Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Supercritical Fluid Extraction and Experimental Design
2.4. Chemical Characterization of the Extracts
2.5. Determination of Total Phenolics Content
2.6. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.7. Antibacterial Susceptibility Testing
2.7.1. Microorganisms and Growth Conditions
2.7.2. Minimum Inhibitory Concentration (MIC), Growth Inhibition and 50% Growth Reduction (IC50)
2.8. Statistical Data Processing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamatou, G.P.P.; Viljoen, A.M.; Gono-Bwalya, A.B.; van Zyl, R.L.; van Vuuren, S.F.; Lourens, A.C.U.; Başer, K.H.C.; Demirci, B.; Lindsey, K.L.; van Staden, J.; et al. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J. Ethnopharmacol. 2005, 102, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz, O.; Koeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Vuković-Gacić, B.; Nikcević, S.; Berić-Bjedov, T.; Knezević-Vukcević, J.; Simić, D. Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem. Toxicol. 2006, 44, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Pedro, D.F.N.; Ramos, A.A.; Lima, C.F.; Baltazar, F.; Pereira-Wilson, C. Colon Cancer Chemoprevention by Sage Tea Drinking: Decreased DNA Damage and Cell Proliferation. Phytother. Res. 2016, 30, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Prior, E.; Aeschbach, R. Evaluation of Antioxidant Activity of Rosemary Extracts, Carnosol and Carnosic Acid in Bulk Vegetable Oils and Fish Oil and Their Emulsions. J. Sci. Food Agric. 1996, 72, 201–208. [Google Scholar] [CrossRef]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2017, 34, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Schwarz, K.; Alegre, L. Response of abietane diterpenes to stress in Rosmarinus officinalis L.: New insights into the function of diterpenes in plants. Free Radic. Res. 1999, 31, S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Gajhede, M.; Anthoni, U.; Per Nielsen, H.; Pedersen, E.J.; Christophersen, C. Carnosol. Crystal structure, absolute configuration, and spectroscopic properties of a diterpene. J. Crystallogr. Spectrosc. Res. 1990, 20, 165–171. [Google Scholar] [CrossRef]
- Wenkert, E.; Fuchs, A.; McChesney, J.D. Chemical Artifacts from the Family Labiatae. J. Org. Chem. 1965, 30, 2931–2934. [Google Scholar] [CrossRef]
- Schwarz, K.; Ternes, W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis. II. Isolation of carnosic acid and formation of other phenolic diterpenes. Z. Lebensm. Unters. Forsch. 1992, 195, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.E.; Müller, M.; Alegre, L.; Munné-Bosch, S. Phenolic diterpene and α-tocopherol contents in leaf extracts of 60 Salvia species. J. Sci. Food Agric. 2008, 88, 2648–2653. [Google Scholar] [CrossRef]
