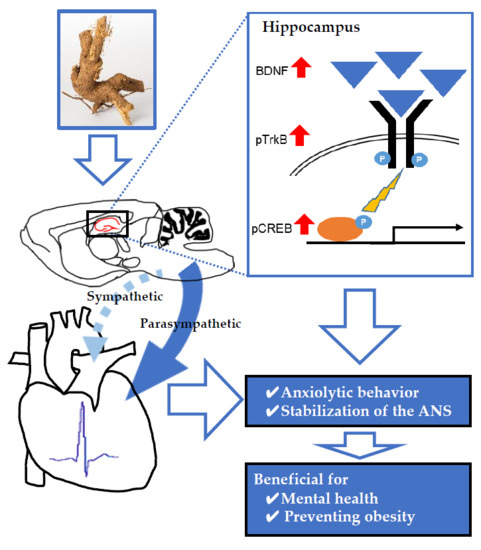
Anxiolytic Effects of Acanthopanax senticosus HARMS Occur via Regulation of Autonomic Function and Activate Hippocampal BDNF–TrkB Signaling
Abstract
1. Introduction
2. Results
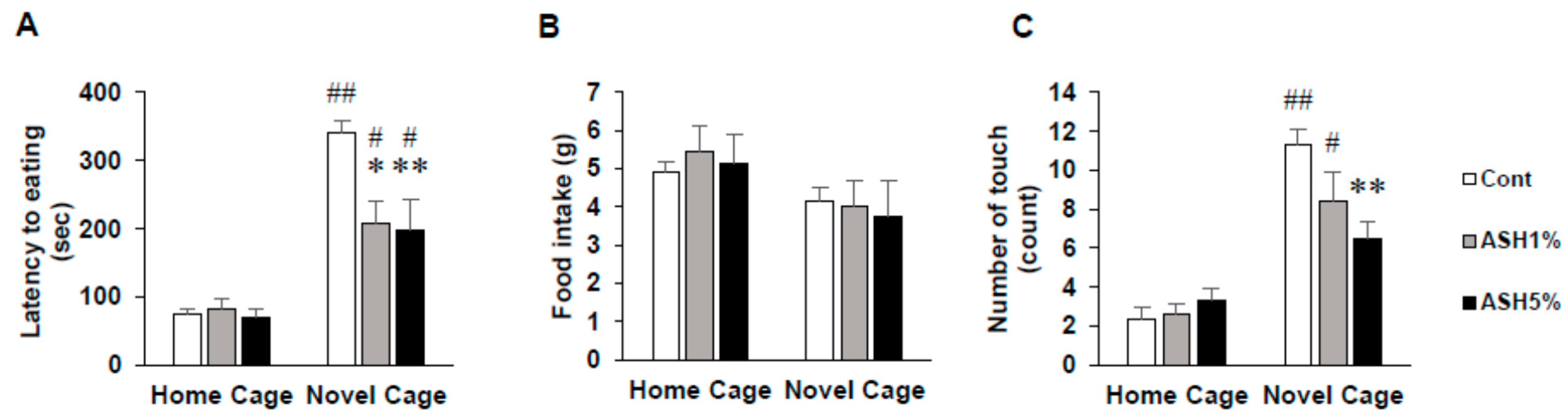
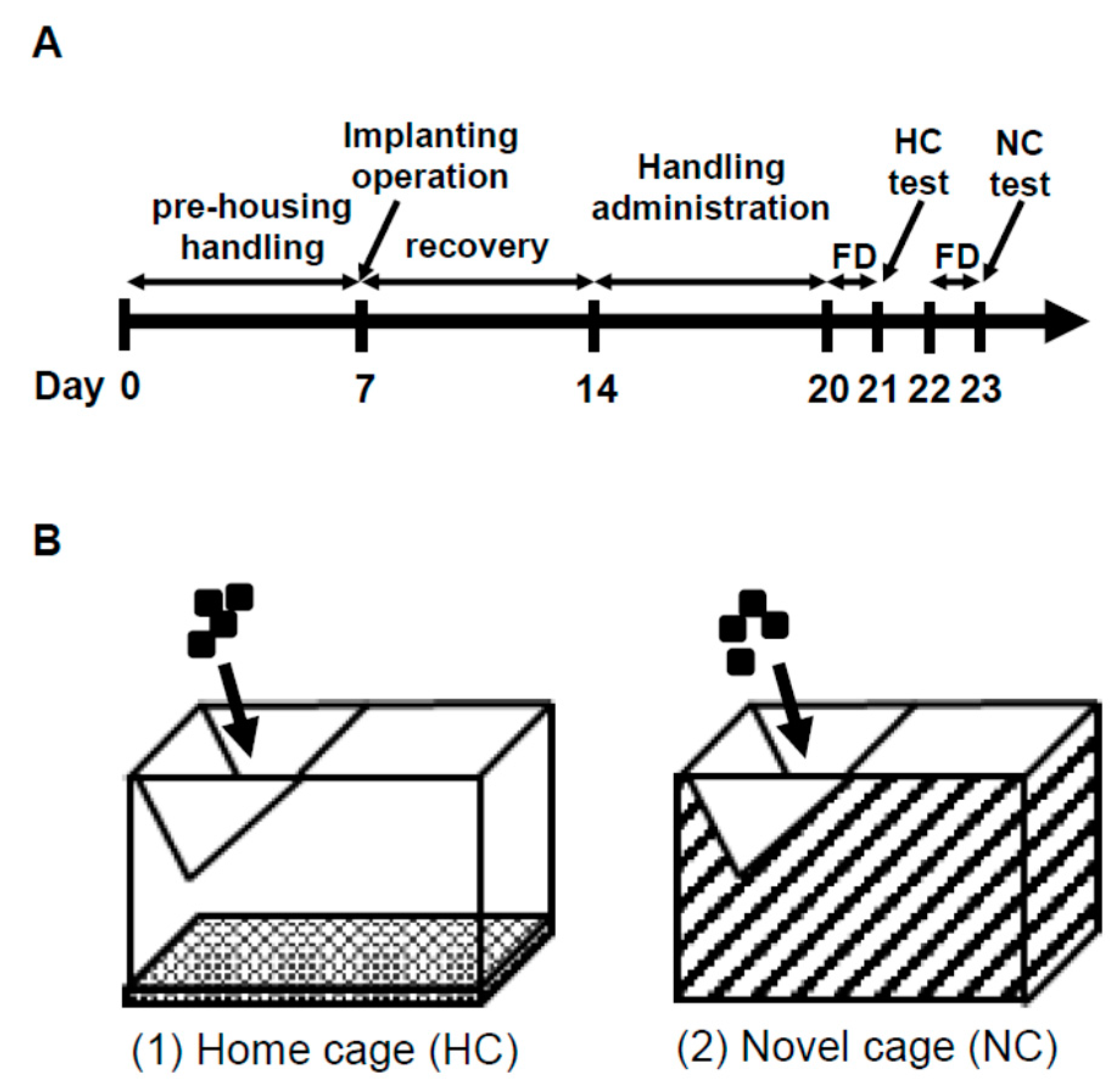
2.1. NSF Test
2.1.1. Behavior in the NSF Test
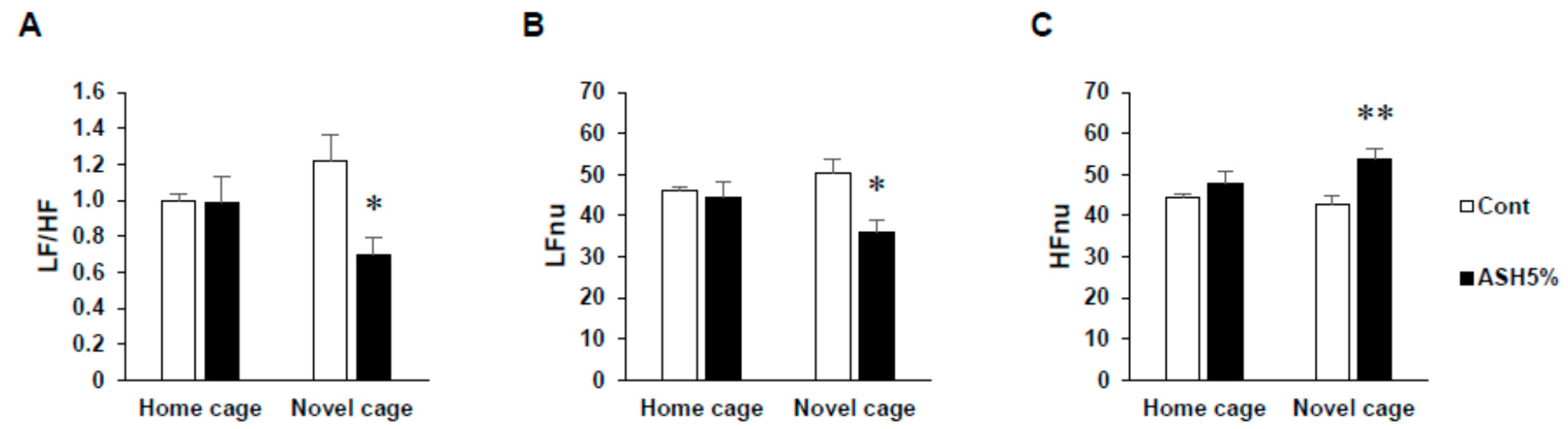
2.1.2. ANS Activity (Heart Rate Variability)
2.2. Comparison of Three Conditions in Heart Rate Variability
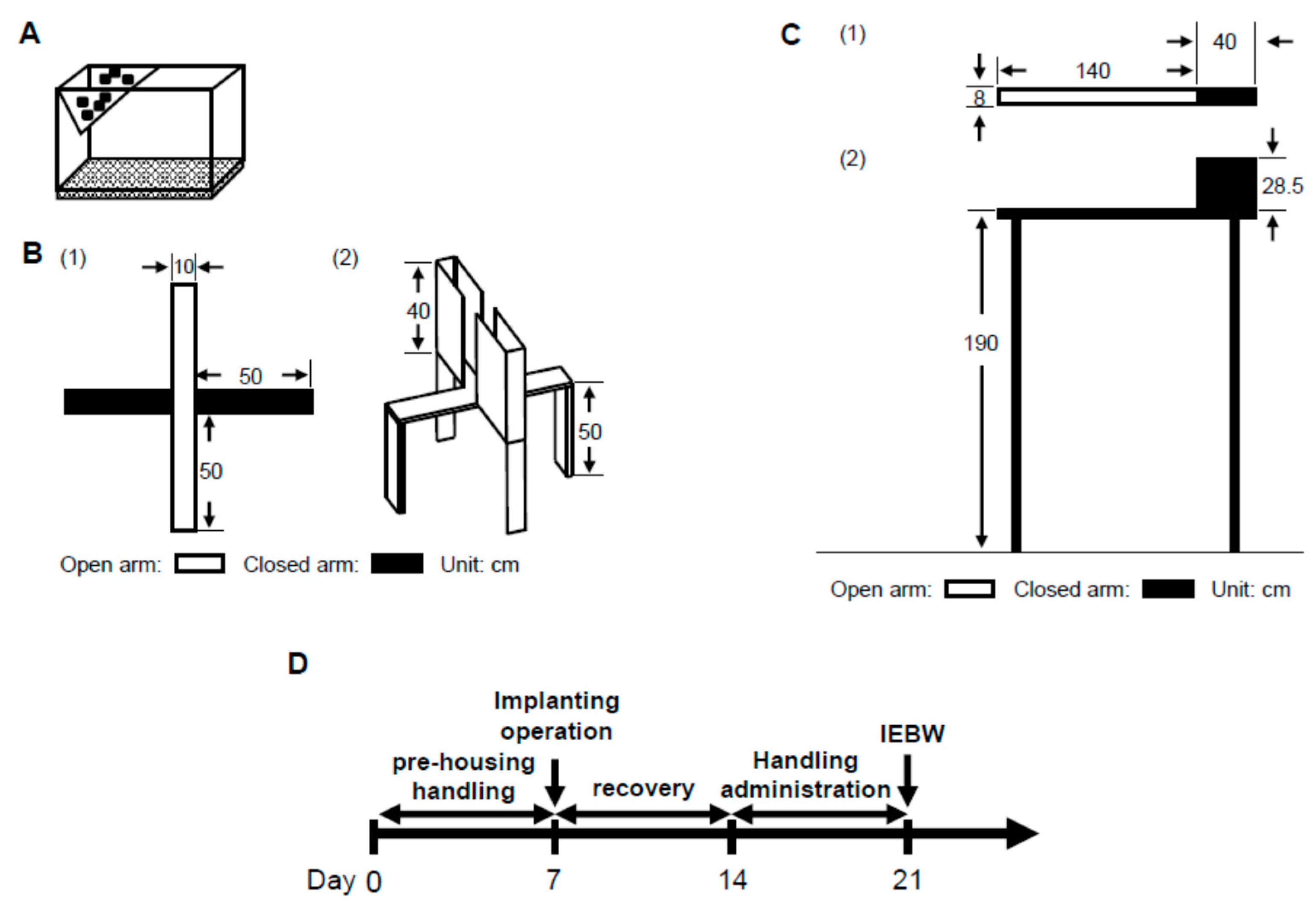
2.3. IEBW Test
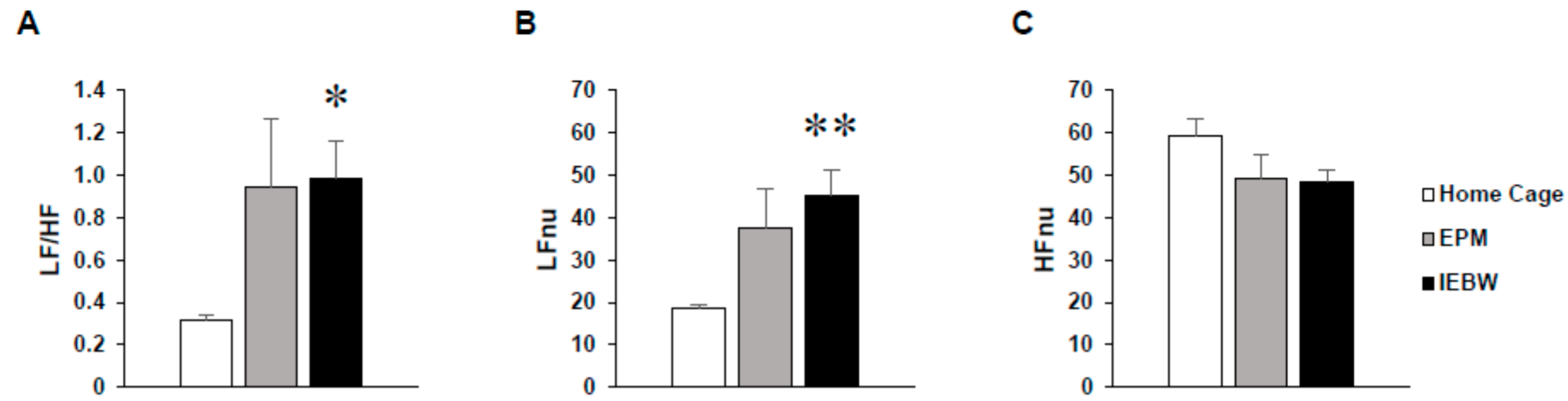
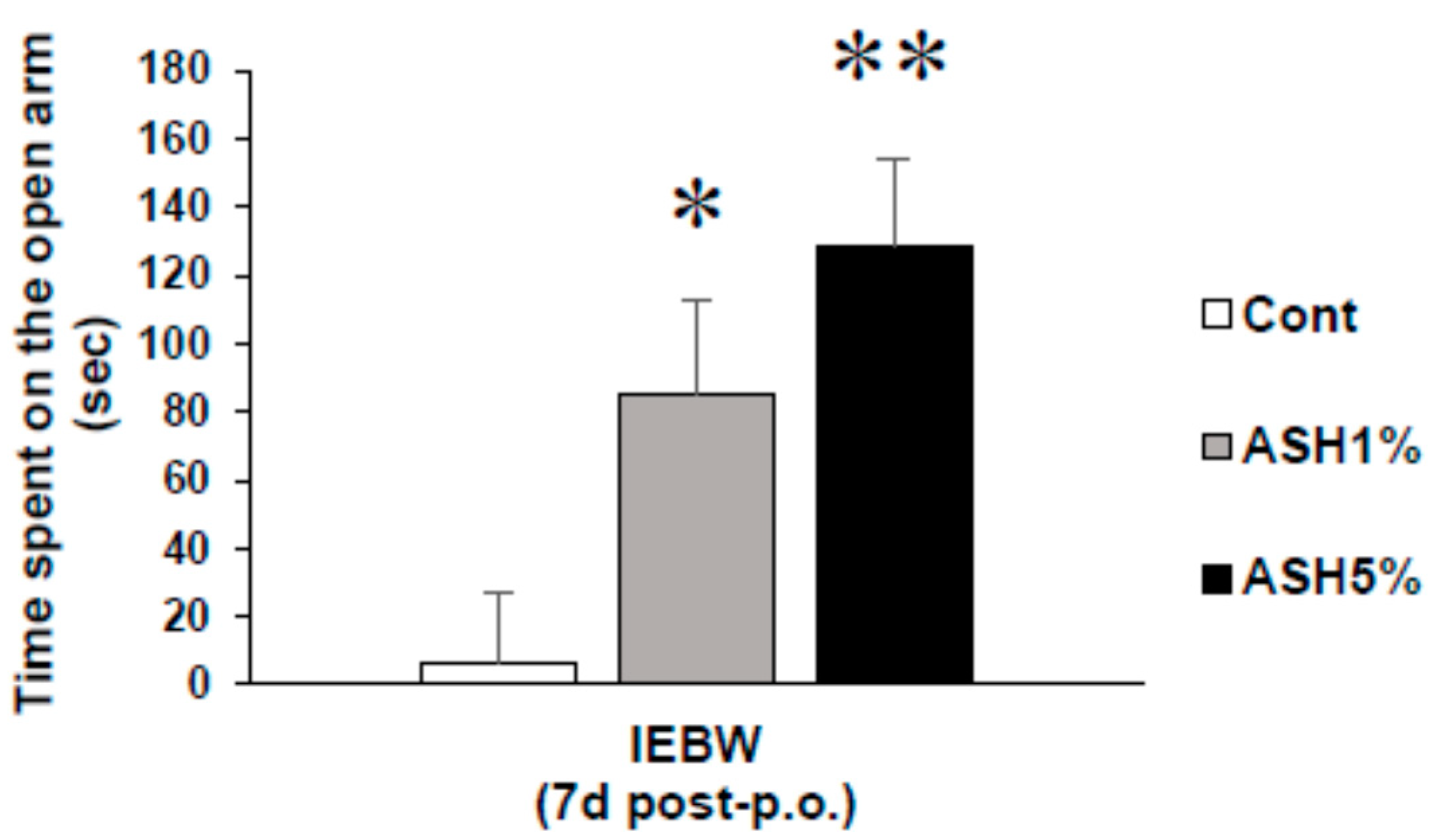
2.3.1. Time Spent in the Open Arm of the IEBW
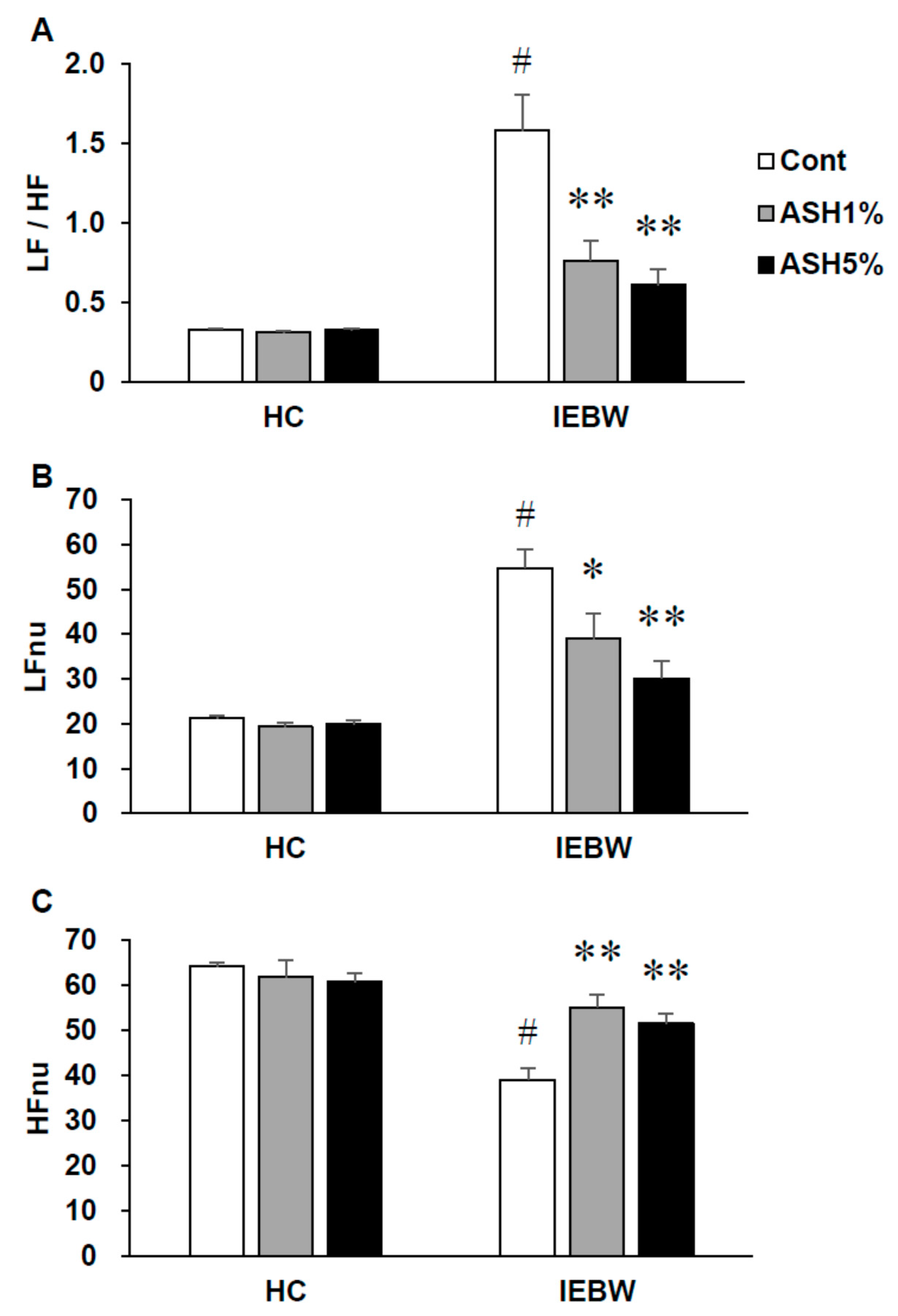
2.3.2. ANS Activity (Heart Rate Variability)
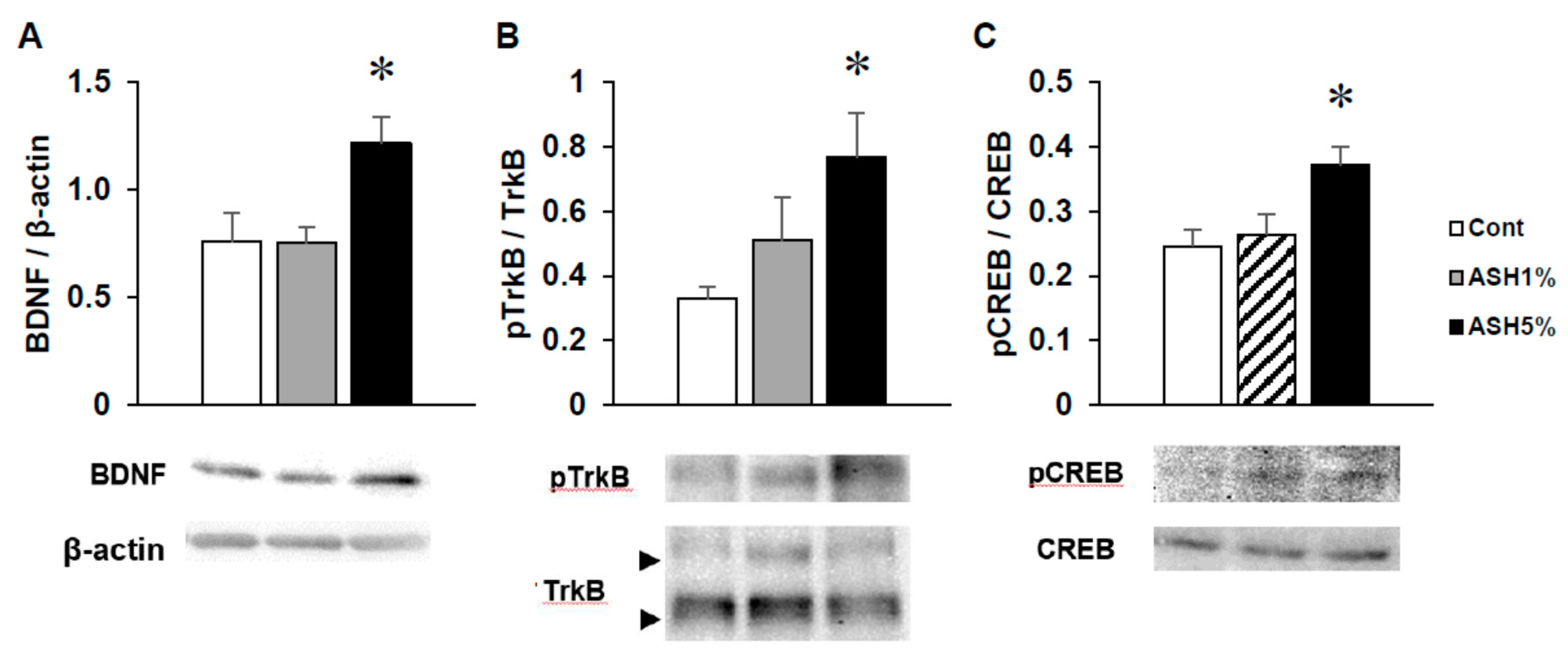
2.4. Western Blotting
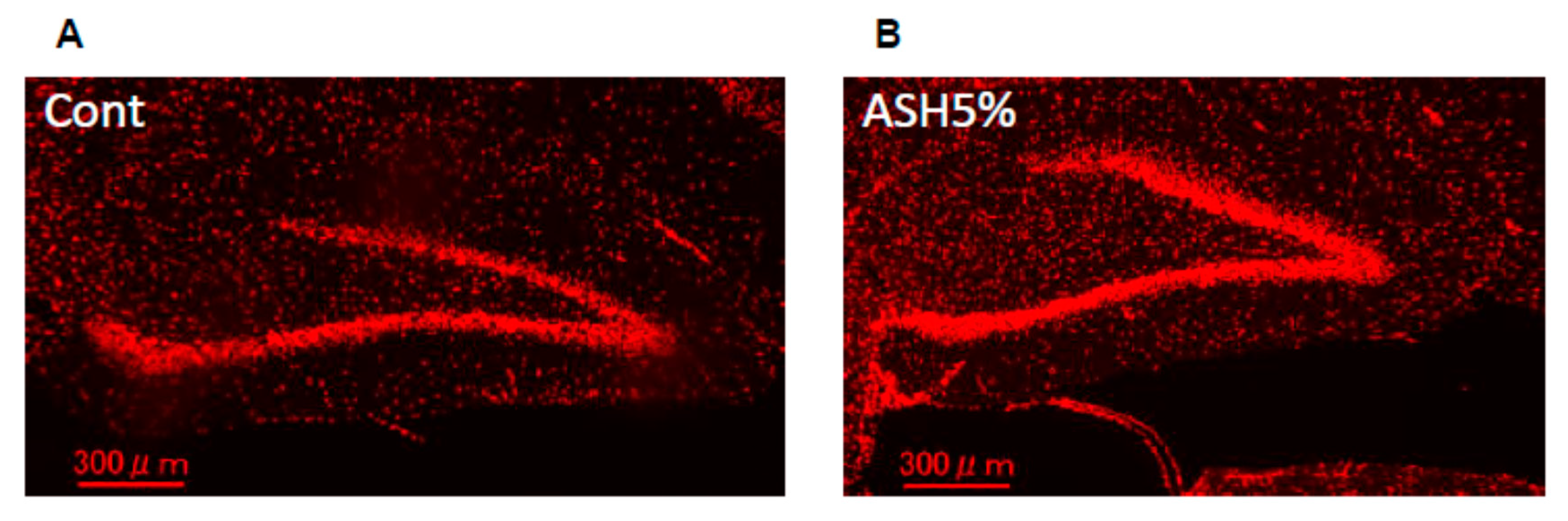
2.5. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Plant Extract
4.2. Ethics Statement
4.3. Animals
4.4. Surgery
4.5. Administration
4.6. Behavioral Studies
4.6.1. NSF Test
4.6.2. IEBW Test
4.7. Brain Tissue Preparation
4.8. Assessment of Cardiac Autonomic Activity (Heart Rate Variability)
4.9. Western Blotting
4.10. Immunohistochemistry
4.11. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shougaku-kan-hen. Chuyaku Encyclopedia 2nd; Shanghai Science Technology Publishing Company: Tokyo, Japan, 1985; ISBN 9784095830018. [Google Scholar]
- Ren, C.C. Zusetsu Kanpo-Iyaku Daijiten (1); Kodansha: Tokyo, Japan, 1982; ISBN 9784061444911. [Google Scholar]
- Nishibe, S.; Chie, F. Ezoukogi no Tyouryoku; The Mainichi Newspapers Co., Ltd.: Tokyo, Japan, 1998; ISBN 9784620312149. [Google Scholar]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C.; Peng, W.; Qin, L. