A Thick-Skulled Troodontid Theropod from the Late Cretaceous of Mexico
Abstract
1. Introduction



Geological Setting
2. Materials and Methods
2.1. Materials
2.2. Phylogenetic Analysis
2.3. Nomenclatural Act
3. Results
3.1. Systematic Paleontology
3.2. Description and Comparisons


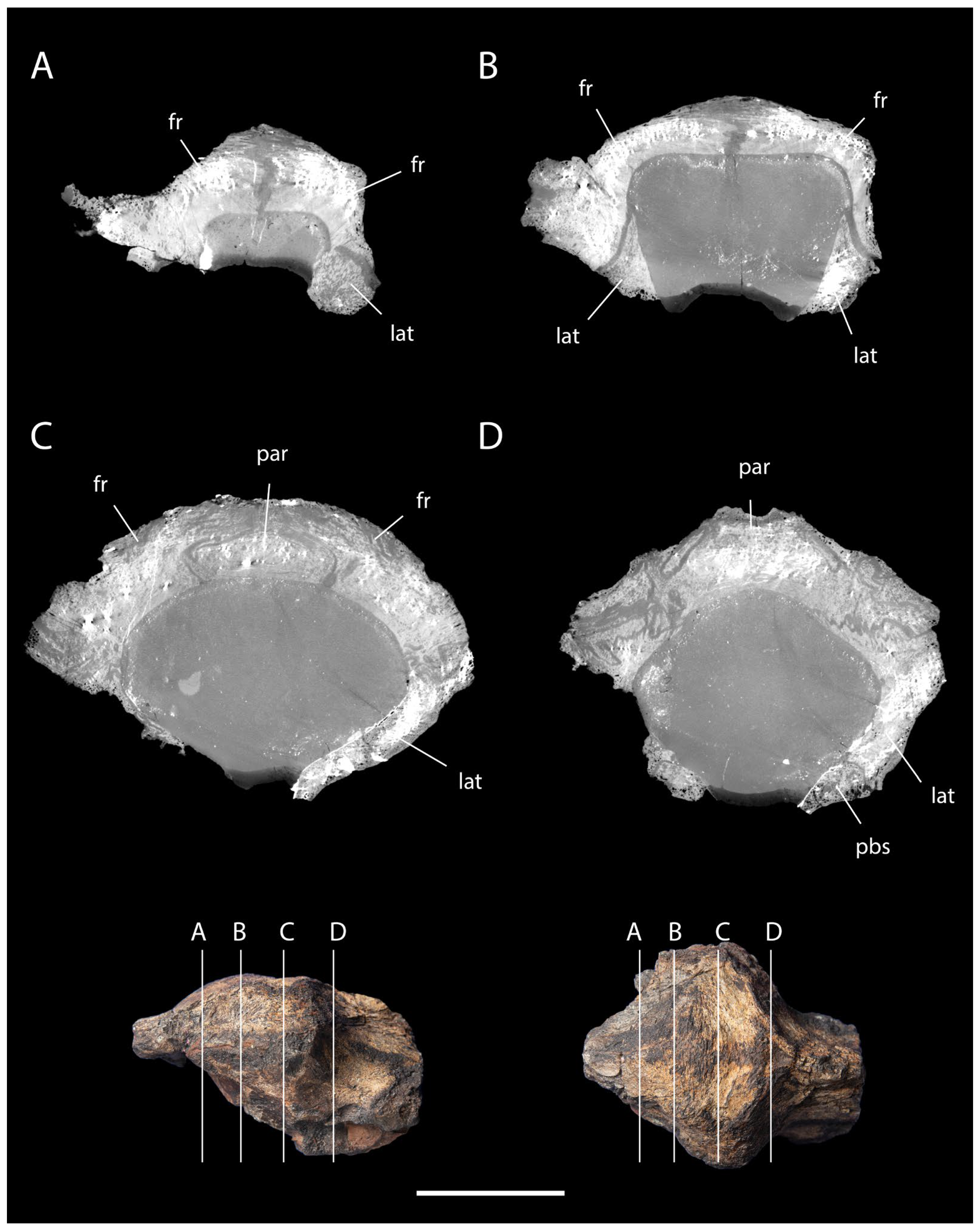


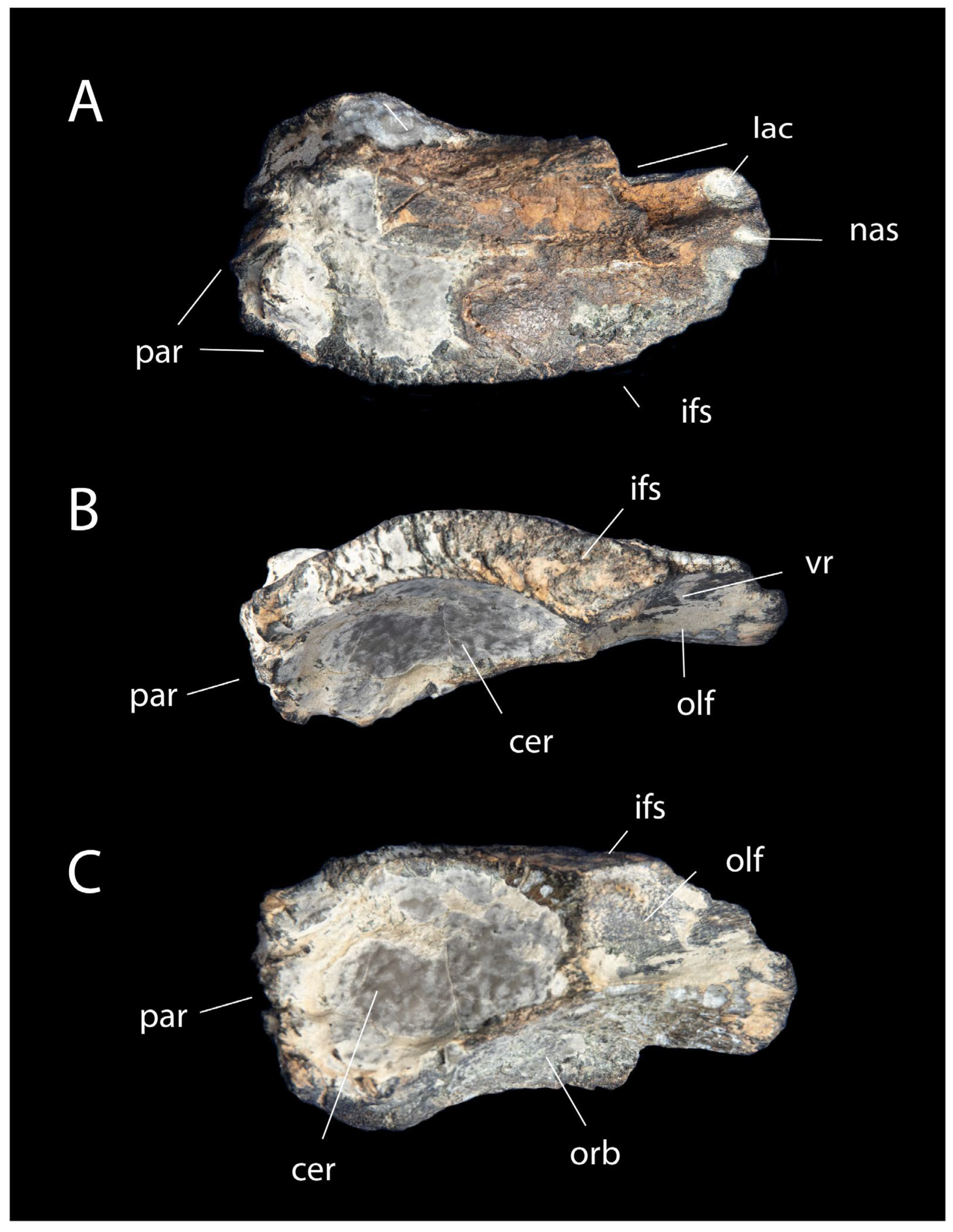
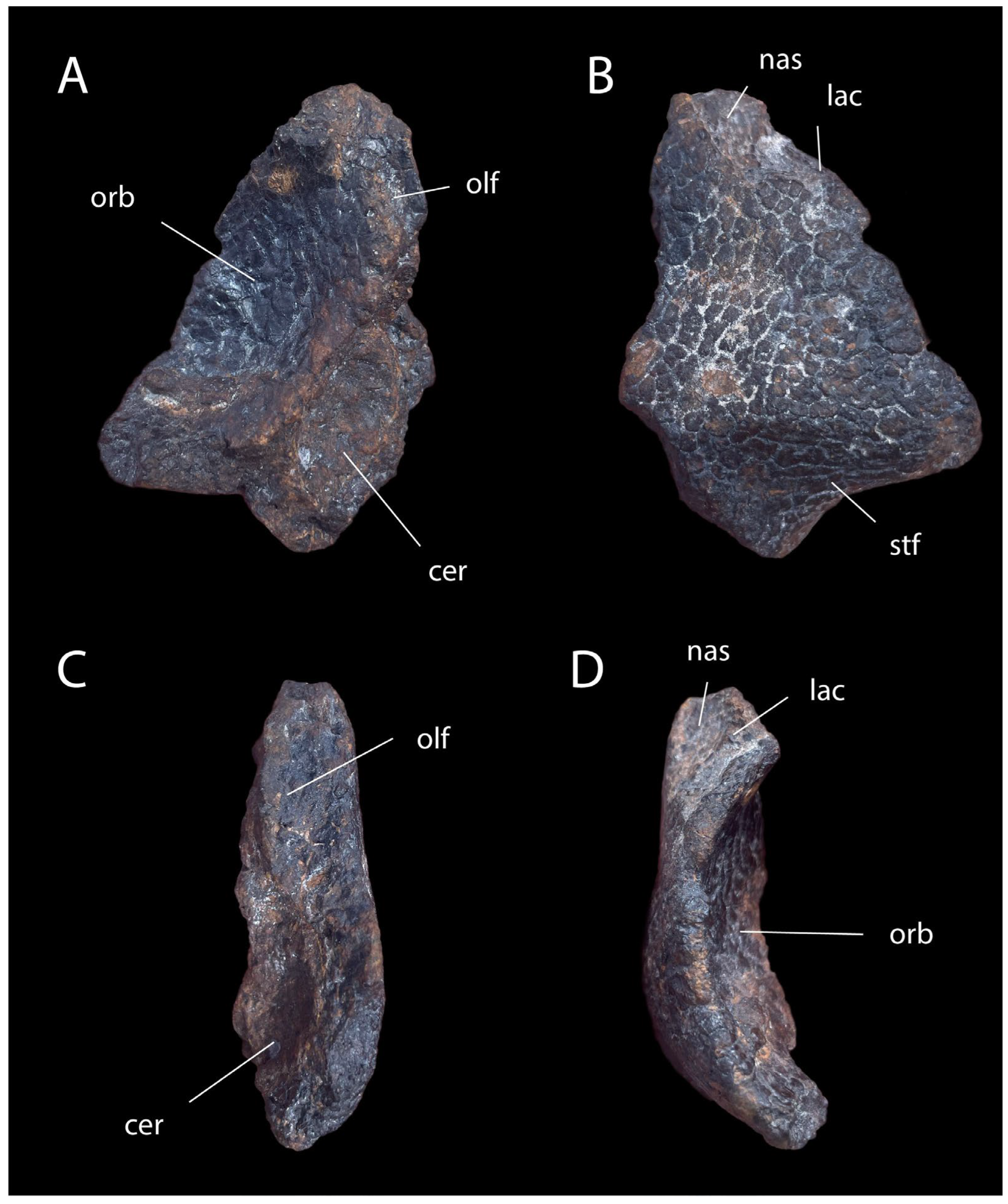
3.3. Phylogenetic Analysis
4. Discussion
4.1. Systematics of the Cerro del Pueblo Troodontids
4.2. Variation Within Xenovenator
- (i)
- Variation between distinct species;
- (ii)
- Ontogeny within a single species;
- (iii)
- Sexual dimorphism within a single species.
4.3. Paleobiology
4.4. Sexual Selection in Dinosaurs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Darwin, C. The Descent of Man and Selection in Relation to Sex; Murray: London, UK, 1871. [Google Scholar]
- Andersson, M.; Iwasa, Y. Sexual selection. Trends Ecol. Evol. 1996, 11, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Knell, R.J.; Naish, D.; Tomkins, J.L.; Hone, D.W.E. Sexual selection in prehistoric animals: Detection and implications. Trends Ecol. Evol. 2013, 28, 38–47. [Google Scholar] [CrossRef]
- Osmólska, H.; Currie, P.J.; Barsbold, R. Oviraptorosauria. In The Dinosauria; Weishampel, D.B., Dodson, P., Osmolska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 165–183. [Google Scholar]
- Dodson, P.; Forster, C.A.; Sampson, S.D. Ceratopsidae. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmolska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 494–513. [Google Scholar]
- Horner, J.R.; Weishampel, D.B.; Forster, C.A. Hadrosauridae. In The Dinosauria; Weishampel, D.B., Dodson, P., Osmolska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 438–463. [Google Scholar]
- Bonaparte, J.F.; Novas, F.; Coria, R. Carnotaurus sastrei Bonaparte, the horned, lightly built carnosaur from the Middle Cretaceous of Patagonia. Contrib. Sci. Nat. Hist. Mus. Los Angeles Cty. 1990, 416, 1–42. [Google Scholar] [CrossRef]
- Maryanska, T.; Chapman, R.E.; Weishampel, D.B. Pachycephalosauria. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmolska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 464–477. [Google Scholar]
- Sampson, S.D.; Witmer, L.M. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the late Cretaceous of Madagascar. J. Vertebr. Paleontol. 2007, 27, 32–104. [Google Scholar] [CrossRef]
- Lamanna, M.C.; Sues, H.-D.; Schachner, E.R.; Lyson, T.R. A New Large-Bodied Oviraptorosaurian Theropod Dinosaur from the Latest Cretaceous of Western North America. PLoS ONE 2014, 9, e92022. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Schlaikjer, E.M. The skeleton of Leptoceratops with the description of a new species. Am. Mus. Novit. 1942, 1169, 1–15. [Google Scholar]
- Brown, B.; Schlaikjer, E.M. The structure and relationships of Protoceratops. Ann. N. Y. Acad. Sci. 1940, 40, 133–266. [Google Scholar] [CrossRef]