- Tounekti, T.; Munné-Bosch, S. Enhanced Phenolic Diterpenes Antioxidant Levels Through Non-transgenic Approaches. Crit. Rev. Plant Sci. 2012, 31, 505–519. [Google Scholar] [CrossRef]
- Tounekti, T.; Munné-Bosch, S.; Vadel, A.M.; Chtara, C.; Khemira, H. Influence of ionic interactions on essential oil and phenolic diterpene composition of Dalmatian sage (Salvia officinalis L.). Plant Physiol. Biochem. 2010, 48, 813–821. [Google Scholar] [CrossRef]
- Tounekti, T.; Vadel, A.M.; Ennajeh, M.; Khemira, H.; Munné-Bosch, S. Ionic interactions and salinity affect monoterpene and phenolic diterpene composition in rosemary (Rosmarinus officinalis). J. Plant Nutr. Soil Sci. 2011, 174, 504–514. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Yang, L.; Zu, Y.; Wang, H.; Qu, S.; Zhang, Y. Oxidative stability of fish oil supplemented with carnosic acid compared with synthetic antioxidants during long-term storage. Food Chem. 2011, 128, 93–99. [Google Scholar] [CrossRef]
- Guitard, R.; Paul, J.-F.; Nardello-Rataj, V.; Aubry, J.-M. Myricetin, rosmarinic and carnosic acids as superior natural antioxidant alternatives to α-tocopherol for the preservation of omega-3 oils. Food Chem. 2016, 213, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.; Amiot, M.J.; Nguyen-The, C. Antimicrobial effect of rosemary extracts. J. Food Prot. 2000, 63, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Potentiation of antimicrobial activity of aminoglycosides by carnosol from Salvia officinalis. Biol. Pharm. Bull. 2007, 30, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Aeschbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P.H. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Dent, M.; Dragović, V.; Penić, M. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) Extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar] [CrossRef]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simões, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef]
- Ollanketo, M.; Peltoketo, A.; Hartonen, K.; Hiltunen, R.; Riekkola, M.-L. Extraction of sage (Salvia officinalis L.) by pressurized hot water and conventional methods: Antioxidant activity of the extracts. Eur. Food Res. Technol. 2002, 215, 158–163. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Sališová, M.; Toma, Š.; Mason, T.J. Comparison of conventional and ultrasonically assisted extractions of pharmaceutically active compounds from Salvia officinalis. Ultrason. Sonochem. 1997, 4, 131–134. [Google Scholar] [CrossRef]