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Pharmazie 2011, 66, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishida, M.; Saito, M.; Tanabe, A.; Eitsuka, T.; Yuan, S.H.; Ikekawa, N.; Nishida, H. The fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms improves insulin resistance and hepatic lipid accumulation by modulation of liver adenosine monophosphate-activated protein kinase activity and lipogenic gene expression in high-fat diet-fed obese mice. Nutr. Res. 2016, 36, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kondo, M.; Shimizu, T.; Saito, T.; Sato, S.; Hirayama, M.; Konishi, T.; Nishida, H. Antihyperlipidemic effect of Acanthopanax senticosus (Rupr. et Maxim) Harms leaves in high-fat-diet fed mice. J. Sci. Food Agric. 2016, 96, 3717–3722. [Google Scholar] [CrossRef] [PubMed]
- Leem, K.H.; Lee, S.; Kim, H.K. Extrusion process enhances the anti-inflammatory effect of Acanthopanax senticosus leaves. Food Sci. Biotechnol. 2014, 23, 911–916. [Google Scholar] [CrossRef]
- Zhang, X.L.; Ren, F.; Huang, W.; Ding, R.T.; Zhou, Q.S.; Liu, X.W. Anti-fatigue activity of extracts of stem bark from Acanthopanax senticosus. Molecules 2011, 16, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Davydov, M.; Krikorian, A.D. Eleutherococcus senticosus (Rupr. and Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. J. Ethnopharmacol. 2000, 72, 345–393. [Google Scholar] [CrossRef]
- Brekhman, I.I.; Dardymov, I.V. New substances of plant origin which increase nonspecific resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, B.T.; Hügel, H.M.; Rich, P.A. The effects of Eleutherococcus senticosus and Panax ginseng on steroidal hormone indices of stress and lymphocyte subset numbers in endurance athletes. Life Sci. 2001, 70, 431–442. [Google Scholar] [CrossRef]