- Bell, P.R.; Fanti, F.; Currie, P.J.; Arbour, V.M. A mummified duck-billed dinosaur with a soft-tissue cock’s comb. Curr. Biol. 2014, 24, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Schlaikjer, E.M. A study of the troödont dinosaurs with a description of a new genus and four new species. Bull. Am. Mus. Nat. Hist. 1943, 82, 115–149. [Google Scholar]
- Stromer, E. Ergebnisse der Forschungsreisen Prof. E. Stromers in den Wüsten Ägyptens. Wirbeltier-Reste der Baharîje-Stufe (unterstes Cenoman). 3. Das Original des Theropoden Spinosaurus aegyptiacus nov. gen. spec. Abh. Der Königlichen Bayer. Akad. Der Wiss. Math.-Phys. Kl. 1915, 28, 1–32. [Google Scholar]
- Andersson, M. Female choice selects for extreme tail length in a widowbird. Nature 1982, 299, 818–820. [Google Scholar] [CrossRef]
- Fiorillo, A.R.; Tykoski, R.S. A new Maastrichtian species of the centrosaurine ceratopsid Pachyrhinosaurus from the North Slope of Alaska. Acta Palaeontol. Pol. 2012, 57, 561–573. [Google Scholar] [CrossRef]
- Ryan, M.J.; Eberth, D.A.; Brinkman, D.; Currie, P.J.; Tanke, D.H. A new Pachyrhinosaurus-like ceratopsid from the upper Dinosaur park formation (Late Campanian) of Southern Alberta, Canada. In New Perspectives on Horned Dinosaurs: The Royal Tyrrell Museum Ceratopsian Symposium; Ryan, M.J., Chinnery-Allgeier, B.J., Eberth, D.A., Eds.; Indiana University Press: Bloomington, IN, USA, 2010; pp. 277–290. [Google Scholar]
- Sternberg, R.M. Pachyrhinosaurus canadensis, representing a new family of the Ceratopsia, from southern Alberta. Natl. Mus. Can. Bull. 1950, 118, 109–120. [Google Scholar]
- Hieronymus, T.L.; Witmer, L.M.; Tanke, D.H.; Currie, P.J. The facial integument of centrosaurine ceratopsids: Morphological and histological correlates of novel skin structures. Anat. Rec. 2009, 292, 1370–1396. [Google Scholar] [CrossRef]
- Sullivan, R.M. A taxonomic review of the Pachycephalosauridae (Dinosauria: Ornithischia). New Mex. Mus. Sci. Bull. 2006, 35, 347–365. [Google Scholar]
- Peterson, J.E.; Vittore, C.P. Cranial pathologies in a specimen of Pachycephalosaurus. PLoS ONE 2012, 7, e36227. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.E.; Dischler, C.; Longrich, N.R. Distributions of cranial pathologies provide evidence for head-butting in dome-headed dinosaurs (Pachycephalosauridae). PLoS ONE 2013, 8, e68620. [Google Scholar] [CrossRef]
- Farke, A.A.; Wolff, E.D.S.; Tanke, D.H. Evidence of combat in Triceratops. PLoS ONE 2009, 4, e4252. [Google Scholar] [CrossRef]
- Makovicky, P.J.; Norell, M.A. Troodontidae. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmolska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 184–195. [Google Scholar]
- Hartman, S.; Mortimer, M.; Wahl, W.R.; Lomax, D.R.; Lippincott, J.; Lovelace, D.M. A new paravian dinosaur from the Late Jurassic of North America supports a late acquisition of avian flight. PeerJ 2019, 7, e7247. [Google Scholar] [CrossRef]
- Averianov, A.; Sues, H.-D. A new troodontid (Dinosauria: Theropoda) from the Cenomanian of Uzbekstan, wth a review of troodontid records from the territories of the former Soviet Union. J. Vertebr. Paleontol. 2007, 27, 87–98. [Google Scholar] [CrossRef]