- Velicković, D.T.; Milenović, D.M.; Ristić, M.S.; Veljković, V.B. Kinetics of ultrasonic extraction of extractive substances from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage. Ultrason. Sonochem. 2006, 13, 150–156. [Google Scholar] [CrossRef]
- Dauksas, E.; Venskutonis, P.R.; Povilaityte, V.; Sivik, B. Rapid screening of antioxidant activity of sage (Salvia officinalis L.) extracts obtained by supercritical carbon dioxide at different extraction conditions. Nahrung 2001, 45, 338–341. [Google Scholar] [CrossRef]
- Fornari, T.; Ruiz-Rodriguez, A.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Kinetic study of the supercritical CO2 extraction of different plants from Lamiaceae family. J. Supercrit. Fluids 2012, 64, 1–8. [Google Scholar] [CrossRef]
- Menaker, A.; Kravets, M.; Koel, M.; Orav, A. Identification and characterization of supercritical fluid extracts from herbs. C. R. Chim. 2004, 7, 629–633. [Google Scholar] [CrossRef]
- Reverchon, E.; Taddeo, R.; Porta, G.D. Extraction of sage oil by supercritical C02: Influence of some process parameters. J. Supercrit. Fluids 1995, 8, 302–309. [Google Scholar] [CrossRef]
- Jokić, S.; Vidović, S.; Aladić, K. Supercritical Fluid Extraction of Edible Oils. In Supercritical Fluids: Fundamentals, Properties and Application; Osborn, J., Ed.; Nova Publishers: New York, NY, USA, 2014; pp. 205–228. ISBN 978-1-63321-946-5. [Google Scholar]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B (Methodol.) 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Jakovljević, M.; Aladić, K.; Jerković, I. Optimization of supercritical CO2 extraction of Salvia officinalis L. leaves targeted on Oxygenated monoterpenes, α-humulene, viridiflorol and manool. J. Supercrit. Fluids 2018, 133, 253–262. [Google Scholar] [CrossRef]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar] [CrossRef]
- Glisic, S.; Ivanovic, J.; Ristic, M.; Skala, D. Extraction of sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluids 2010, 52, 62–70. [Google Scholar] [CrossRef]
- Jokić, S.; Horvat, G.; Aladić, K. Design of SFE System Using a Holistic Approach: Problems and Challenges. In Supercritical Fluid Extraction: Technology, Applications and Limitations; Jason, L., Ed.; Nova Publishers: New York, NY, USA, 2015; pp. 95–122. ISBN 978-1-63463-353-6. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Shih, M.-H.; Su, Y.-S.; Wu, C.-L. Syntheses of Aromatic Substituted Hydrazino-thiazole Derivatives to Clarify Structural Characterization and Antioxidant Activity between 3-Arylsydnonyl and Aryl Substituted Hydrazino-thiazoles. Chem. Pharm. Bull. 2007, 55, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, S. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Medicinal Chem. 2010, 45, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.; Pavić, V.; Šarkanj, B.; Čačić, M.; Vuković, D.; Klenkar, J. Mono-and bis-dipicolinic acid heterocyclic derivatives–thiosemicarbazides, triazoles, oxadiazoles and thiazolidinones as antifungal and antioxidant agents. Heterocycl. Commun. 2017, 23, 35–42. [Google Scholar] [CrossRef]
- Wellwood, C.R.L.; Cole, R.A. Relevance of carnosic acid concentrations to the selection of rosemary, Rosmarinus officinalis (L.), accessions for optimization of antioxidant yield. J. Agric. Food Chem. 2004, 52, 6101–6107. [Google Scholar] [CrossRef]
- Masuda, T.; Inaba, Y.; Maekawa, T.; Takeda, Y.; Tamura, H.; Yamaguchi, H. Recovery mechanism of the antioxidant activity from carnosic acid quinone, an oxidized sage and rosemary antioxidant. J. Agric. Food Chem. 2002, 50, 5863–5869. [Google Scholar] [CrossRef]
- Matsingou, T.C.; Petrakis, N.; Kapsokefalou, M.; Salifoglou, A. Antioxidant Activity of Organic Extracts from Aqueous Infusions of Sage. J. Agric. Food Chem. 2003, 51, 6696–6701. [Google Scholar] [CrossRef]
- Okamura, N.; Fujimoto, Y.; Kuwabara, S.; Yagi, A. High-performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus officinalis and Salvia officinalis. J. Chromatogr. A 1994, 679, 381–386. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Tena, M.T.; Valcárcel, M.; Hidalgo, P.J.; Ubera, J.L. Supercritical Fluid Extraction of Natural Antioxidants from Rosemary: Comparison with Liquid Solvent Sonication. Anal. Chem. 1997, 69, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, M. Natural Extracts Using Supercritical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-1-4200-4169-9. [Google Scholar]
- Caldera, G.; Figueroa, Y.; Vargas, M.; Santos, D.; Marquina-Chidsey, G. Optimization of Supercritical Fluid Extraction of Antioxidant Compounds from Venezuelan Rosemary Leaves. Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Chafer, A.; Fornari, T.; Berna, A.; Ibáñez, E.; Reglero, G. Solubility of solid carnosic acid in supercritical CO2 with ethanol as a co-solvent. J. Supercrit. Fluids 2005, 34, 323–329. [Google Scholar] [CrossRef]
- Cojocaru, C.; Khayet, M.; Zakrzewska-Trznadel, G.; Jaworska, A. Modeling and multi-response optimization of pervaporation of organic aqueous solutions using desirability function approach. J. Hazard. Mater. 2009, 167, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef]