- Fujikawa, T.; Yamaguchi, A.; Morita, I.; Takeda, H.; Nishibe, S. Protective effects of Acanthopanax senticosus Harms from Hokkaido and its components on gastric ulcer in restrained cold water stressed rats. Biol. Pharm. Bull. 1996, 19, 1227–1230. [Google Scholar] [CrossRef]
- Nishibe, S.; Kinoshita, H.; Takeda, H.; Okano, G. Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stressed rats. Chem. Pharm. Bull. 1990, 38, 1763–1765. [Google Scholar] [CrossRef]
- Jin, L.; Wu, F.; Li, X.; Li, H.; Du, C.; Jiang, Q.; You, J.; Li, S.; Xu, Y. Anti-depressant effects of aqueous extract from Acanthopanax senticosus in mice. Phytother. Res. 2013, 27, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.; Lim, D. Antidepressant effects of Radix et Caulis Acanthopanacis Santicosi extracts on rat models with depression in terms of immobile behavior. J. Tradit. Chin. Med. 2014, 34, 317–323. [Google Scholar] [CrossRef]
- Ito, R.; Lee, A.C.H. The role of the hippocampus in approach-avoidance conflict decision-making: Evidence from rodent and human studies. Behav. Brain Res. 2016, 313, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lanza, A.M.; Abad Martínez, M.J.; Fernández Matellano, L.; Recuero Carretero, C.; Villaescusa Castillo, L.; Silván Sen, A.M.; Bermejo Benito, P. Lignan and phenylpropanoid glycosides from Phillyrea latifolia and their in vitro anti-inflammatory activity. Planta Med. 2001, 67, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, M.S.; Hyun, C.K. Syringin attenuates insulin resistance via adiponectin-mediated suppression of low-grade chronic inflammation and ER stress in high-fat diet-fed mice. Biochem. Biophys. Res. Commun. 2017, 488, 40–45. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, X.; Zhao, C.; Qie, Y.; Yan, Z.; Zhu, X. Eleutheroside E ameliorates arthritis severity in collagen-induced arthritis mice model by suppressing inflammatory cytokine release. Inflammation 2014, 37, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Shimosaka, S.; Sasaki, H.; Matsumura, T.; Tukiyama, T.; Tokiwa, T. (+)-Syringaresinol-di-O-β-d-glucoside, a phenolic compound from Acanthopanax senticosus Harms, suppresses proinflammatory mediators in SW982 human synovial sarcoma cells by inhibiting activating protein-1 and/or nuclear factor-κB activities. Toxicol. In Vitro 2007, 21, 1530–1537. [Google Scholar] [CrossRef]
- Bu, Y.; Jin, Z.H.; Park, S.Y.; Baek, S.; Rho, S.; Ha, N.; Park, S.K.; Kim, S.Y.; Kim, H. Siberian ginseng reduces infarct volume in transient focal cerebral ischaemia in Sprague-Dawley rats. Phytother. Res. 2005, 19, 167–169. [Google Scholar] [CrossRef]
- Ahn, J.; Um, M.Y.; Lee, H.; Jung, C.H.; Heo, S.H.; Ha, T.Y. Eleutheroside E, An active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evid. Based Complement. Altern. Med. 2013, 2013, 934183. [Google Scholar] [CrossRef]
- Yamazaki, T.; Tokiwa, T. Isofraxidin, a coumarin component from Acanthopanax senticosus, inhibits matrix metalloproteinase-7 expression and cell invasion of human hepatoma cells. Biol. Pharm. Bull. 2010, 33, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Li, Z.; Zhang, L.; Liu, Y.; Ding, H.; Yin, S. Isofraxidin, a coumarin component improves high-fat diet induced hepatic lipid homeostasis disorder and macrophage inflammation in mice. Food Funct. 2017, 8, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Cho, M.L.; Kim, D.B.; Shin, G.H.; Lee, J.H.; Lee, J.S.; Park, S.O.; Lee, S.J.; Shin, H.M.; Lee, O.H. The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Z.; Gao, Y.; Wu, Y.; Li, Z.; Liu, H.; Zhang, C. Bidirectional crosstalk between stress-induced gastric ulcer and depression under chronic stress. PLoS ONE 2012, 7, e51148. [Google Scholar] [CrossRef]
