3.1. Results
Here, the biophysical processes of the water uptake, transpiration, plastic and elastic wall deformations are analyzed for fungal, algal, and plant cells using dimensionless Π parameters. The Π parameters are obtained from dimensionless biophysical equations describing the net water uptake rate and the total cell wall deformation rate; Equations (4) and (5) respectively. The use of dimensionless Π parameters provides an assessment of the magnitude of each biophysical process during the expansive growth for each cell type considered. In
Table 2, reading the values down each column, it can be seen that the magnitude of Π
wv is the largest dimensionless coefficient in Equations (4) and (5) for each species of walled cell (the first four values in each column).
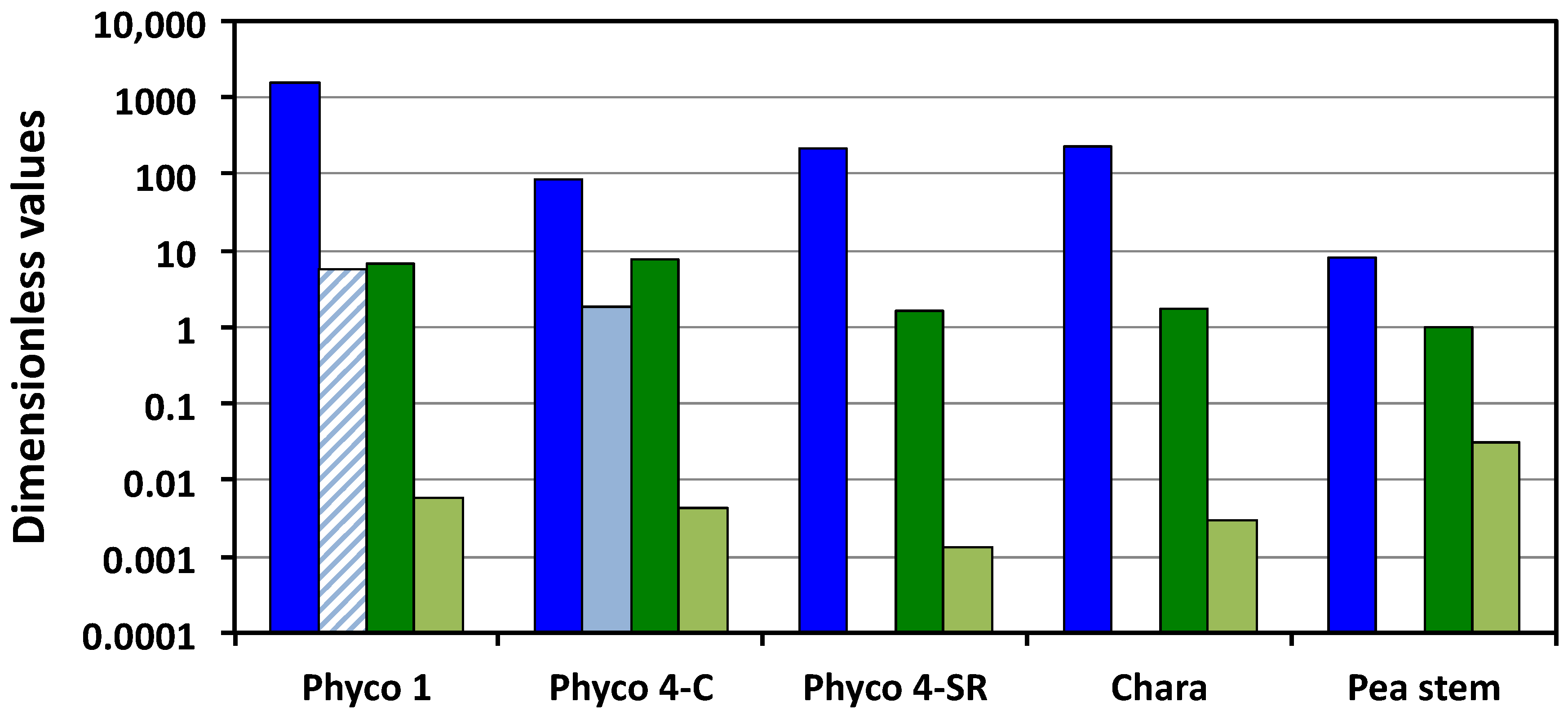
Figure 1 presents a visual comparison of the relevant Π parameters, i.e., Π
wv, Π
Tv, Π
pv and Π
ev. Π
wv is the ratio of the relative volumetric water uptake rate and the relative volumetric growth rate, so the large magnitudes (greater than unity) indicate that the biophysical processes responsible for the water uptake are capable of transporting water into the cells at a much faster rate than they are growing. The magnitudes of Π