- Osborn, H.F. Three new Theropoda, Protoceratops zone. Am. Mus. Novit. 1924, 144, 1–12. [Google Scholar]
- Norell, M.A.; Makovicky, P.J.; Bever, G.S.; Balanoff, A.M.; Clark, J.M.; Barsbold, R.; Rowe, T. A review of the Mongolian cretaceous dinosaur Saurornithoides (Troodontidae: Theropoda). Am. Mus. Novit. 2009, 2009, 1–63. [Google Scholar] [CrossRef]
- Varricchio, D.J.; Hogan, J.D.; Gardner, J.D. Troodontid specimens from the Cretaceous Two Medicine Formation of Montana (USA) and the validity of Troodon formosus. J. Paleontol. 2025, 99, 219–240. [Google Scholar] [CrossRef]
- Russell, D.A. A new specimen of Stenonychosaurus from the Oldman Formation (Cretaceous) of Alberta. Can. J. Earth Sci. 1969, 6, 595–612. [Google Scholar] [CrossRef]
- van der Reest, A.J.; Currie, P.J. Troodontids (Theropoda) from the Dinosaur Park Formation, Alberta, with a description of a unique new taxon: Implications for deinonychosaur diversity in North America. Can. J. Earth Sci. 2017, 54, 919–935. [Google Scholar] [CrossRef]
- Sankey, J.T.; Brinkman, D.B.; Guenther, M.; Currie, P.J. Small theropod and bird teeth from the Late Cretaceous (Late Campanian) Judith River Group, Alberta. J. Paleontol. 2002, 76, 751–763. [Google Scholar]
- Longrich, N.R. Small theropod teeth from the Lance Formation of Wyoming. In The Unique Role of Vertebrate Microfossil Assemblages in Paleoecology and Paleobiology; Sankey, J.T., Baszio, S., Eds.; Indiana University Press: Bloomington, IN, USA, 2008; pp. 135–158. [Google Scholar]
- Carpenter, K. Baby dinosaurs from the Late Cretaceous Lance and Hell Creek formations and a description of a new species of theropod. Contrib. Geol. Univ. Wyo. 1982, 20, 123–134. [Google Scholar]
- Currie, P.J. Bird like characteristics of the jaws and teeth of troodontid theropods (Dinosauria:Saurischia). J. Vertebr. Paleontol. 1987, 7, 72–81. [Google Scholar] [CrossRef]
- Yun, C.-G. Jaw biomechanics of Troodontidae and their implications for the palaeobiology of this lineage of bird-like theropod dinosaurs. Lethaia 2025, 58, 1–12. [Google Scholar] [CrossRef]
- Holtz, T.R.; Brinkman, D.L.; Chandler, C.L. Denticle morphometrics and a possibly omnivorous feeding habit for the theropod dinosaur Troodon. Gaia 1998, 15, 159–166. [Google Scholar]
- Cullen, T.M.; Cousens, B.L. New biogeochemical insights into Mesozoic terrestrial paleoecology and evidence for omnivory in troodontid dinosaurs. Geol. Soc. Am. Bull. 2024, 136, 2689–2701. [Google Scholar]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; JHU Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Russell, D.A.; Seguin, R. Reconstruction of the small Cretaceous theropod Stenonychosaurus inequalis and a hypothetical dinosauroid. Syllogeus 1982, 37. [Google Scholar]
- Aguillón-Martínez, M.C.; Rivera-Sylva, H.E. A Troodontid (Theropoda: Troodontidae) Neurocranium from the Cerro del Pueblo Formation (Late Campanian) of Coahuila, Mexico. Paleontol. Mex. 2023, 12, 99–105. [Google Scholar]
