In-Vivo Characterization of Healthy Retinal Pigment Epithelium and Photoreceptor Cells from AO-(T)FI Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study
2.1.1. Design
2.1.2. Recruitment
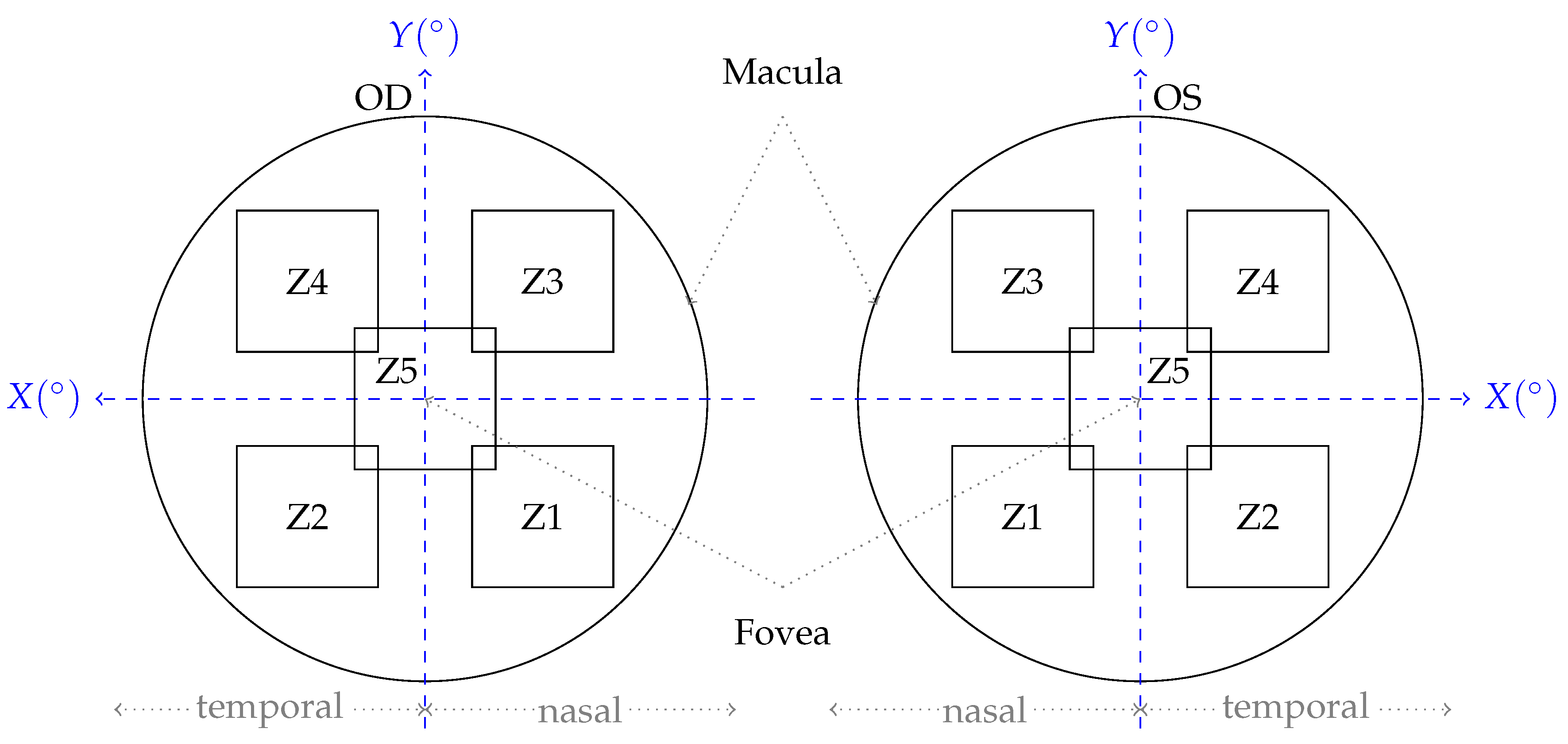
2.1.3. Ocular Examination and Image Acquisition
2.2. Image Analysis and Quantification
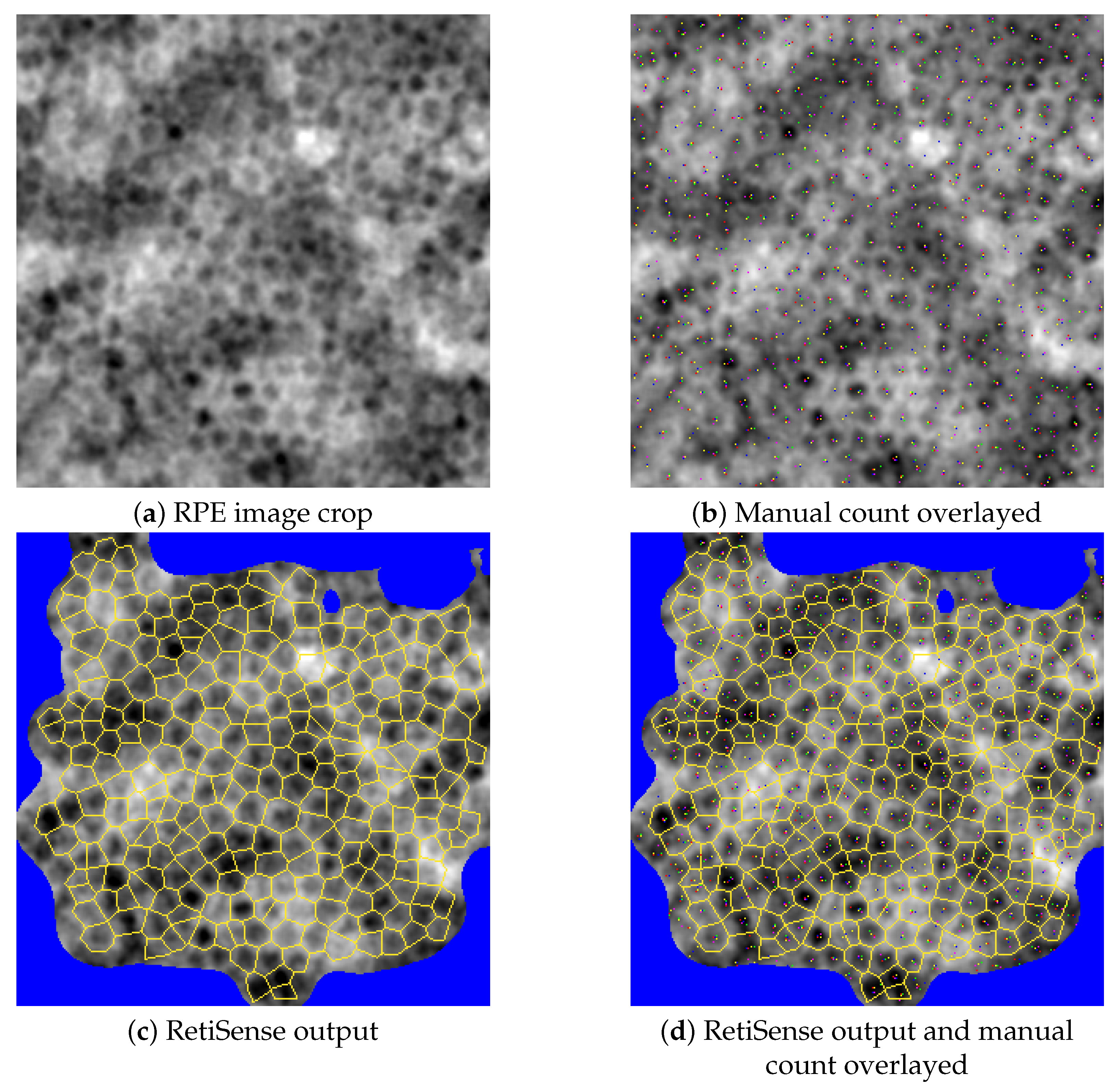
2.2.1. The RetiSense Software Package
2.2.2. Manual Validation of Cell Counting
2.2.3. Analysis of the “CEL01LUKS-Normative” Set
3. Results
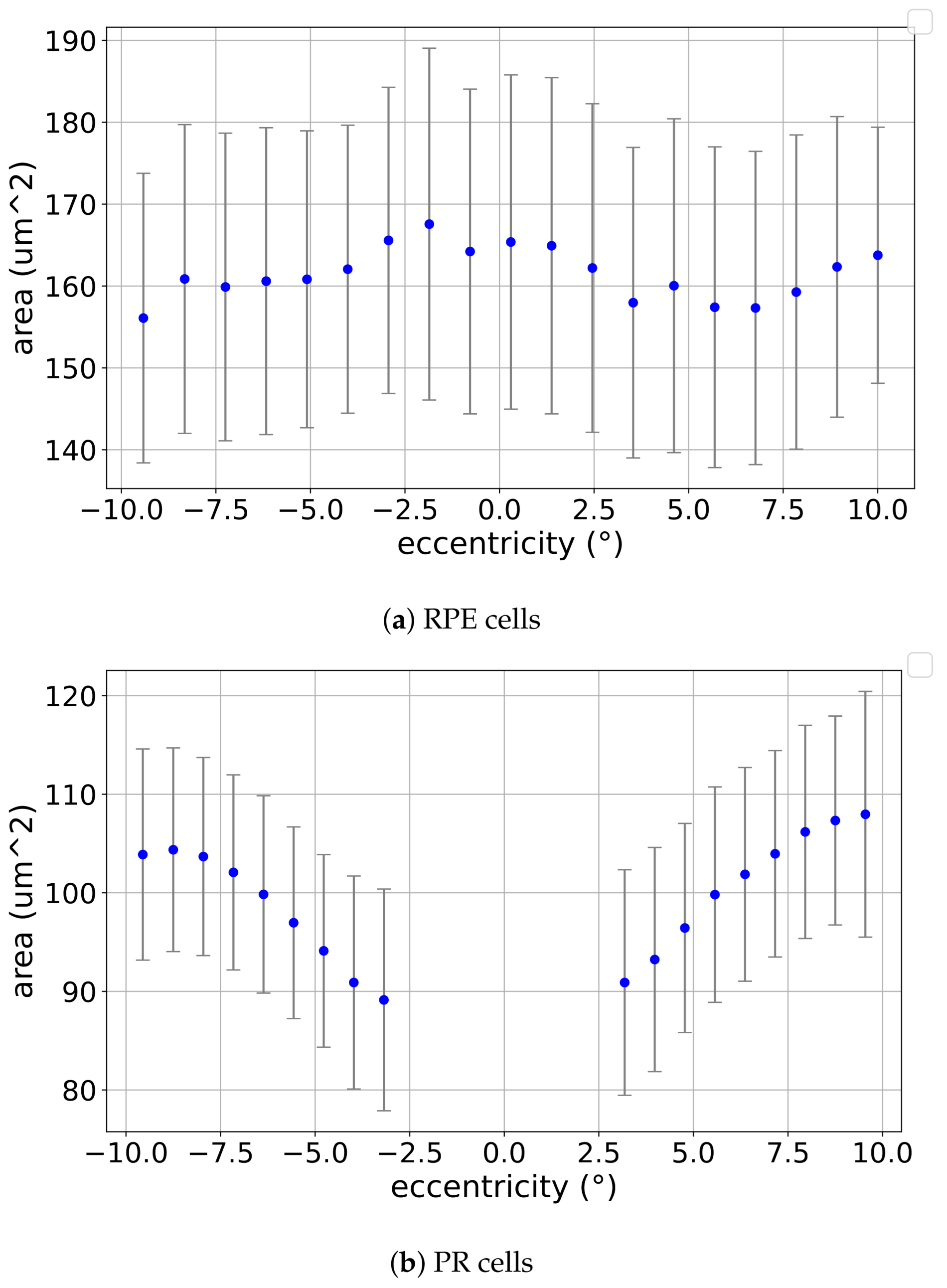
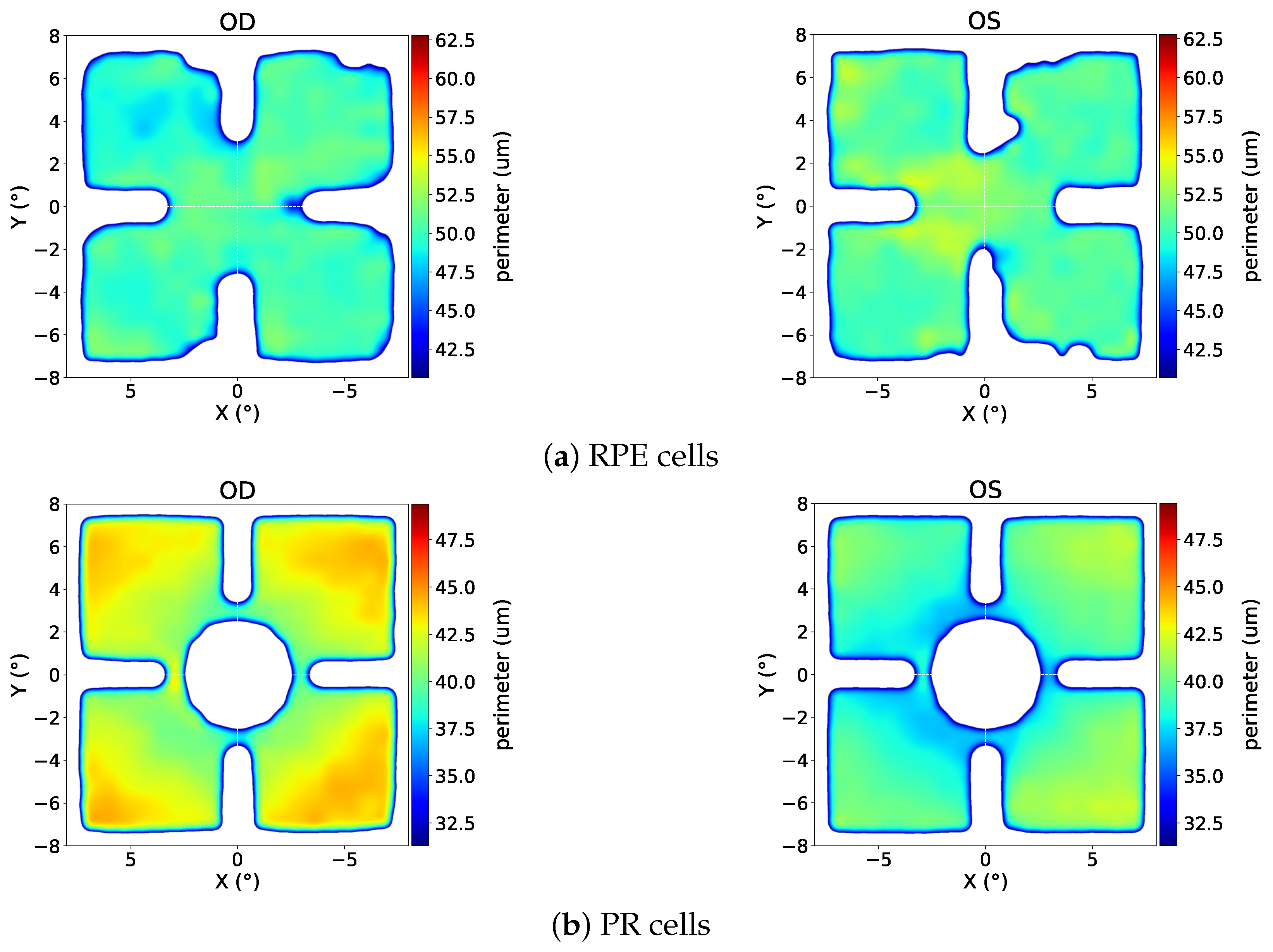
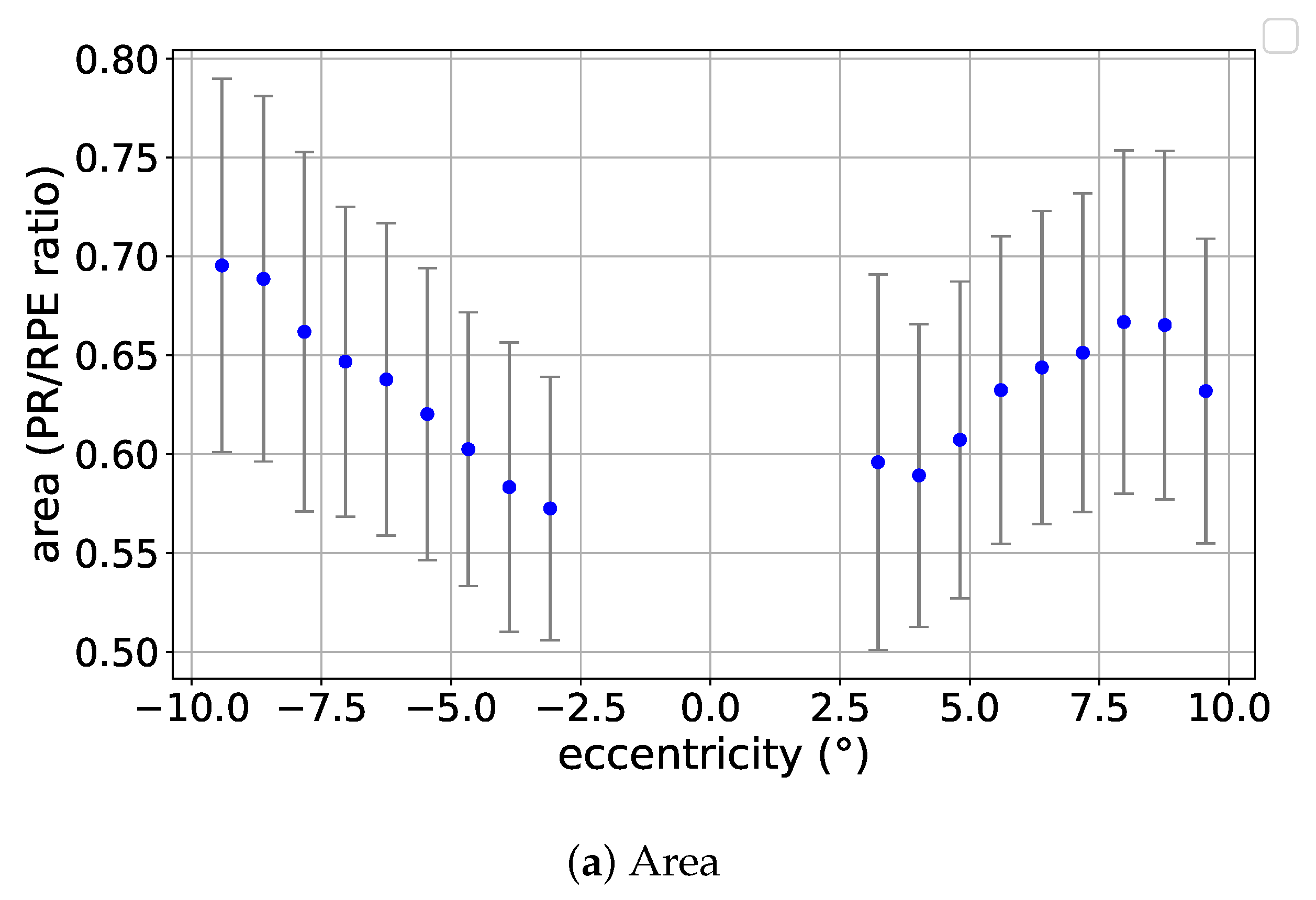
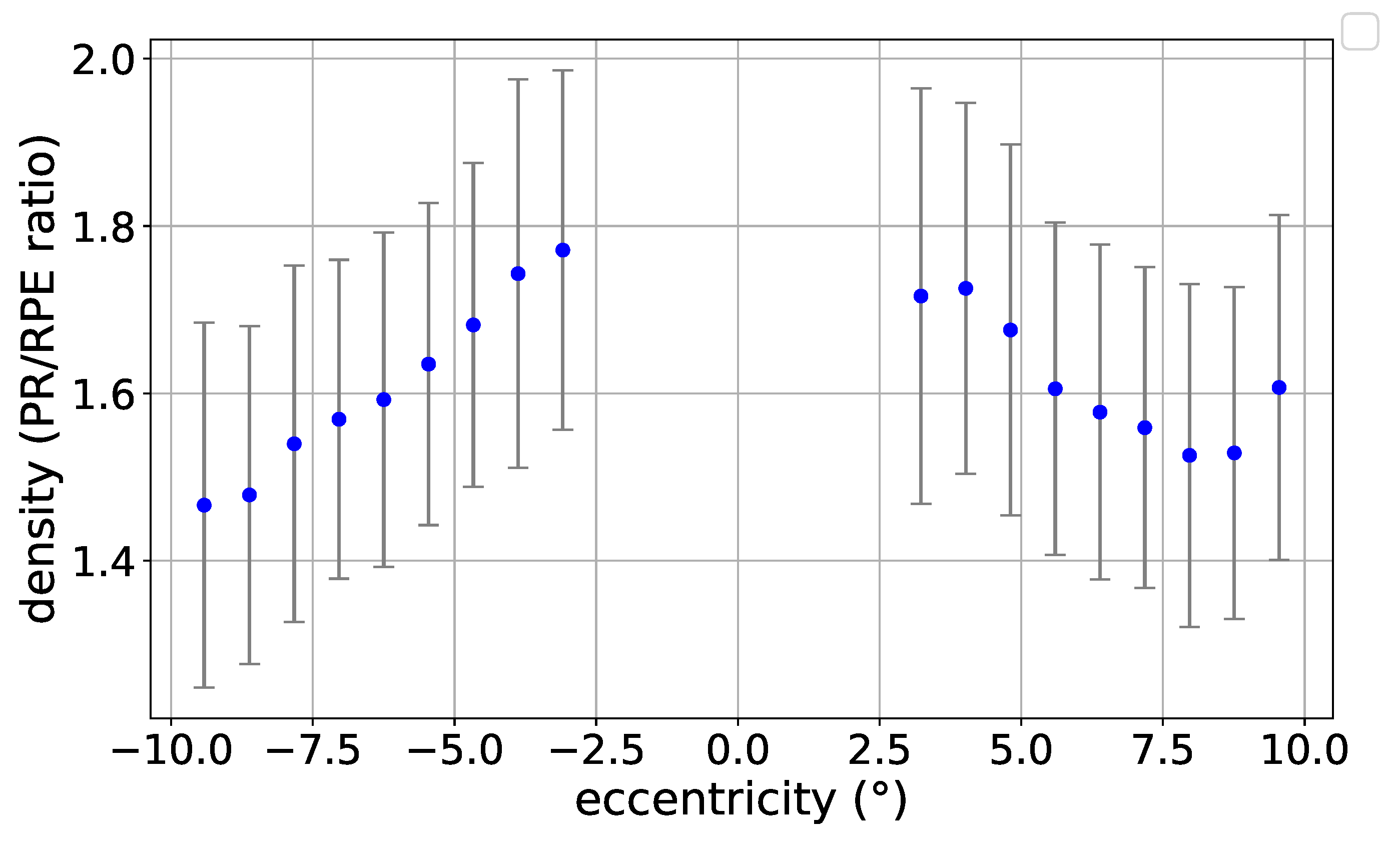
3.1. Eccentricity Profile
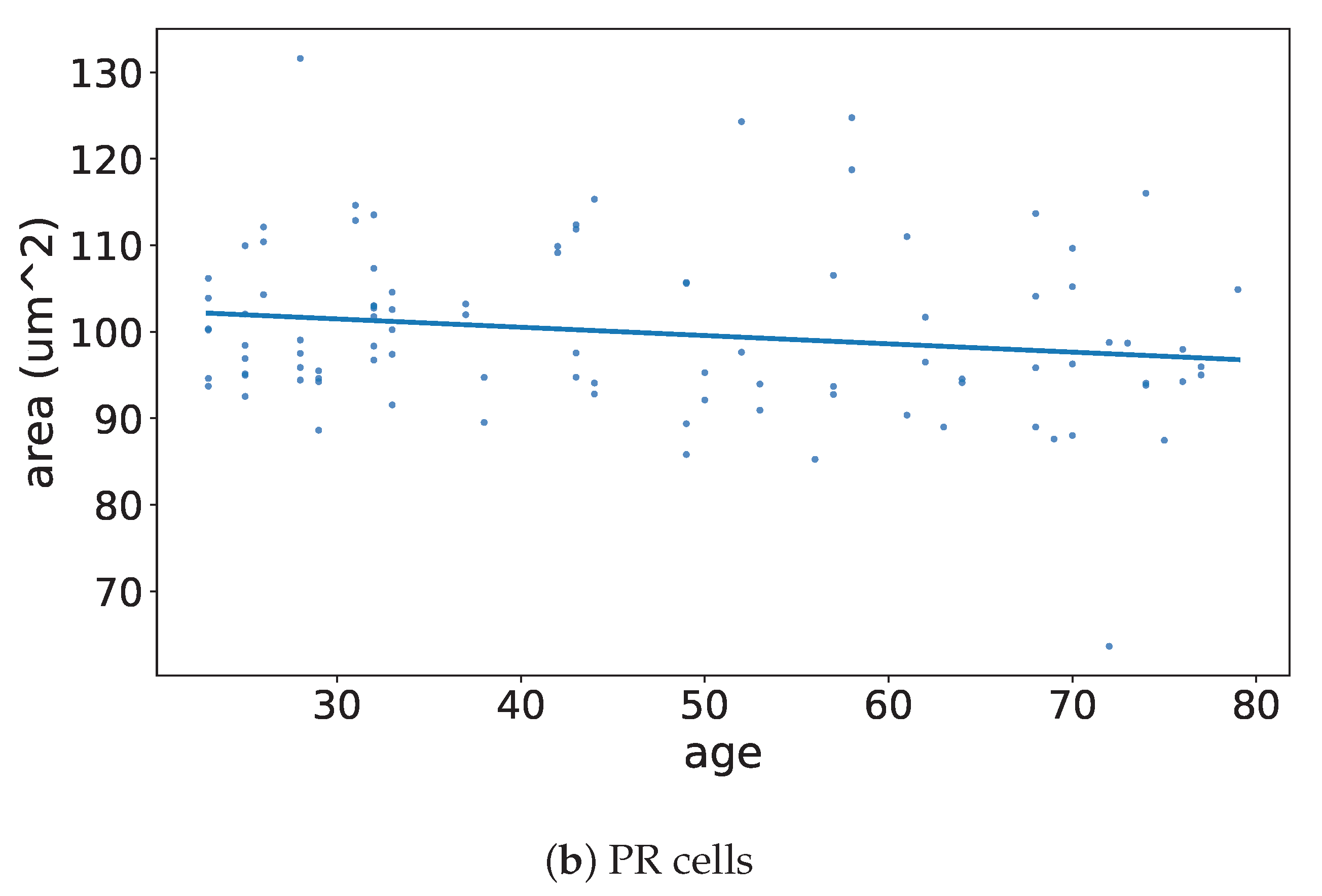
3.2. Age Profile
3.3. Correlation with Independent Variables
4. Discussion
4.1. Results in the Context of the Literature
4.2. Technological Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RPE | Retinal Pigment Epithelium |
| PR | Photoreceptor |
| AO | Adaptive Optics |
| AO-FI | Adaptive Optics Flood Illumination |
| AO-TFI | Adaptive Optics Transscleral Flood Illumination |
| AMD | Age-Related Macular degeneration |
| DR | Diabetic Retinopathy |
| ML | Machine Learning |
| OCT | Optical Coherence Tomography |
Appendix A









References
- Wang, S.; Li, W.; Chen, M.; Cao, Y.; Lu, W.; Li, X. The retinal pigment epithelium: Functions and roles in ocular diseases. Fundam. Res. 2023, 4, 1710–1718. [Google Scholar] [CrossRef]
- Storm, T.; Burgoyne, T.; Futter, C. Membrane trafficking in the retinal pigment epithelium at a glance. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Hurley, J. Retina Metabolism and Metabolism in the Pigmented Epithelium: A Busy Intersection. Annu. Rev. Vis. Sci. 2021, 7, 665–692. [Google Scholar] [CrossRef]