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Macit, C.; Mercanoglu, G.; Safran, N.; Gungor, M.; Eroglu, L. Time to onset of anxiety- and depression-like behaviors in rats after myocardial infarction and association with autonomic control of heart. Neuroanatomy 2009, 8, 20–25. [Google Scholar]
- Kreibig, S.D. Autonomic nervous system activity in emotion: A review. Biol. Psychol. 2010, 84, 394–421. [Google Scholar] [CrossRef]
- Beauchaine, T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev. Psychopathol. 2001, 13, 183–214. [Google Scholar] [CrossRef]
- Friedman, B.H.; Thayer, J.F. Anxiety and autonomic flexibility: A cardiovascular approach. Biol. Psychol. 1998, 49, 303–323. [Google Scholar] [CrossRef]
- Nijsen, M.J.; Croiset, G.; Diamant, M.; Stam, R.; Delsing, D.; de Wied, D.; Wiegant, V.M. Conditioned fear-induced tachycardia in the rat: Vagal involvement. Eur. J. Pharmacol. 1998, 350, 211–222. [Google Scholar] [CrossRef]
- Hashimoto, K. Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin. Neurosci. 2010, 64, 341–357. [Google Scholar] [CrossRef]
- Duman, C.H.; Schlesinger, L.; Russell, D.S.; Duman, R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008, 1199, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Deltheil, T.; Guiard, B.P.; Cerdan, J.; David, D.J.; Tanaka, K.F.; Repérant, C.; Guilloux, J.P.; Coudoré, F.; Hen, R.; Gardier, A.M. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology 2008, 55, 1006–1014. [Google Scholar] [CrossRef]
- Pardon, M.C. Role of neurotrophic factors in behavioral processes: Implications for the treatment of psychiatric and neurodegenerative disorders. Vitam. Horm. 2010, 82, 185–200. [Google Scholar] [CrossRef]
- Schaaf, M.J.; De Kloet, E.R.; Vreugdenhil, E. Corticosterone effects on BDNF expression in the hippocampus implications for memory formation. Stress 2000, 3, 201–208. [Google Scholar] [CrossRef]