- Klancnik, A.; Guzej, B.; Kolar, M.H.; Abramovic, H.; Mozina, S.S. In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J. Food Prot. 2009, 72, 1744–1752. [Google Scholar] [CrossRef]
- Bubonja-Sonje, M.; Giacometti, J.; Abram, M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011, 127, 1821–1827. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef] [PubMed]
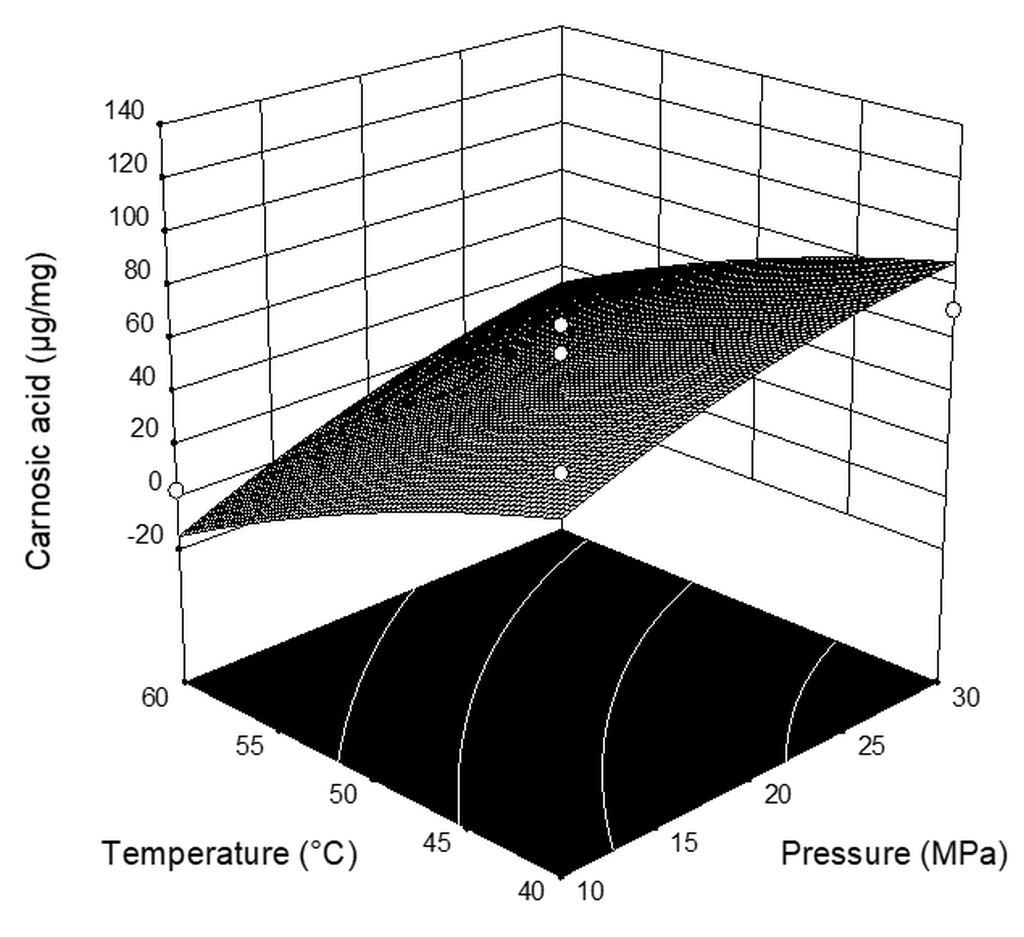
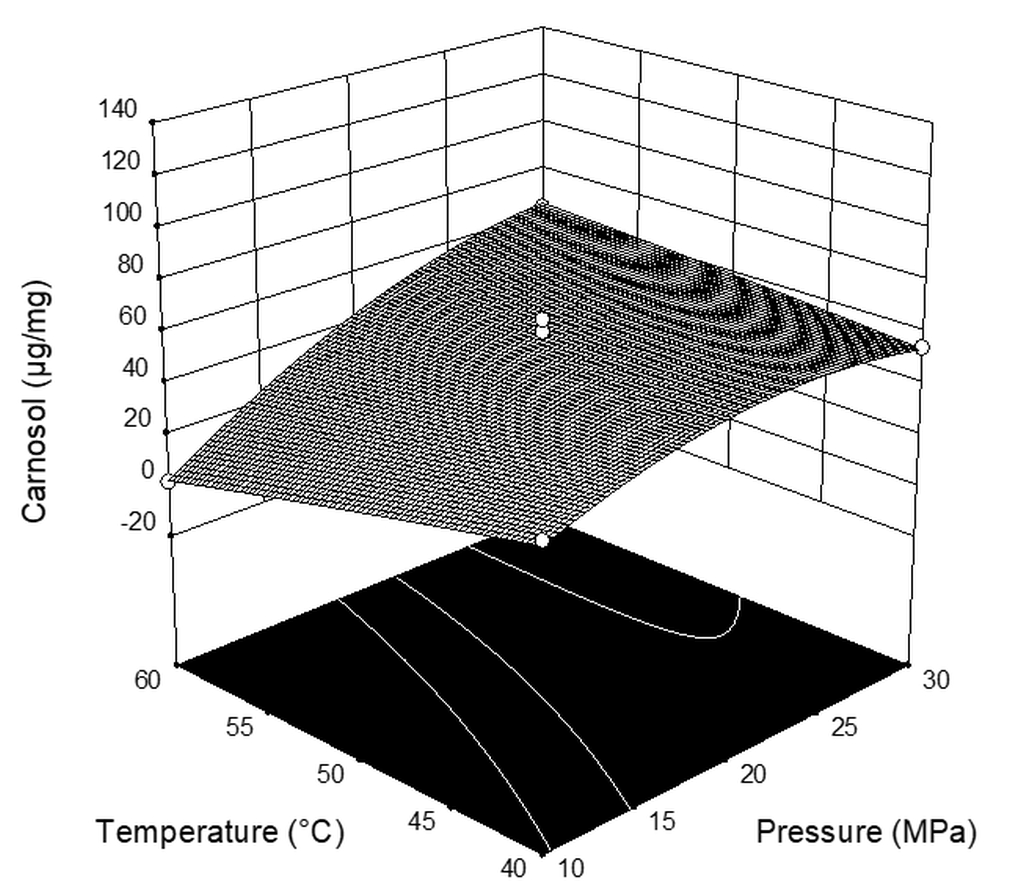
| Independent Variable | Symbol | Level | ||
|---|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | ||
| Pressure (MPa) | X1 | 10 | 20 | 30 |
| Temperature (°C) | X2 | 40 | 50 | 60 |
| CO2 flow rate (kg h−1) | X3 | 1 | 2 | 3 |
| Run | Pressure (MPa) | Temperature (°C) | CO2 Flow Rate (kg h−1) | Extraction Yield * (%) | Carnosic Acid (µg mg−1extract) | Carnosol (µg mg−1extract) |
|---|---|---|---|---|---|---|
| 1 | 10 | 40 | 2 | 0.659 | 67.94 | 40.71 |
| 2 | 20 | 40 | 1 | 3.385 | 48.04 | 61.70 |
| 3 | 20 | 40 | 3 | 4.026 | 120.05 | 51.08 |
| 4 | 10 | 50 | 3 | 1.144 | 25.49 | 39.77 |
| 5 | 20 | 50 | 2 | 3.768 | 47.56 | 65.51 |
| 6 | 30 | 50 | 3 | 7.361 | 116.25 | 60.63 |
| 7 | 30 | 50 | 1 | 5.238 | 61.78 | 61.47 |
| 8 | 20 | 60 | 3 | 5.477 | 38.53 | 58.00 |
| 9 | 10 | 50 | 1 | 0.242 | 0.29 | 0.46 |
| 10 | 10 | 60 | 2 | 0.365 | 2.93 | 1.80 |
| 11 | 20 | 50 | 2 | 3.305 | 39.78 | 37.28 |
| 12 | 30 | 60 | 2 | 4.316 | 18.93 | 64.19 |
| 13 | 20 | 50 | 2 | 2.552 | 55.61 | 46.72 |
| 14 | 30 | 40 | 2 | 3.803 | 71.94 | 54.75 |
| 15 | 20 | 60 | 1 | 4.891 | 1.64 | 35.19 |
| 16 | 20 | 50 | 2 | 4.528 | 66.20 | 61.02 |
| Term | Coefficients | Standard Error | F-Value | p-Value * |
|---|---|---|---|---|
| Carnosic acid | ||||
| Intercept | 52.99 | 10.58 | ||
| X1 | 21.53 | 7.48 | 8.28 | 0.0282 |
| X2 | −30.74 | 7.48 | 16.87 | 0.0063 |
| X3 | 23.57 | 7.48 | 9.92 | 0.0198 |
| X12 | −6.48 | 10.58 | 0.38 | 0.5628 |
| X22 | −5.37 | 10.58 | 0.26 | 0.6298 |
| X32 | −5.15 | 10.58 | 0.24 | 0.6439 |
| X1X2 | 3.00 | 10.58 | 0.080 | 0.7865 |
| X1X3 | 7.32 | 10.58 | 0.48 | 0.5153 |
| X2X3 | −8.78 | 10.58 | 0.69 | 0.4385 |
| R2 = 0.8611 | ||||
| Carnosol | ||||
| Intercept | 52.63 | 5.04 | ||
| X1 | 19.79 | 3.56 | 30.85 | 0.0014 |
| X2 | −6.13 | 3.56 | 2.96 | 0.1360 |
| X3 | 6.33 | 3.56 | 3.16 | 0.1258 |
| X12 | −11.59 | 5.04 | 5.29 | 0.0534 |
| X22 | −0.68 | 5.04 | 0.018 | 0.0934 |
| X32 | −0.46 | 5.04 | 0.03 | 0.1481 |
| X1X2 | 12.09 | 5.04 | 5.75 | 0.0611 |
| X1X3 | −10.04 | 5.04 | 3.97 | 0.8973 |
| X2X3 | −0.46 | 5.04 | 2.75 | 0.9300 |
| R2 = 0.9013 | ||||
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value * |
|---|---|---|---|---|---|
| Carnosic acid | |||||
| The recovery | |||||
| Model | 16663.23 | 9 | 1851.47 | 4.13 | 0.0491 |