- Taylor, A.W.; Hsu, S.X.; Ng, T. The Role of Retinal Pigment Epithelial Cells in Regulation of Macrophages/Microglial Cells in Retinal Immunobiology. Front. Immunol. 2021, 12, 724601. [Google Scholar] [CrossRef]
- Amram, B.; Cohen-Tayar, Y.; David, A.; Ashery-Padan, R. The retinal pigmented epithelium – from basic developmental biology research to translational approaches. Int. J. Dev. Biol. 2017, 61, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Cuenca, N.; Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Kutsyr, O.; Albertos-Arranz, H.; Fernández-Sánchez, L.; Martínez-Gil, N.; Noailles, A.; López-Garrido, J.A.; López-Gálvez, M.; et al. Interpretation of OCT and OCTA images from a histological approach: Clinical and experimental implications. Prog. Retin. Eye Res. 2020, 77, 100828. [Google Scholar] [CrossRef] [PubMed]
- Somasundaran, S.; Constable, I.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Zhou, M.; Geathers, J.S.; Grillo, S.; Weber, S.R.; Wang, W.; Zhao, Y.; Sundstrom, J. Role of Epithelial–Mesenchymal Transition in Retinal Pigment Epithelium Dysfunction. Front. Cell Dev. Biol. 2020, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.C.; Ma, J.Y.; Jobling, A.; Brandli, A.; Greferath, U.; Fletcher, E.; Vessey, K.A. Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front. Neurosci. 2022, 16, 1009599. [Google Scholar] [CrossRef]
- Hoy, S.M. Pegcetacoplan: First Approval. Drugs 2021, 81, 1423–1430. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.S.; Metlapally, R.; Joshi, P. Pegcetacoplan treatment for geographic atrophy due to age-related macular degeneration: a plain language summary of the FILLY study. Immunotherapy 2022, 14, 995–1006. [Google Scholar] [CrossRef]