pv indicate that the cell walls are capable of relative volumetric plastic deformation rates that are equal to or slightly greater than the relative volumetric growth rate, but all values are of the same order of magnitude, between unity and 7.7. In contrast, the small magnitudes of Π
ev indicate that during expansive growth, the walls are capable of only small relative volumetric elastic deformation rates, much smaller than the relative volumetric growth rate; Π
ev << 1 (
Figure 1). In the one case where transpiration rates were measured,
P. blakesleeanus stage IV (C), it can be seen that the sporangiophores capability for the water uptake rate is much greater than that for water loss through transpiration, by more than an order of magnitude. It can be seen that for the growing cells of pea stems (
P. satinis L.), the internodes of
C. corallina, and the sporangiophores of
P. blakesleeanus (stage I and stage IV), the magnitudes of the Π parameters representing the water uptake rates are larger than those representing the transpiration rates, the plastic wall deformation rates, and the elastic wall deformation rates by one to five orders of magnitude (
Figure 1). This finding indicates that the rate of water uptake is the dominant process during the expansive growth of these cells. Also, it is found that the magnitudes of the plastic wall deformation rates are larger than the magnitudes of the elastic wall deformation rates by one to three orders of magnitude (Π
pv and Π
ev in
Figure 1).
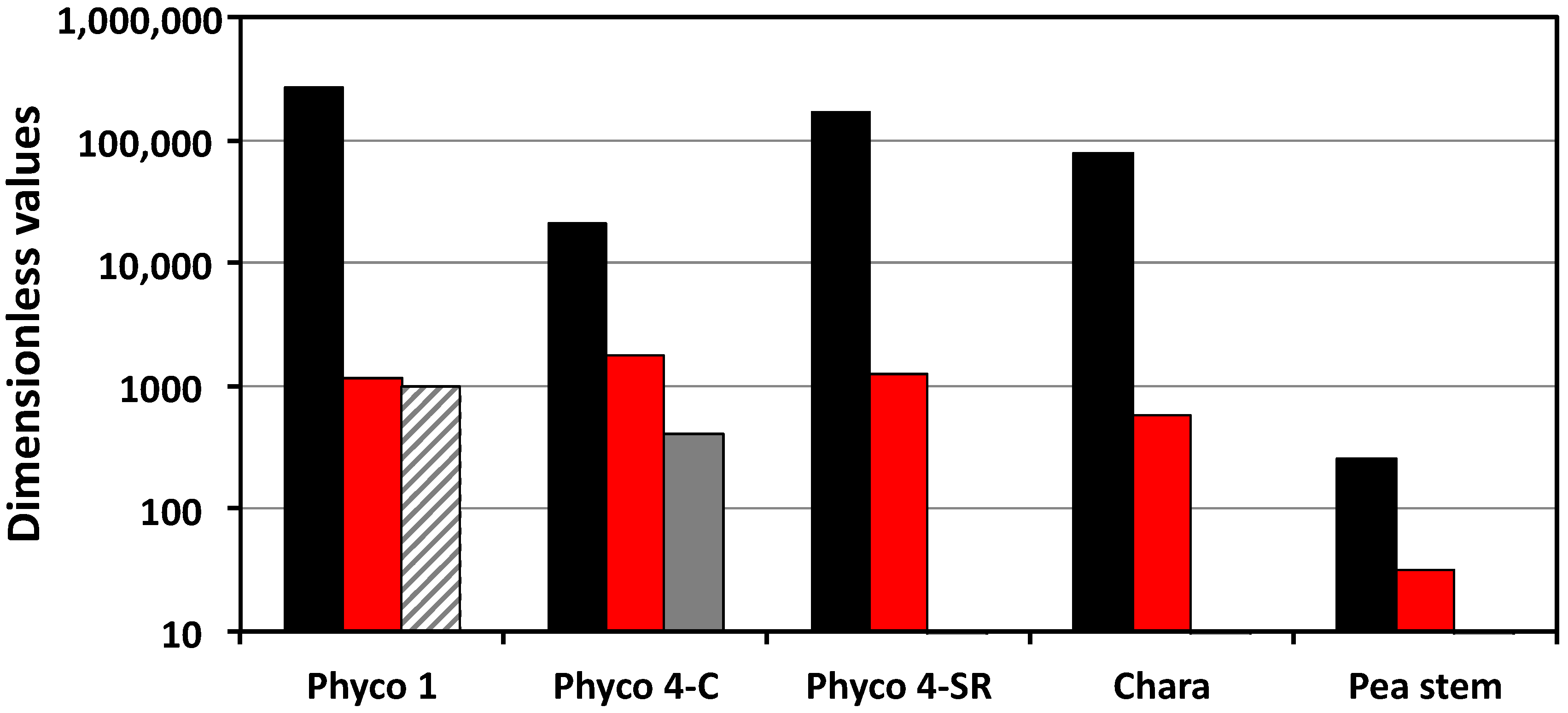
The dimensionless rate of change of the turgor pressure is a function of Π
we, Π
Te, and Π
pe (Equation (6)). The results presented in
Table 2 and
Figure 2 show that the values of Π
we are larger than those of Π
Te and Π
pe by one to two orders of magnitude. It is expected that Π
we is large because Π
wv is generally large (Π
wv >> 1) and Π
ev is generally small (Π
ev << 1), and Π
we = Π
wv/Π
ev. The large magnitude of Π
we indicates that a high rate of water uptake can be achieved and sustained with a relatively small rate of elastic wall deformation. The magnitude of Π
Te was only determined for the intact stage IV sporangiophores, and its magnitude is nearly the same as that of Π
pe. The results presented in
Figure 2 indicate that the dimensionless rate of change of the turgor pressure is dominated by the magnitude of Π
we (black bars). During normal growth, changes in the transpiration rate and the expansive growth rate (stress relaxation rate) produce relatively small changes in the rate of change of turgor pressure and in the magnitude of equilibrium turgor pressure.