- Murray, F.; Smith, D.W.; Hutson, P.H. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 2008, 583, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Sterner, E.Y.; Kalynchuk, L.E. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: Relevance to depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, H.; Zhao, L.; Li, X.; You, J.; Jiang, Q.; Li, S.; Jin, L.; Xu, Y. Protective effects of aqueous extract from Acanthopanax senticosus against corticosterone-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2013, 148, 861–868. [Google Scholar] [CrossRef]
- Fujikawa, T.; Miguchi, S.; Kanada, N.; Nakai, N.; Ogata, M.; Suzuki, I.; Nakashima, K. Acanthopanax senticosus Harms as a prophylactic for MPTP-induced Parkinson’s disease in rats. J. Ethnopharmacol. 2005, 97, 375–381. [Google Scholar] [CrossRef]
- Fujikawa, T.; Kanada, N.; Shimada, A.; Ogata, M.; Suzuki, I.; Hayashi, I.; Nakashima, K. Effect of sesamin in Acanthopanax senticosus HARMS on behavioral dysfunction in rotenone-induced parkinsonian rats. Biol. Pharm. Bull. 2005, 28, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Soya, H.; Deocaris, C.C.; Yamaguchi, K.; Ohiwa, N.; Saito, T.; Nishijima, T.; Kato, M.; Tateoka, M.; Matsui, T.; Okamoto, M.; et al. Extract from Acanthopanax senticosus harms (Siberian ginseng) activates NTS and SON/PVN in the rat brain. Biosci. Biotechnol. Biochem. 2008, 72, 2476–2480. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Soya, H.; Hibasami, H.; Kawashima, H.; Takeda, H.; Nishibe, S.; Nakashima, K. Effect of Acanthopanax senticosus Harms on biogenic monoamine levels in the rat brain. Phytother. Res. 2002, 16, 474–478. [Google Scholar] [CrossRef]