- Diniz, B.; Ribeiro, R.; Heussen, F.M.; Maia, M.; Sadda, S. Drusen measurements comparison by fundus photograph manual delineation versus optical coherence tomography retinal pigment epithelial segmentation automated analysis. Retina 2014, 34, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 369–390. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Regatieri, C.V.; Branchini, L.; Duker, J.S. The Role of Spectral-Domain OCT in the Diagnosis and Management of Neovascular Age-Related Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2011, 42, S56–S66. [Google Scholar] [CrossRef]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Klimscha, S.; Waldstein, S.M.; Bogunović, H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 2017, 31, 26–44. [Google Scholar] [CrossRef]
- Spaide, R.F. Improving the age-related macular degeneration construct: A New Classification System. Retina 2018, 38, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Peng, L.; Varadarajan, A.V.; Keane, P.A.; Burlina, P.M.; Chiang, M.F.; Schmetterer, L.; Pasquale, L.R.; Bressler, N.M.; Webster, D.R.; et al. Deep learning in ophthalmology: The technical and clinical considerations. Prog. Retin. Eye Res. 2019, 72, 100759. [Google Scholar] [CrossRef] [PubMed]
- Turgut, B. Past and Present Terminology for the Retinal and Choroidal Structures in Optical Coherence Tomography. Eur. Ophthalmic Rev. 2017, 11, 59–61. [Google Scholar] [CrossRef]
- Morgan, J.I.W.; Chui, T.Y.P.; Grieve, K. Twenty-five years of clinical applications using adaptive optics ophthalmoscopy [Invited]. Biomed. Opt. Express 2023, 14, 387–428. [Google Scholar] [CrossRef]
- Akyol, E.; Hagag, A.M.; Sivaprasad, S.; Lotery, A.J. Adaptive optics: Principles and applications in ophthalmology. Eye 2021, 35, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.A.; Elsner, A.E.; Sapoznik, K.A.; Warner, R.L.; Gast, T.J. Adaptive Optics Imaging of the Human Retina. Prog. Retin. Eye Res. 2019, 68, 1–30. [Google Scholar] [CrossRef]
- Liu, T.; Jung, H.; Liu, J.; Droettboom, M.; Tam, J. Noninvasive near infrared autofluorescence imaging of retinal pigment epithelial cells in the human retina using adaptive optics. Biomed. Opt. Express 2017, 8, 4348–4360. [Google Scholar] [CrossRef]