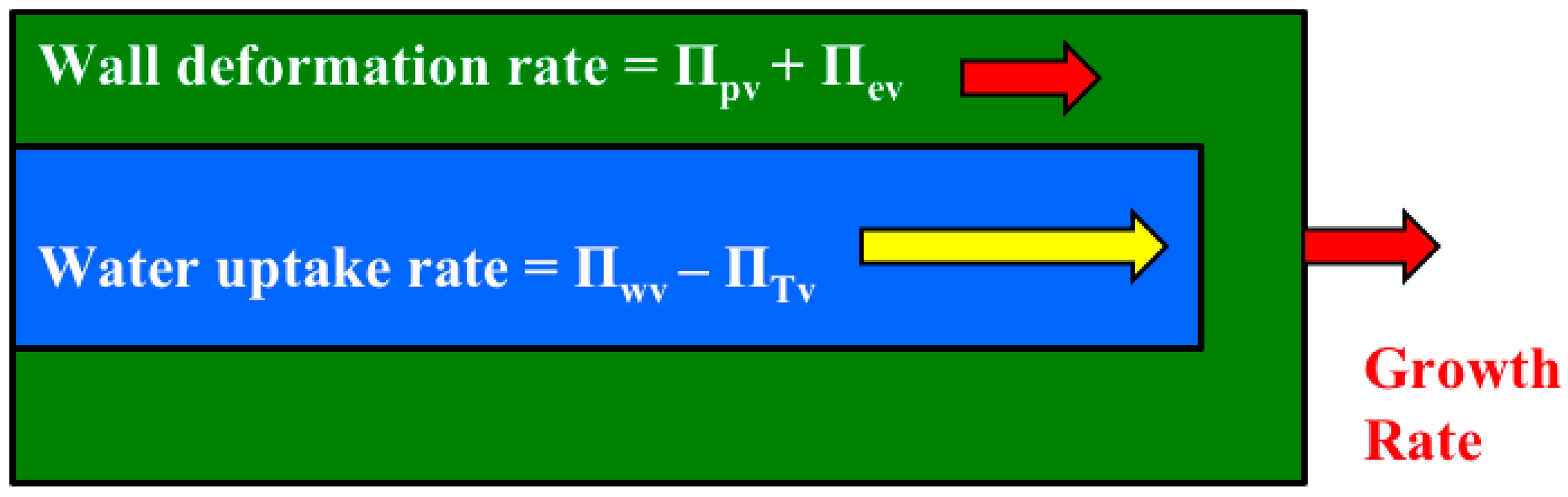
It is shown that the relative magnitudes of whole equations can be determined by simply summing the magnitudes of the Π parameters that precede the dimensionless term in each dimensionless equation (
Figure 3). Another dimensionless number, Π
wd, was obtained to determine the relative magnitudes of the
net water uptake rate and the
total wall deformation rate [
5], see Equation (7). The magnitudes of Π
wd for the cell species and types presented in
Table 2 are calculated (
Appendix B) and presented in
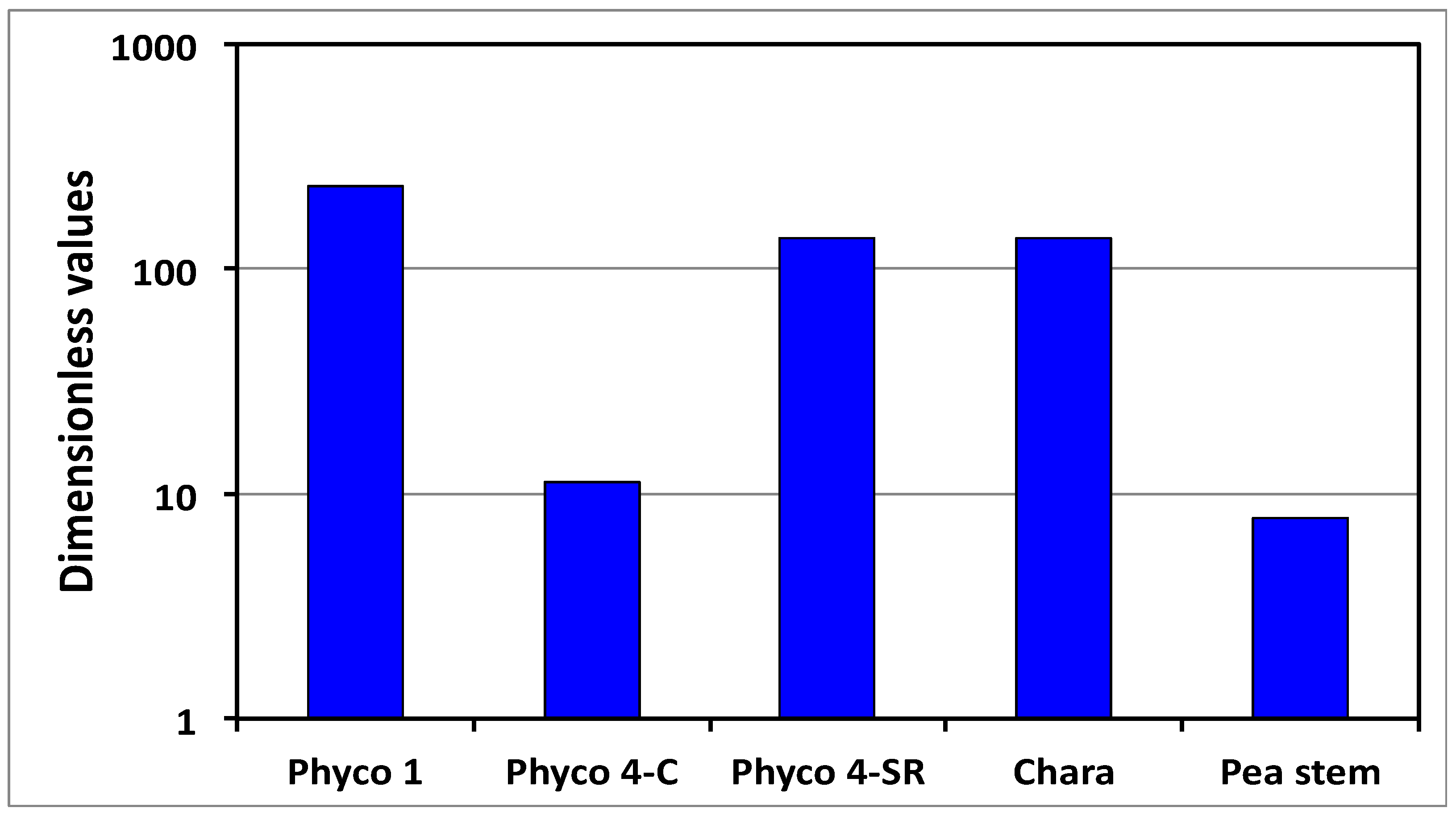
Figure 4. The results demonstrate that Π
wd is approximately one to two orders of magnitude greater than unity for all the cells in
Table 2. Thus, it is concluded for these cells that the smaller wall deformation rate limits and governs the expansive growth rate. This conclusion indicates that either Equation (2) or Equation (5) may be used as the sole governing equation for the expansive growth rate for these cells in normal growing conditions.
3.2. Stress Relaxation and the Πpe Parameter
When Π
wd > 1, as shown in
Figure 4, the expansive growth rate is limited and governed by the cell wall deformation rate; Equation (2) or Equation (5). Prior research demonstrates that the in vivo expansive growth rate requires stress relaxation of the cell wall in order to produce the decrease in turgor pressure that creates the water potential difference necessary to drive water uptake [
1,
5]. Stress relaxation experiments are conducted by eliminating the water uptake and transpiration for the growing walled cell and measuring the decreasing turgor pressure as a function of time using a pressure probe [
6,
10]. The dimensionless governing equation for the turgor pressure relaxation and the wall stress relaxation can be obtained from Equation (5) by recognizing that
vcw* =
vw* = 0 when both water uptake and transpiration rates are zero for these experimental conditions. Then, the dimensionless rate of change of the turgor pressure is reduced to Equation (8) [
3,
5].
Equation (8) is integrated to obtain a solution for the dimensionless turgor pressure as a function of dimensionless time, Equation (9) [
3,
5].
Equation (9) demonstrates that the dimensionless turgor pressure,
P*, decays exponentially from an initial value,
Pi*, to unity with a dimensionless time constant of
tc* = (Π
pe)
−1. So, the dimensionless stress relaxation rate is determined by the magnitude of the dimensionless number, Π