| Residual | 2688.70 | 6 | 448.12 | ||
| Lack of fit | 2305.19 | 3 | 768.40 | 6.01 | 0.0874 |
| Pure error | 383.51 | 3 | 127.84 | ||
| Total | 19351.94 | 15 | |||
| Carnosol | |||||
| The recovery | |||||
| Model | 5560.32 | 9 | 617.81 | 6.09 | 0.0198 |
| Residual | 609.14 | 6 | 101.52 | ||
| Lack of fit | 102.25 | 3 | 34.08 | 0.20 | 0.8893 |
| Pure error | 506.89 | 3 | 168.96 | ||
| Total | 6169.46 | 15 |
| Run | Total Phenolics Content (TPC) (mgGAE gDM−1) | DPPH Radical Scavenging Activity (%) |
|---|---|---|
| 1 | 2.79 ± 0.06 | 57.71 ± 0.33 |
| 2 | 3.08 ± 0.05 | 38.99 ± 0.32 |
| 3 | ND | ND |
| 4 | 2.26 ± 0.01 | 46.87 ± 3.60 |
| 5 | 3.97 ± 0.13 | 53.53 ± 3.37 |
| 6 | 4.32 ± 0.03 | 47.05 ± 1.77 |
| 7 | ND | ND |
| 8 | ND | ND |
| 9 | ND | ND |
| 10 | ND | ND |
| 11 | 2.72 ± 0.07 | 56.72 ± 0.65 |
| 12 | 1.02 ± 0.02 | 26.91 ± 0.91 |
| 13 | 3.17 ± 0.01 | 48.01 ± 0.86 |
| 14 | 9.15 ± 0.09 | 79.98 ± 0.68 |
| 15 | 2.65 ± 0.07 | 42.62 ± 1.69 |
| 16 | 2.96 ± 0.01 | 50.54 ± 1.98 |
| Run | MIC (µg mL−1) | IC50 (µg mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | B. subtilis | S. aureus | E. coli | P. aeruginosa | B. subtilis | S. aureus | |
| 1 | 31.25 | 31.25 | 15.625 | 31.25 | 29.53 ± 0.16 | 28.54 ± 0.05 | 12.45 ± 0.08 | 17.17 ± 0.09 |
| 2 | 31.25 | 31.25 | 15.625 | 31.25 | 27.89 ± 0.07 | 26.28 ± 1.76 | 12.69 ± 0.07 | 23.90 ± 0.10 |
| 3 | ND | ND | ND | ND | ND | ND | ND | ND |
| 4 | 31.25 | 31.25 | 15.625 | 15.625 | 27.03 ± 0.55 | 25.75 ± 0.52 | 13.97 ± 0.07 | 14.53 ± 0.04 |
| 5 | 31.25 | 31.25 | 15.625 | 31.25 | 31.18 ± 0.05 | 16.33 ±0.26 | 12.77 ± 0.06 | 22.75 ± 0.13 |
| 6 | 31.25 | 31.25 | 15.625 | 15.625 | 31.08 ± 0.08 | 17.82 ± 0.39 | 10.82 ± 0.02 | 14.61 ± 0.08 |
| 7 | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | ND | ND | ND | ND | ND | ND | ND | ND |
| 9 | ND | ND | ND | ND | ND | ND | ND | ND |
| 10 | ND | ND | ND | ND | ND | ND | ND | ND |
| 11 | 31.25 | 31.25 | 15.625 | 15.625 | 28.43 ± 0.36 | 22.23 ± 0.61 | 12.70 ± 0.08 | 14.10 ± 0.10 |
| 12 | 62.50 | 31.25 | 15.625 | 15.625 | 39.68 ± 0.21 | 22.36 ± 0.79 | 14.02 ± 0.16 | 14.12 ± 0.18 |
| 13 | 62.50 | 31.25 | 15.625 | 15.625 | 37.80 ± 0.83 | 23.78 ± 0.44 | 13.18 ± 0.08 | 12.46 ± 0.10 |
| 14 | 31.25 | 31.25 | 15.625 | 15.625 | 24.85 ± 0.08 | 23.57 ± 1.21 | 12.51 ± 0.11 | 15.04 ± 0.13 |
| 15 | 31.25 | 31.25 | 15.625 | 15.625 | 20.29 ± 0.62 | 27.98 ± 1.24 | 13.01 ± 0.16 | 13.75 ± 0.04 |
| 16 | 62.50 | 31.25 | 15.625 | 15.625 | 40.44 ± 0.19 | 23.61 ± 0.53 | 13.43 ± 0.04 | 17.91 ± 0.67 |
| G | 0.976 | 0.976 | 1.953 | 3.906 | 0.58 ± 0.10 | 0.91 ± 0.07 | 1.83 ± 0.01 | 3.06 ± 0.06 |