- Rossi, E.A.; Norberg, N.; Eandi, C.; Chaumette, C.; Kapoor, S.; Le, L.; Snyder, V.C.; Martel, J.N.; Gautier, J.; Gocho, K.; et al. A New Method for Visualizing Drusen and Their Progression in Flood-Illumination Adaptive Optics Ophthalmoscopy. Transl. Vis. Sci. Technol. 2021, 10, 19. [Google Scholar] [CrossRef]
- Laforest, T.; Künzi, M.; Kowalczuk, L.; Carpentras, D.; Behar-Cohen, F.; Moser, C. Transscleral optical phase imaging of the human retina. Nat. Photonics 2020, 14, 439–445. [Google Scholar] [CrossRef]
- Ach, T.; Huisingh, C.; McGwin, G.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Smith, R.T.; Sloan, K.R.; et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4832–4841. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Rashid, A.; Chrenek, M.A.; Zhang, Q.; Bruce, B.B.; Klein, M.; Boatright, J.H.; Jiang, Y.; Grossniklaus, H.E.; Nickerson, J.M. Analysis of RPE morphometry in human eyes. Mol. Vis. 2016, 22, 898. [Google Scholar]
- Ortolan, D.; Sharma, R.; Volkov, A.; Maminishkis, A.; Hotaling, N.A.; Huryn, L.A.; Cukras, C.; Di Marco, S.; Bisti, S.; Bharti, K. Single-cell resolution map of human retinal pigment epithelium helps discover subpopulations with differential disease sensitivity. Proc. Natl. Acad. Sci. USA 2022, 119, e2117553119. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A 1997, 14, 2884–2892. [Google Scholar] [CrossRef]
- Roorda, A.; Williams, D.R. The arrangement of the three cone classes in the living human eye. Nature 1999, 397, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.K.; Jian, Y.; Cua, M.; Bonora, S.; Zawadzki, R.J.; Sarunic, M.V. In vivo imaging of human photoreceptor mosaic with wavefront sensorless adaptive optics optical coherence tomography. Biomed. Opt. Express 2015, 6, 580–590. [Google Scholar] [CrossRef]