- Soegaard, K.; Ye, H.; Halik, N.; Daniels, A.T.; Arney, J.; Garrick, S. Stratigraphic Evolution of Latest Cretaceous to Early Tertiary Difunta Foreland Basin in Northeast Mexico: Influence of Salt Withdrawal on Tectonically Induced Subsidence by the Sierra Madre Oriental Fold and Thrust Belt. 2003. Available online: https://archives.datapages.com/data/specpubs/memoir79/CHAPTER16/CHAPTER16.HTM (accessed on 10 December 2025).
- de Jesús, C.R.D.; Eberth, D.A.; de la Rosa, R.A.R.; Lerbekmo, J.F.; Brinkman, D.B.; Sampson, S.D. Cerro del Pueblo Fm (Difunta Group, Upper Cretaceous), Parras Basin, southern Coahuila, Mexico: Reference sections, age, and correlation. Rev. Mex. De Cienc. Geol. 2004, 21, 335–352. [Google Scholar]
- Vogt, M.; Stinnesbeck, W.; Zell, P.; Kober, B.; Kontny, J.; Herzer, N.; Frey, E.; Rivera-Sylva, H.E.; Gutierrez, J.M.P.; Amezcua, N. Age and depositional environment of the “dinosaur graveyard” at Las Águilas, southern Coahuila, NE Mexico. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 441, 758–769. [Google Scholar] [CrossRef]
- Vivas González, R. Paleoecología de Dinosaurios Hadrosauridos (Ornithischia: Ornithopoda) de la Formación Cerro del Pueblo (Cretácico Tardío: Campaniano), Coahuila, México; Universidad Autónoma de Nuevo León: San Nicolás de los Garza, Mexico, 2013. [Google Scholar]
- Stinnesbeck, W.; Frey, E.; Rivera-Sylva, H.; Carpenter, K. Paleogeography and paleoenvironment of Mexico during the Mesozoic. In Dinosaurs and Other Reptiles from the Mesozoic of Mexico; Indiana University Press: Bloomington, IN, USA, 2014; pp. 13–29. [Google Scholar]
- Wang, S.; Ding, N.; Tan, Q.; Yang, R.; Zhang, Q.; Tan, L. A new Urbacodon (Theropoda, Troodontidae) from the Upper Cretaceous Iren Dabasu Formation, China: Implications for troodontid phylogeny and tooth biology. Cladistics 2025, 41, 104–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Currie, P.; Pittman, M.; Xing, L.; Meng, Q.; Lü, J.; Hu, D.; Yu, C. Mosaic evolution in an asymmetrically feathered troodontid dinosaur with transitional features. Nat. Commun. 2017, 8, 14972. [Google Scholar] [CrossRef] [PubMed]
- Tsuihiji, T.; Barsbold, R.; Watabe, M.; Tsogtbaatar, K.; Chinzorig, T.; Fujiyama, Y.; Suzuki, S. An exquisitely preserved troodontid theropod with new information on the palatal structure from the Upper Cretaceous of Mongolia. Naturwissenschaften 2014, 101, 131–142. [Google Scholar] [CrossRef]
- Kubota, K.; Kobayashi, Y.; Ikeda, T. Early Cretaceous troodontine troodontid (Dinosauria: Theropoda) from the Ohyamashimo Formation of Japan reveals the early evolution of Troodontinae. Sci. Rep. 2024, 14, 16392. [Google Scholar] [CrossRef]
- Benton, M.J.; Hitchin, R. Congruence between phylogenetic and stratigraphic data on the history of life. Proc. Biol. Sci. 1997, 264, 885–890. [Google Scholar] [CrossRef]
- Ezcurra, M.D. Exploring the effects of weighting against homoplasy in genealogies of palaeontological phylogenetic matrices. Cladistics 2024, 40, 242–281. [Google Scholar] [CrossRef] [PubMed]