- Lee, Y.B.; Yu, J.; Choi, H.H.; Jeon, B.S.; Kim, H.K.; Kim, S.W.; Kim, S.S.; Park, Y.G.; Chae, H.S. The association between peptic ulcer diseases and mental health problems: A population-based study: A STROBE compliant article. Medicine (Baltimore) 2017, 96, e7828. [Google Scholar] [CrossRef] [PubMed]
- Bodnoff, S.R.; Suranyi-Cadotte, B.; Quirion, R.; Meaney, M.J. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology 1989, 97, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E.; Iwata, J.; Cicchetti, P.; Reis, D.J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988, 8, 2517–2529. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Fukumoto, K.; Iijima, M.; Chaki, S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav. Brain Res. 2013, 238, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Liu, B.B.; Li, J.; Luo, L.; Liu, Q.; Geng, D.; Tang, Y.; Xia, Y.; Wu, D. BDNF signaling is necessary for the antidepressant-like effect of naringenin. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Stringer, T.P.; Guerrieri, D.; Vivar, C.; van Praag, H. Plant-derived flavanol (−)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Transl. Psychiatry 2015, 5, e493. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Imbe, H.; Morikawa, Y.; Kubo, C.; Senba, E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005, 53, 129–139. [Google Scholar] [CrossRef]
- Finkbeiner, S.; Tavazoie, S.F.; Maloratsky, A.; Jacobs, K.M.; Harris, K.M.; Greenberg, M.E. CREB: A major mediator of neuronal neurotrophin responses. Neuron 1997, 19, 1031–1047. [Google Scholar] [CrossRef]
- Pentkowski, N.S.; Blanchard, D.C.; Lever, C.; Litvin, Y.; Blanchard, R.J. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur. J. Neurosci. 