- Heitkotter, H.; Patterson, E.J.; Woertz, E.N.; Cava, J.A.; Gaffney, M.; Adhan, I.; Tam, J.; Cooper, R.F.; Carroll, J. Extracting spacing-derived estimates of rod density in healthy retinae. Biomed. Opt. Express 2022, 14, 1–17. [Google Scholar] [CrossRef]
- Wooning, S.; Heutinck, P.A.T.; Liman, K.; Hennekam, S.; van Haute, M.; van den Broeck, F.; Leroy, B.; Sampson, D.M.; Roshandel, D.; Chen, F.K.; et al. Automated Cone Photoreceptor Detection in Adaptive Optics Flood Illumination Ophthalmoscopy. Ophthalmol. Sci. 2025, 5, 100675. [Google Scholar] [CrossRef]
- Vienola, K.V.; Zhang, M.; Snyder, V.C.; Sahel, J.A.; Dansingani, K.K.; Rossi, E.A. Microstructure of the retinal pigment epithelium near-infrared autofluorescence in healthy young eyes and in patients with AMD. Sci. Rep. 2020, 10, 9561. [Google Scholar] [CrossRef]
- Kowalczuk, L.; Dornier, R.; Künzi, M.; Iskandar, A.; Misutkova, Z.; Gryczka, A.; Navarro, A.; Jeunet, F.; Mantel, I.; Behar-Cohen, F.; et al. In Vivo Retinal Pigment Epithelium Imaging using Transscleral Optical Imaging in Healthy Eyes. Ophthalmol. Sci. 2023, 3, 100234. [Google Scholar] [CrossRef]
- Granger, C.E.; Yang, Q.; Song, H.; Saito, K.; Nozato, K.; Latchney, L.R.; Leonard, B.T.; Chung, M.M.; Williams, D.R.; Rossi, E.A. Human Retinal Pigment Epithelium: In Vivo Cell Morphometry, Multispectral Autofluorescence, and Relationship to Cone Mosaic. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5705. [Google Scholar] [CrossRef]
- Liu, Z.; Aghayee, S.; Soltanian-Zadeh, S.; Kovalick, K.; Agrawal, A.; Saeedi, O.; Cukras, C.; Chew, E.Y.; Farsiu, S.; Hammer, D.X. Quantification of Human Photoreceptor–Retinal Pigment Epithelium Macular Topography with Adaptive Optics–Optical Coherence Tomography. Diagnostics 2024, 14, 1518. [Google Scholar] [CrossRef] [PubMed]
