, Mp: 198–200 °C. 1H-NMR (400 MHz, D2O): δ 3.31 (br, 2H, piperazinyl-H), 3.65 (br,4H, piperazinyl-H), 4.12 (br, 2H, piperazinyl-H), 4.78 (s, 2H, -CH2-),7.16 (d, 1H, J = 6.9 Hz, ArH), 7.29–7.32 (m, 2H, ArH), 7.39–7.62 (m, 6H, ArH), 7.87 (d, 1H, J = 8.4 Hz, ArH), 7.93 (d, 1H, J = 6.9 Hz, ArH). HRMS calcd for C22H22ClN3 (M + H)+, 364.1575; found, 364.1576.
(E)-N-((4-Chlorophenyl)(piperazin-1-yl)methylene)-1-(naphthalen-1-yl)methanaminehydrochloride (II-7): Yield 41%, Mp: 210–212 °C. 1H-NMR (400 MHz, D2O): δ 3.19 (t, 2H, J = 5.1 Hz, J = 5.1 Hz, piperazinyl-H), 3.43 (t, 2H, J = 6.9 Hz, J = 3.9 Hz, piperazinyl-H), 3.50 (t, 2H, J = 5.7 Hz, piperazinyl-H), 4.12 (t, 2H, J = 6.0 Hz, piperazinyl-H), 4.75 (s, 2H, -CH2-), 7.09 (d, 1H, J = 6.6 Hz, ArH), 7.20 (d, 2H, J = 8.4 Hz, ArH), 7.30–7.35 (m, 1H, ArH), 7.38 (d, 2H, J = 8.4 Hz, ArH), 7.41–7.54 (m, 3H, ArH), 7.76–7.85 (m, 2H, ArH). HRMS calcd for C22H22ClN3 (M + H)+, 364.1575; found, 364.1577.
(E)-4-(((Naphthalen-1-ylmethyl)imino)(piperazin-1-yl)methyl)benzonitrilehydrochloride (II-8): Yield 30%, Mp: 158–160 °C. 1H-NMR (400 MHz, D2O): δ 3.34 (br, 2H, piperazinyl-H), 3.63 (br,4H, piperazinyl-H), 4.18 (br, 2H, piperazinyl-H), 4.84 (s, 2H, -CH2-), 7.19 (d, 1H, J = 7.2 Hz, ArH), 7.40–7.63 (m, 6H, ArH), 7.77 (d, 2H, J = 8.4 Hz, ArH), 7.88 (d, 1H, J = 8.1 Hz, ArH), 7.94 (d, 1H, J = 9.0 Hz, ArH). HRMS calcd for C23H22N4 (M + H)+, 355.1917; found, 355.1921.
(E)-3-(((Naphthalen-1-ylmethyl)imino)(piperazin-1-yl)methyl)benzonitrilehydrochloride (II-9): Yield 11%, Mp: 168–170 °C. 1H-NMR (400 MHz, D2O): δ 3.16 (br, 2H, piperazinyl-H), 3.45 (br,4H, piperazinyl-H), 4.04 (br, 2H, piperazinyl-H), 4.72 (d, 1H, J = 13.5 Hz, -CH′-), 4.78 (d, 1H, J = 13.5 Hz, -CH′′-), 6.85 (d, 1H, J = 5.7 Hz, ArH), 7.01–7.05 (m, 2H, ArH), 7.24–7.29 (m, 2H, ArH), 7.35–7.52 (m, 3H, ArH), 7.73–7.90 (m, 3H, ArH). HRMS calcd for C23H22N4 (M + H)+, 355.1917; found, 355.1911.
(E)-1-(Naphthalen-1-yl)-N-(piperazin-1-yl(p-tolyl)methylene)methanaminehydrochloride (II-10): Yield 49%, Mp: 178–180 °C. 1H-NMR (400 MHz, D2O): δ 2.38 (s, 3H, -CH3), 3.19 (t, 2H, J = 5.4 Hz, piperazinyl-H), 3.42 (t, 2H, J = 5.7 Hz, piperazinyl-H), 3.51 (t, 2H, J = 5.4 Hz, piperazinyl-H), 3.96 (t, 2H, J = 5.4 Hz, piperazinyl-H), 4.74 (s, 2H, -CH2-), 7.10–7.15 (m, 3H, ArH), 7.21 (d, 2H, J = 8.1 Hz, ArH), 7.30–7.35 (m, 1H, ArH), 7.37–7.44 (m, 2H, ArH), 7.46–7.52 (m, 1H, ArH), 7.77 (d, 1H, J = 8.1 Hz, ArH), 7.83 (d, 1H, J = 9.3 Hz, ArH). HRMS calcd for C23H25N3 (M + H)+, 344.2121; found, 344.2108.
(E)-N-((5-Nitronaphthalen-1-yl)(piperazin-1-yl)methylene)-1-phenylmethanaminehydrochloride(III-1): Yield 14%, Mp: 173–175 °C. 1H-NMR (400 MHz, D2O): δ 3.14–3.26 (m, 2H, piperazinyl-H), 3.51–-3.57 (m, 2H, piperazinyl-H), 3.71 (br, 2H, piperazinyl-H), 4.34 (br, 2H, piperazinyl-H), 4.23 (d, 1H, J = 15.0 Hz, -CH′-), 4.36 (d, 1H, J = 15.0 Hz, -CH′′-), 6.73 (d, 2H, J = 6.6 Hz, ArH), 7.06–7.16 (m, 3H, ArH), 7.59 (t, 1H, J = 7.8 Hz, ArH), 7.80–7.86 (m, 2H, ArH), 7.94 (t, 1H, J=7.2Hz, ArH), 7.29 (d, 1H, J = 7.8 Hz, ArH), 8.74 (d, 1H, J = 8.7 Hz, ArH). HRMS calcd for C22H22N4O2 (M + H)+, 375.1816; found, 375.1815.