- Seeley, H.G. On the classification of the fossil animals commonly named Dinosauria. Proc. R. Soc. Lond. 1888, 43, 165–171. [Google Scholar] [CrossRef]
- Marsh, O.C. Classification of the Dinosauria. Am. J. Sci. 1881, 23, 81–86. [Google Scholar] [CrossRef]
- Paul, G.S. Predatory Dinosaurs of the World; Simon and Schuster: New York, NY, USA, 1988; p. 464. [Google Scholar]
- Huene, F.V. The dinosaurs not a natural order. Am. J. Sci. 1914, 38, 145–146. [Google Scholar] [CrossRef]
- Gauthier, J. Saurischian monophyly and the origin of birds. Mem. Calif. Acad. Sci. 1986, 8, 1–55. [Google Scholar]
- Colbert, E.; Russell, D.A. The small Cretaceous dinosaur Dromaeosaurus. Am. Mus. Novit. 1969, 2380, 1–49. [Google Scholar]
- Gilmore, C.W. On Troodon validus, an orthopodous dinosaur from the Belly River Cretaceous of Alberta, Canada. Alta. Univ. Bull. 1924, 1, 1–43. [Google Scholar]
- Evans, D.C.; Cullen, T.M.; Larson, D.W.; Rego, A. A new species of troodontid theropod (Dinosauria: Maniraptora) from the Horseshoe Canyon Formation (Maastrichtian) of Alberta, Canada. Can. J. Earth Sci. 2017, 54, 813–826. [Google Scholar] [CrossRef]
- Currie, P.J. Cranial anatomy of Stenonychosaurus inequalis (Saurischia, Theropoda) and its bearing on the origin of birds. Can. J. Earth Sci. 1985, 22, 1643–1658. [Google Scholar] [CrossRef]
- Evans, D.C.; Larson, D.W.; Cullen, T.M.; Sullivan, R.M. “Saurornitholestes” robustus is a troodontid (Dinosauria: Theropoda). Can. J. Earth Sci. 2014, 51, 730–734. [Google Scholar] [CrossRef]
- Sullivan, R.M. Saurornitholestes robustus, n. sp. (Theropoda: Dromaeosauridae) from the Upper Cretaceous Kirtland Formation (De-Na-Zin Member), San Juan Basin, New Mexico. N. M. Mus. Nat. Hist. Sci. Bull. 2006, 35, 253–256. [Google Scholar]
- Longrich, N.R. A new, large ornithomimid from the Dinosaur Park Formation of Alberta, Canada: Implications for the study of dissociated dinosaur remains. Palaeontology 2008, 51, 983–997. [Google Scholar] [CrossRef]
- Cullen, T.M.; Zanno, L.; Larson, D.W.; Todd, E.; Currie, P.J.; Evans, D.C. Anatomical, morphometric, and stratigraphic analyses of theropod biodiversity in the Upper Cretaceous (Campanian) Dinosaur Park Formation1. Can. J. Earth Sci. 2021, 58, 870–884. [Google Scholar] [CrossRef]
- Mallon, J.C.; Evans, D.C.; Ryan, M.J.; Anderson, J.S. Megaherbivorous dinosaur turnover in the Dinosaur Park Formation (upper Campanian) of Alberta, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 350–352, 124–138. [Google Scholar] [CrossRef]
- Zanno, L.E.; Varricchio, D.J.; O’Connor, P.M.; Titus, A.L.; Knell, M.J. A new troodontid theropod, Talos sampsoni gen. et sp. nov., from the Upper Cretaceous Western Interior Basin of North America. PLoS ONE 2011, 6, e24487. [Google Scholar] [CrossRef] [PubMed]
- Schott, R.K.; Evans, D.C.; Goodwin, M.B.; Horner, J.R.; Brown, C.M.; Longrich, N.R. Cranial Ontogeny in Stegoceras validum (Dinosauria: Pachycephalosauria): A Quantitative Model of Pachycephalosaur Dome Growth and Variation. PLoS ONE 2011, 6, e21092. [Google Scholar] [CrossRef] [PubMed]