- ISO 14155:2020; Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice. International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/71690.html (accessed on 10 July 2025).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.G.; Rudnicka, A.R.; Edgar, D.F. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefe’s Arch. Clin. Exp. Ophthalmol. 1994, 232, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.; Millican, C.L.; Allen, K.; Kalina, R. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Gill, J.S.; Moosajee, M.; Dubis, A.M. Cellular imaging of inherited retinal diseases using adaptive optics. Eye 2019, 33, 1683–1698. [Google Scholar] [CrossRef]
- Godara, P.; Dubis, A.M.; Roorda, A.; Duncan, J.L.; Carroll, J. Adaptive optics retinal imaging: Emerging clinical applications. Optom. Vis. Sci. 2010, 87, 930–941. [Google Scholar] [CrossRef]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal photoreceptor density decreases with age. Ophthalmology 1995, 102, 1853–1859. [Google Scholar] [CrossRef]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am. J. Ophthalmol. 1996, 121, 181–189. [Google Scholar] [CrossRef]
- Baraas, R.C.; Pedersen, H.R.; Knoblauch, K.; Gilson, S.J. Human foveal cone and RPE cell topographies and their correspondence with foveal shape. Investig. Ophthalmol. Vis. Sci. 2022, 63, 8. [Google Scholar] [CrossRef]
- Roshandel, D.; Sampson, D.M.; Mackey, D.A.; Chen, F.K. Impact of reference center choice on adaptive optics imaging cone mosaic analysis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.A.; Dubis, A.M.; Cooper, R.F.; Summerfelt, P.; Dubra, A.; Carroll, J. Assessing the spatial relationship between fixation and foveal specializations. Vis. Res. 2017, 132, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Heitkotter, H.; Salmon, A.; Linderman, R.; Porter, J.; Carroll, J. Theoretical versus empirical measures of retinal magnification for scaling AOSLO images. J. Opt. Soc. Am. A 