pe. The mean values of Π
pe are presented in
Table 2 and plotted in
Figure 2 (red bars). It is shown that the Π
pe values for the sporangiophores of
P. blakesleeanus are an order of magnitude larger than those of the internode cells of
C. corallina and two orders of magnitude larger than those of the cells in the stem tissue of
P. satinis L.
Previously, it was shown that if the magnitude of Π
pe,
ϕ, and
ε are known, the steady expansive growth rate,
vs, can be calculated with good accuracy [
5]. These calculations demonstrate for the growing sporangiophores of
P. blakesleeanus that a single constant value for Π
pe (Π
pe = 1524) can be used to determine the expansive growth rate of the sporangiophore during different developmental stages (stage I and stage IV) and different growth conditions (plucked from the mycelium and growing with its base in pure water) [
5]. Similarly, for plant cells of the growing stem of
P. satinis L., it was found that a single constant value for Π
pe (Π
pe = 32) can be used to determine the expansive growth rate of the stem after the application of the growth hormone IAA and during different growth conditions (incised and growing in water, and just cut from the plant) [
5]. These findings suggest that the magnitude of Π
pe is a constant characteristic of the wall of each cell species and individual cell. The implications of this finding are significant because it demonstrates that the dimensionless steady and quasi-steady expansive growth rate of the walled cell is directly related to the magnitude of Π
pe. Furthermore, this finding indicates that the ‘wall stress relaxation’ similarity can be achieved by matching the magnitude of Π
pe [
5].
The large magnitude of Π
pe and the large differences in values obtained for the cells of
P. blakesleeanus,
C. corallina, and
P. satinis L. were previously discussed [
5]. A few observations are noted from this comparison of Π
pe values (
Figure 2). (a) All the magnitudes of Π
pe for these cells are much greater than unity (Π
pe >> 1), indicating that the plastic deformation rate is much greater than the elastic deformation rate. This finding draws into question modeling the cell wall with constitutive equations that only describe the elastic wall deformation. (b) Mechanical energy is continually dissipated by the cell wall at a relatively large rate during the normal expansive growth and the cell walls of the sporangiophores of
P. blakesleeanus dissipate mechanical energy at a significantly higher rate than the cell walls in the pea stems of
P. satinis L. (c) The large differences in the magnitudes of Π
pe for the different cell species suggest that wall loosening chemistry may be qualitatively different for the fungal sporangiophores, algal internodes, and plant tissue. There is experimental evidence indicating that the molecular wall loosening mechanisms for plant and algal cells are different [
14,
15]. Little is known about wall loosening in the cell walls of the sporangiophores of
P. blakesleeanus other than low pH can elicit creep [
16]. However, because the molecular compositions of fungal cell walls are chitin-based [
17] and different from those of the plants and algae (cellulose based), it is reasonable to think that the molecular agents for wall loosening might be different.
The large differences in values of Π
pe obtained for cells of
P. blakesleeanus,
C. corallina, and
P. satinis L. can be further analyzed using the new results presented here. Two dimensionless Π parameters, Π
pv and Π
ev, contribute to the magnitude of Π
pe; Π
pe = Π
pv/Π
ev. The results presented in
Table 2 and
Figure 1 reveal that all the values of Π
pv are relatively similar, i.e., of the same order of magnitude, varying between unity and 7.7. The values of Π
ev are slightly more variable, varying by over an order of magnitude. Overall, some of the differences in the values of Π
pe are because of the variability in the relative volumetric plastic deformation rate of the wall. However, the variability of the relative volumetric elastic deformation rate of the wall contributes equally (or slightly more) to the differences in the magnitude of Π
pe and the dimensionless stress relaxation rate of the different species of cells analyzed.
Here, it is hypothesized that both the plastic and elastic wall deformation rates are regulated during the expansive growth. The variability of Π
pv and Π
ev presented in
Table 2 and
Figure 1 supports this hypothesis. Additional support is obtained from the algal internode cells of
C. corallina, where it is found that the longitudinal volumetric elastic modulus of the wall decreased in magnitude (thus increasing the wall elastic deformation rate) as the elongation growth rate increased in magnitude [
8]. For these internode algal cells, both the plastic and the elastic deformation rates of the wall increase as the elongation rate increases. It is generally thought that the plastic deformation rate of the wall is regulated at a microscopic level by breaking load-bearing bonds between wall polymers. Controlling the number of unbroken and load-bearing bonds between polymers, and making the wall polymers deform elastically when the wall is stressed, can regulate the elastic deformation rate. It is envisioned that the cell wall’s plastic and elastic deformation rates may be regulated at the microscopic level by controlling the rate of detachment of load-bearing bonds,
kd, and controlling the rate of attachment,
ka, and the formation of new bonds to become load-bearing [
18]. Future research should determine whether the elastic deformation rate is regulated independently, or is a function of the plastic deformation rate regulation. It is noted that this finding draws into question the modeling of the cell wall with constitutive equations that only describe the plastic wall deformation.