- Longrich, N.R.; Field, D. Torosaurus is not Triceratops: Ontogeny in Chasmosaurine Ceratopsids as a Case Study in Dinosaur Taxonomy. PLoS ONE 2012, 7, e32623. [Google Scholar] [CrossRef]
- Plateau, O.; Foth, C. Common patterns of skull bone fusion and their potential to discriminate different ontogenetic stages in extant birds. Front. Ecol. Evol. 2021, 9, 737199. [Google Scholar] [CrossRef]
- Saitta, E.T.; Stockdale, M.T.; Longrich, N.R.; Bonhomme, V.; Benton, M.J.; Cuthill, I.C.; Makovicky, P.J. An effect size statistical framework for investigating sexual dimorphism in non-avian dinosaurs and other extinct taxa. Biol. J. Linn. Soc. 2020, 131, 231–273. [Google Scholar] [CrossRef]
- Mayr, G. A survey of casques, frontal humps, and other extravagant bony cranial protuberances in birds. Zoomorphology 2018, 137, 457–472. [Google Scholar] [CrossRef]
- Kinnaird, M.F.; Hadiprakarsa, Y.-Y.; Thiensongrusamee, P. Aerial jousting by helmeted hornbills Rhinoplax vigil: Observations from Indonesia and Thailand. Ibis 2003, 145, 506–508. [Google Scholar] [CrossRef]
- Surapaneni, V.A.; Flaum, B.; Schindler, M.; Hayat, K.; Wölfer, J.; Baum, D.; Hu, R.; Kong, T.F.; Doube, M.; Dean, M.N. The helmeted hornbill casque is reinforced by a bundle of exceptionally thick, rod-like trabeculae. Ann. N. Y. Acad. Sci. 2025, 1544, 78–91. [Google Scholar] [CrossRef]
- Allen, J.A. Ontogenetic and Other Variations in Muskoxen, with a Systematic Review of the Muskox Group, Recent and Extinct; Memoirs of the AMNH; new ser., v. 1, pt. 4; AMNH: New York, NY, USA, 1913. [Google Scholar]
- Hanitsch, R. On a Serow from Annam. J. Straits Branch R. Asiat. Soc. 1918, 78, 59–65. [Google Scholar]
- Ridewood, W. Some observations on the skull of the giraffe. Proc. Zool. Soc. Lond. 1904, 1, 150–157. [Google Scholar] [CrossRef]
- Drake, A.; Donahue, T.L.H.; Stansloski, M.; Fox, K.; Wheatley, B.B.; Donahue, S.W. Horn and horn core trabecular bone of bighorn sheep rams absorbs impact energy and reduces brain cavity accelerations during high impact ramming of the skull. Acta Biomater. 2016, 44, 41–50. [Google Scholar] [CrossRef]
- Cau, A.; Dalla Vecchia, F.M.; Fabbri, M. A thick-skulled theropod (Dinosauria, Saurischia) from the Upper Cretaceous of Morocco with implications for carcharodontosaurid cranial evolution. Cretac. Res. 2013, 40, 251–260. [Google Scholar] [CrossRef]
- Farlow, J.O.; Dodson, P. The behavioral significance of frill and horn morphology in ceratopsian dinosaurs. Evolution 1975, 29, 353–361. [Google Scholar] [CrossRef]
- Cruickshank, A.R.I. On a specimen of the anomodont reptile Kannemeyeria latifrons (Broom) from the Manda Formation of Tanganyika, Tanzania. Proc. Linn. Soc. Lond. 1965, 176, 149–157. [Google Scholar] [CrossRef]
- Benoit, J.; Manger, P.R.; Norton, L.; Fernandez, V.; Rubidge, B.S. Synchrotron scanning reveals the palaeoneurology of the head-butting Moschops capensis (Therapsida, Dinocephalia). PeerJ 2017, 5, e3496. [Google Scholar] [CrossRef] [PubMed]