2021, 38, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Dutt, D.D.; Yazar, S.; Charng, J.; Mackey, D.A.; Chen, F.K.; Sampson, D.M. Correcting magnification error in foveal avascular zone area measurements of optical coherence tomography angiography images with estimated axial length. Eye Vis. 2022, 9, 29. [Google Scholar] [CrossRef]








| Female | Male | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants | Eyes | RPE | PR | Age (Mean) | Age (Std) | Participants | Eyes | RPE | PR | Age (Mean) | Age (Std) | Participants | Eyes | RPE | PR | Age (Mean) | Age (Std) | |
| CEL01LUKS-normative | 58 | 100 | 582 | 492 | 51.8 | 19.1 | 46 | 72 | 447 | 354 | 58.5 | 17.2 | 104 | 172 | 1029 | 846 | 54.8 | 18.5 |
| CEL01LUKS-normative-mosaic | 45 | 76 | 296 | 217 | 47.2 | 17.67 | 37 | 51 | 204 | 148 | 58.7 | 16.97 | 82 | 127 | 500 | 365 | 52.39 | 18.19 |
| CEL01LUKS-normative-mosaic (filtered) | 42 | 71 | 222 | 217 | 45.79 | 17.32 | 33 | 47 | 121 | 148 | 58.55 | 16.73 | 75 | 118 | 343 | 365 | 51.40 | 18.11 |
| Female | Male | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Std | 25% | 75% | Mean | Median | Std | 25% | 75% | Mean | Median | Std | 25% | 75% | |
| local density (mm−2) | 6488.09 | 6422.58 | 741.80 | 5995.38 | 6947.01 | 5886.64 | 5888.19 | 611.53 | 5447.63 | 6281.45 | 6313.56 | 6257.57 | 757.38 | 5823.85 | 6765.04 |
| number of neighbors | 5.99 | 5.99 | 0.02 | 5.98 | 6.00 | 5.99 | 5.99 | 0.02 | 5.98 | 6.00 | 5.99 | 5.99 | 0.02 | 5.98 | 6.00 |
| area (m2) | 156.14 | 155.70 | 17.90 | 143.95 | 166.80 | 171.72 | 169.83 | 17.99 | 159.20 | 183.57 | 160.67 | 159.81 | 19.27 | 147.82 | 171.71 |
| perimeter (m) | 49.18 | 49.19 | 2.83 | 47.30 | 50.88 | 51.46 | 51.22 | 2.71 | 49.56 | 53.37 | 49.85 | 49.77 | 2.98 | 47.92 | 51.62 |
| Female | Male | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Std | 25% | 75% | Mean | Median | Std | 25% | 75% | Mean | Median | Std | 25% | 75% | |