2006, 23, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.L.; Appelhans, B.M.; Whited, M.C.; Oleski, J.; Pagoto, S.L. Trait anxiety, but not trait anger, predisposes obese individuals to emotional eating. Appetite 2010, 55, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Goossens, L.; Braet, C.; Van, V.L.; Mels, S. Loss of control over eating in overweight youngsters: The role of anxiety, depression and emotional eating. Eur. Eat. Disord. Rev. 2009, 17, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Kanoski, S.E.; Schier, L.A.; Clegg, D.J.; Benoit, S.C. A potential role for the hippocampus in energy intake and body weight regulation. Curr. Opin. Pharmacol. 2007, 7, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, S.; Oikawa, H.; Takekoshi, H.; Hoshizaki, M.; Ogata, M.; Fujikawa, T. Anxiolytic Effects of Acanthopanax senticosus HARMS Occur via Regulation of Autonomic Function and Activate Hippocampal BDNF–TrkB Signaling. Molecules 2019, 24, 132. https://doi.org/10.3390/molecules24010132
Miyazaki S, Oikawa H, Takekoshi H, Hoshizaki M, Ogata M, Fujikawa T. Anxiolytic Effects of Acanthopanax senticosus HARMS Occur via Regulation of Autonomic Function and Activate Hippocampal BDNF–TrkB Signaling. Molecules. 2019; 24(1):132. https://doi.org/10.3390/molecules24010132
Chicago/Turabian StyleMiyazaki, Shouhei, Hirotaka Oikawa, Hideo Takekoshi, Masako Hoshizaki, Masato Ogata, and Takahiko Fujikawa. 2019. "Anxiolytic Effects of Acanthopanax senticosus HARMS Occur via Regulation of Autonomic Function and Activate Hippocampal BDNF–TrkB Signaling" Molecules 24, no. 1: 132. https://doi.org/10.3390/molecules24010132
APA StyleMiyazaki, S., Oikawa, H., Takekoshi, H., Hoshizaki, M., Ogata, M., & Fujikawa, T. (2019). Anxiolytic Effects of Acanthopanax senticosus HARMS Occur via Regulation of Autonomic Function and Activate Hippocampal BDNF–TrkB Signaling. Molecules, 24(1), 132. https://doi.org/10.3390/molecules24010132