- Livezey, B.C.; Humphrey, P.S. Sexual dimorphism in continental steamer-ducks. Condor 1984, 86, 368–377. [Google Scholar] [CrossRef]
- Longrich, N.R.; Saitta, E.T. Taxonomic status of Nanotyrannus lancensis (Dinosauria: Tyrannosauroidea)—A distinct taxon of small-bodied tyrannosaur. Foss. Stud. 2024, 2, 1–65. [Google Scholar] [CrossRef]
- Voris, J.T.; Therrien, F.; Zelenitsky, D.K.; Brown, C.M. A new tyrannosaurine (Theropoda: Tyrannosauridae) from the Campanian Foremost Formation of Alberta, Canada, provides insight into the evolution and biogeography of tyrannosaurids. Cretac. Res. 2020, 110, 104388. [Google Scholar] [CrossRef]
- Dalman, S.G.; Loewen, M.A.; Pyron, R.A.; Jasinski, S.E.; Malinzak, D.E.; Lucas, S.G.; Fiorillo, A.R.; Currie, P.J.; Longrich, N.R. A giant tyrannosaur from the Campanian–Maastrichtian of southern North America and the evolution of tyrannosaurid gigantism. Sci. Rep. 2024, 14, 22124. [Google Scholar] [CrossRef]
- Brown, C.M.; Currie, P.J.; Therrien, F. Intraspecific facial bite marks in tyrannosaurids provide insight into sexual maturity and evolution of bird-like intersexual display. Paleobiology 2021, 48, 12–43. [Google Scholar] [CrossRef]
- Evans, D.C.; Ridgely, R.; Witmer, L.M. Endocranial anatomy of lambeosaurine hadrosaurids (Dinosauria: Ornithischia): A sensorineural perspective on cranial crest function. Anat. Rec. 2009, 292, 1315–1337. [Google Scholar] [CrossRef] [PubMed]
- Galton, P.M. Pachycephalosaurids: Dinosaurian battering rams. Discovery 1970, 6, 22–32. [Google Scholar]
- Longrich, N.R.; Sankey, J.T.; Tanke, D.H. Texacephale langstoni, a new genus of pachycephalosaurid (Dinosauria: Ornithischia) from the upper Campanian Aguja Formation, southern Texas, USA. Cretac. Res. 2010, 31, 274–284. [Google Scholar] [CrossRef]
- Rowe, T. A new species of the theropod dinosaur Syntarsus from the Early Jurassic Kayenta Formation of Arizona. J. Vertebr. Paleontol. 1989, 9, 125–136. [Google Scholar] [CrossRef]
- Hammer, W.R.; Hickerson, W.J. A crested theropod dinosaur from Antarctica. Science 1994, 264, 828–830. [Google Scholar] [CrossRef]
- Evans, D.C. Cranial anatomy and systematics of Hypacrosaurus altispinus, and a comparative analysis of skull growth in lambeosaurine hadrosaurids (Dinosauria: Ornithischia). Zool. J. Linn. Soc. 2010, 159, 398–434. [Google Scholar] [CrossRef]
- Gates, T.A.; Evans, D.C.; Sertich, J.J. Description and rediagnosis of the crested hadrosaurid (Ornithopoda) dinosaur Parasaurolophus cyrtocristatus on the basis of new cranial remains. PeerJ 2021, 9, e10669. [Google Scholar] [CrossRef]
- Prieto-Marquez, A. New information on the cranium of Brachylophosaurus canadensis (Dinosauria, Hadrosauridae), with a revision of its phylogenetic position. J. Vertebr. Paleontol. 2005, 25, 144–156. [Google Scholar] [CrossRef]
- Stovall, J.W.; Langston, W. Acrocanthosaurus atokensis, a new genus and species of Lower Cretaceous Theropoda from Oklahoma. Am. Midl. Nat. 1950, 43, 696–728. [Google Scholar] [CrossRef]