3.3. Similarity Analysis
Similarity between qualitatively identical processes is achieved when the dimensionless Π parameters governing the processes are the same magnitude [
19]. This similarity principle was recently employed on the results of constant tension creep experiments. Creep extension was observed for frozen–thawed sporangiophore walls when the pH of the bathing solution was decreased to acidic values [
16]. Furthermore, it was found that the measured creep rates are within the range of the elongation growth rates observed from natural growing sporangiophores. The similarity principle was employed to determine whether this low pH mechanism (acid growth mechanism [
20]) might be responsible for producing wall loosening at a molecular level, and therefore used to initiate, maintain and regulate the expansive growth of the sporangiophore wall. The experimental protocol was slightly modified to obtain the biophysical variables defining the Π
pe parameter [
5]. Then, the magnitude of Π
pe was determined and compared to the values obtained during normal growth [
5]. Wall stress relaxation similarity is achieved when the magnitudes of Π
pe for the two deforming walls (frozen–thawed walls and normal growing walls) are the same. If the Π
pe values are the same, it is evidence that regulating the pH of the cell wall is a viable mechanism for initiating, maintaining, and controlling wall loosening in the sporangiophore during normal growth. However, it was found that the magnitude of Π
pe was an order of magnitude smaller than that obtained during normal growth [
5]. Therefore, it was concluded that lowering the pH may contribute to normal wall extension and regulation, but other agents must be involved to achieve the value of Π
pe observed for normal growing sporangiophore walls. Now, because of the new findings presented here, it is suggested that both the plastic and the elastic wall deformation rates, Π
pv and Π
ev, be determined in future experiments to learn whether the respective rates obtained from the constant tension creep experiments are similar to those obtained from naturally growing sporangiophores. The results could help determine whether one of the wall deformation processes is similar to those obtained from naturally growing sporangiophores and the other is not, or if both wall deformation processes (plastic and elastic) are not similar.
Wall stress relaxation similarity was also employed to guide and validate a local numerical model of the cell wall. Recently, a statistical numerical model was constructed for cell wall extension using the same cell wall loosening mechanism employed by growing cells, i.e., breaking load-bearing bonds between cell wall polymers and making the bonds under zero-load conditions [
18]. Two variables in the model are
kd and
ka (the rate of detachment of load-bearing bonds and the rate of making bonds under zero-load conditions, respectively). In this model, the plastic deformation of the wall is the result of breaking load-bearing bonds between microfibrils and connecting polymer tethers. The elastic deformation of the wall is the result of stretching the polymer tethers between microfibrils. It was found that the experimentally obtained stress relaxation curves for the fungal sporangiophores and plant tissue (even though very different) could be accurately modeled when the Π
pe values that were obtained experimentally for sporangiophores and plant tissue were used in the model [
18]. Using the same Π
pe for each cell species establishes wall stress relaxation similarity between the biological cell wall and the statistical model of the cell wall. In addition, the elongation growth response to steps-up in the turgor pressure could be accurately described by the statistical model when the respective experimentally obtained values of Π
pe for the sporangiophores (
P. blakesleeanus) and the algal cells (
C. corallina) were used [
18]. Interestingly, it was found that Π
pe is related to one of the microscopic variables in the statistical model,
kd (the detachment rate between microfibrils and connecting polymer tethers); Π
pe =
kd/
vs. Future research will explore how the relative magnitudes of
kd and
ka change during different growth responses produced by environmental sensory stimuli and steps-up and steps-down in the turgor pressure.