| local density (mm−2) | 10,323.61 | 10,261.17 | 1214.41 | 9496.71 | 11,033.76 | 10,024.69 | 9919.62 | 1340.54 | 9077.56 | 10,836.12 | 10,207.21 | 10,151.19 | 1273.39 | 9332.74 | 10,969.40 |
| number of neighbors | 5.99 | 5.99 | 0.02 | 5.98 | 6.00 | 5.98 | 5.99 | 0.02 | 5.97 | 6.00 | 5.99 | 5.99 | 0.02 | 5.98 | 6.00 |
| area (m2) | 98.20 | 97.45 | 11.54 | 90.63 | 105.30 | 101.53 | 100.81 | 13.69 | 92.28 | 110.16 | 99.50 | 98.51 | 12.53 | 91.16 | 107.15 |
| perimeter (m) | 38.89 | 38.77 | 2.33 | 37.37 | 40.35 | 39.56 | 39.46 | 2.66 | 37.78 | 41.29 | 39.15 | 39.02 | 2.49 | 37.52 | 40.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdowsi, S.; Eppenberger, L.S.; Mohanna, S.; Pfäffli, O.; Amstutz, C.; Bachmann, L.M.; Thiel, M.A.; Schmid, M.K. In-Vivo Characterization of Healthy Retinal Pigment Epithelium and Photoreceptor Cells from AO-(T)FI Imaging. Vision 2025, 9, 91. https://doi.org/10.3390/vision9040091
Ferdowsi S, Eppenberger LS, Mohanna S, Pfäffli O, Amstutz C, Bachmann LM, Thiel MA, Schmid MK. In-Vivo Characterization of Healthy Retinal Pigment Epithelium and Photoreceptor Cells from AO-(T)FI Imaging. Vision. 2025; 9(4):91. https://doi.org/10.3390/vision9040091
Chicago/Turabian StyleFerdowsi, Sohrab, Leila Sara Eppenberger, Safa Mohanna, Oliver Pfäffli, Christoph Amstutz, Lucas M. Bachmann, Michael A. Thiel, and Martin K. Schmid. 2025. "In-Vivo Characterization of Healthy Retinal Pigment Epithelium and Photoreceptor Cells from AO-(T)FI Imaging" Vision 9, no. 4: 91. https://doi.org/10.3390/vision9040091
APA StyleFerdowsi, S., Eppenberger, L. S., Mohanna, S., Pfäffli, O., Amstutz, C., Bachmann, L. M., Thiel, M. A., & Schmid, M. K. (2025). In-Vivo Characterization of Healthy Retinal Pigment Epithelium and Photoreceptor Cells from AO-(T)FI Imaging. Vision, 9(4), 91. https://doi.org/10.3390/vision9040091