- Salgado, L.; Bonaparte, J. A new dicraeosaurid sauropod, Amargasaurus cazaui gen. et. sp. nov., from the La Amarga Formation, Neocomian of Neuquen Province, Argentina. Ameghiniana 1991, 28, 333–346. [Google Scholar]
- Taquet, P. Géologie et Paléontologie du Gisement de Gadoufaoua (Aptien du Niger); Editions du Centre National de la Recherque Scientifique: Paris, France, 1976; 191p. [Google Scholar]
- Prieto-Márquez, A.; Chiappe, L.M.; Joshi, S.H. The lambeosaurine dinosaur Magnapaulia laticaudus from the Late Cretaceous of Baja California, Northwestern Mexico. PLoS ONE 2012, 7, e38207. [Google Scholar] [CrossRef]
- Lü, J.; Li, G.; Kundrát, M.; Lee, Y.-N.; Sun, Z.; Kobayashi, Y.; Shen, C.; Teng, F.; Liu, H. High diversity of the Ganzhou Oviraptorid Fauna increased by a new “cassowary-like” crested species. Sci. Rep. 2017, 7, 6393. [Google Scholar] [CrossRef]
- Norell, M.A.; Clark, J.M.; Weintraub, R.; Chiappe, L.M.; Demberelyin, D. A nesting dinosaur. Nature 1995, 278, 247–248. [Google Scholar] [CrossRef]
- Clark, J.M.; Norell, M.A.; Chiappe, L.M. An oviraptorid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avianlike brooding position over an oviraptorid nest. Am. Mus. Novit. 1999, 3265, 1–36. [Google Scholar]
- Eberth, D.A.; Getty, M.A. Ceratopsian bonebeds: Occurrence, origins, and significance. In Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed; Currie, P.J., Koppelhus, E.B., Eds.; Indiana University Press: Bloomington, IN, USA, 2005; pp. 501–536. [Google Scholar]
- Funston, G.F.; Currie, P.J.; Eberth, D.A.; Ryan, M.J.; Chinzorig, T.; Badamgarav, D.; Longrich, N.R. The first oviraptorosaur (Dinosauria: Theropoda) bonebed: Evidence of gregarious behaviour in a maniraptoran theropod. Sci. Rep. 2016, 6, 35782. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Lu, J.-C. A new ornithomimid dinosaur with gregarious habits from the Late Cretaceous of China. Acta Palaeontol. Pol. 2003, 48, 235–259. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Rivera-Sylva, H.E.; Aguillón-Martinez, M.C.; Flores-Ventura, J.; Sánchez-Uribe, I.E.; Guzman-Gutierrez, J.R.; Longrich, N.R. A Thick-Skulled Troodontid Theropod from the Late Cretaceous of Mexico. Diversity 2026, 18, 38. https://doi.org/10.3390/d18010038
Rivera-Sylva HE, Aguillón-Martinez MC, Flores-Ventura J, Sánchez-Uribe IE, Guzman-Gutierrez JR, Longrich NR. A Thick-Skulled Troodontid Theropod from the Late Cretaceous of Mexico. Diversity. 2026; 18(1):38. https://doi.org/10.3390/d18010038
Chicago/Turabian StyleRivera-Sylva, Hector E., Martha C. Aguillón-Martinez, Jose Flores-Ventura, Ivan E. Sánchez-Uribe, Jose Ruben Guzman-Gutierrez, and Nicholas R. Longrich. 2026. "A Thick-Skulled Troodontid Theropod from the Late Cretaceous of Mexico" Diversity 18, no. 1: 38. https://doi.org/10.3390/d18010038
APA StyleRivera-Sylva, H. E., Aguillón-Martinez, M. C., Flores-Ventura, J., Sánchez-Uribe, I. E., Guzman-Gutierrez, J. R., & Longrich, N. R. (2026). A Thick-Skulled Troodontid Theropod from the Late Cretaceous of Mexico. Diversity, 18(1), 38. https://doi.org/10.3390/d18010038