| Run | Growth Inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | B. subtilis | S. aureus | |||||
| 62.5 µg mL−1 | 15.625 µg mL−1 | 62.5 µg mL−1 | 15.625 µg mL−1 | 62.5 µg mL−1 | 15.625 µg mL−1 | 62.5 µg mL−1 | 15.625 µg mL−1 | |
| 1 | 98.34 ± 0.35 | 16.73 ± 0.51 | 96.62 ± 1.84 | 31.93 ± 3.22 | 98.84 ± 0.29 | 72.19 ± 0.22 | 98.32 ± 0.06 | 46.33 ± 0.15 |
| 2 | 93.04 ± 0.19 | 20.10 ± 0.59 | 91.42 ± 1.56 | 35.39 ± 0.57 | 98.26 ± 0.44 | 71.80 ± 0.83 | 97.56 ± 0.22 | 21.97 ± 1.17 |
| 3 | ND | ND | ND | ND | ND | ND | ND | ND |
| 4 | 97.17 ± 0.28 | 14.45 ± 1.18 | 97.36 ± 0.30 | 38.30 ± 0.79 | 99.48 ± 010 | 61.62 ± 0.43 | 98.31 ± 0.12 | 57.57 ± 0.36 |
| 5 | 95.82 ± 0.28 | 23.91 ± 0.34 | 93.30 ± 0.65 | 49.41 ± 0.22 | 97.39 ± 0.42 | 68.05 ± 0.74 | 96.57 ± 0.87 | 32.24 ± 0.16 |
| 6 | 99.05 ± 1.19 | 39.22 ± 0.06 | 95.91 ± 0.96 | 49.63 ± 0.08 | 98.33 ± 0.38 | 76.79 ± 0.88 | 97.80 ± 0.57 | 55.47 ± 0.77 |
| 7 | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | ND | ND | ND | ND | ND | ND | ND | ND |
| 9 | ND | ND | ND | ND | ND | ND | ND | ND |
| 10 | ND | ND | ND | ND | ND | ND | ND | ND |
| 11 | 95.75 ± 1.62 | 28.04 ± 1.18 | 95.07 ± 1.29 | 44.10 ± 1.02 | 98.88 ± 0.22 | 72.31 ± 0.39 | 97.74 ± 0.19 | 56.69 ± 0.59 |
| 12 | 94.96 ± 0.35 | 20.21 ± 3.00 | 97.78 ± 1.37 | 31.93 ± 3.22 | 99.00 ± 0.22 | 60.99 ± 1.31 | 97.98 ± 0.09 | 60.77 ± 1.56 |
| 13 | 97.41 ± 0.82 | 26.80 ± 3.62 | 98.07 ± 0.41 | 41.50 ± 3.88 | 98.72 ± 0.22 | 67.30 ± 0.10 | 97.37 ± 0.66 | 74.93 ± 0.30 |
| 14 | 97.03 ± 0.24 | 37.76 ± 0.25 | 99.15 ± 0.15 | 39.71 ± 2.95 | 99.28 ± 0.18 | 71.42 ± 0.64 | 97.55 ± 0.18 | 52.30 ± 0.60 |
| 15 | 97.47 ± 1.10 | 40.72 ± 1.30 | 97.45 ± 0.70 | 42.32 ± 2.04 | 99.41 ± 0.45 | 69.85 ± 0.65 | 98.24 ± 0.29 | 61.11 ± 0.29 |
| 16 | 96.77 ± 0.41 | 28.47 ± 3.18 | 97.26 ± 2.11 | 40.38 ± 1.19 | 98.81 ± 0.11 | 65.04 ± 0.58 | 97.00 ± 0.86 | 46.29 ± 1.28 |
| G | 98.18 ± 0.76 | 95.00 ± 0.17 | 99.83 ± 0.02 | 96.83 ± 0.02 | 99.86 ± 0.14 | 94.83 ± 0.71 | 98.58 ± 0.56 | 97.00 ± 0.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. https://doi.org/10.3390/plants8010016
Pavić V, Jakovljević M, Molnar M, Jokić S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants. 2019; 8(1):16. https://doi.org/10.3390/plants8010016
Chicago/Turabian StylePavić, Valentina, Martina Jakovljević, Maja Molnar, and Stela Jokić. 2019. "Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity" Plants 8, no. 1: 16. https://doi.org/10.3390/plants8010016
APA StylePavić, V., Jakovljević, M., Molnar, M., & Jokić, S. (2019). Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants, 8(1), 16. https://doi.org/10.3390/plants8010016








