Immunogenicity and Integration of a Decellularized Extracellular Matrix-Based Scaffold for the Reconstruction of Human Foreskin: A Preclinical Animal Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Decellularization Protocol
2.3. Characteristics of Decellularized Foreskin
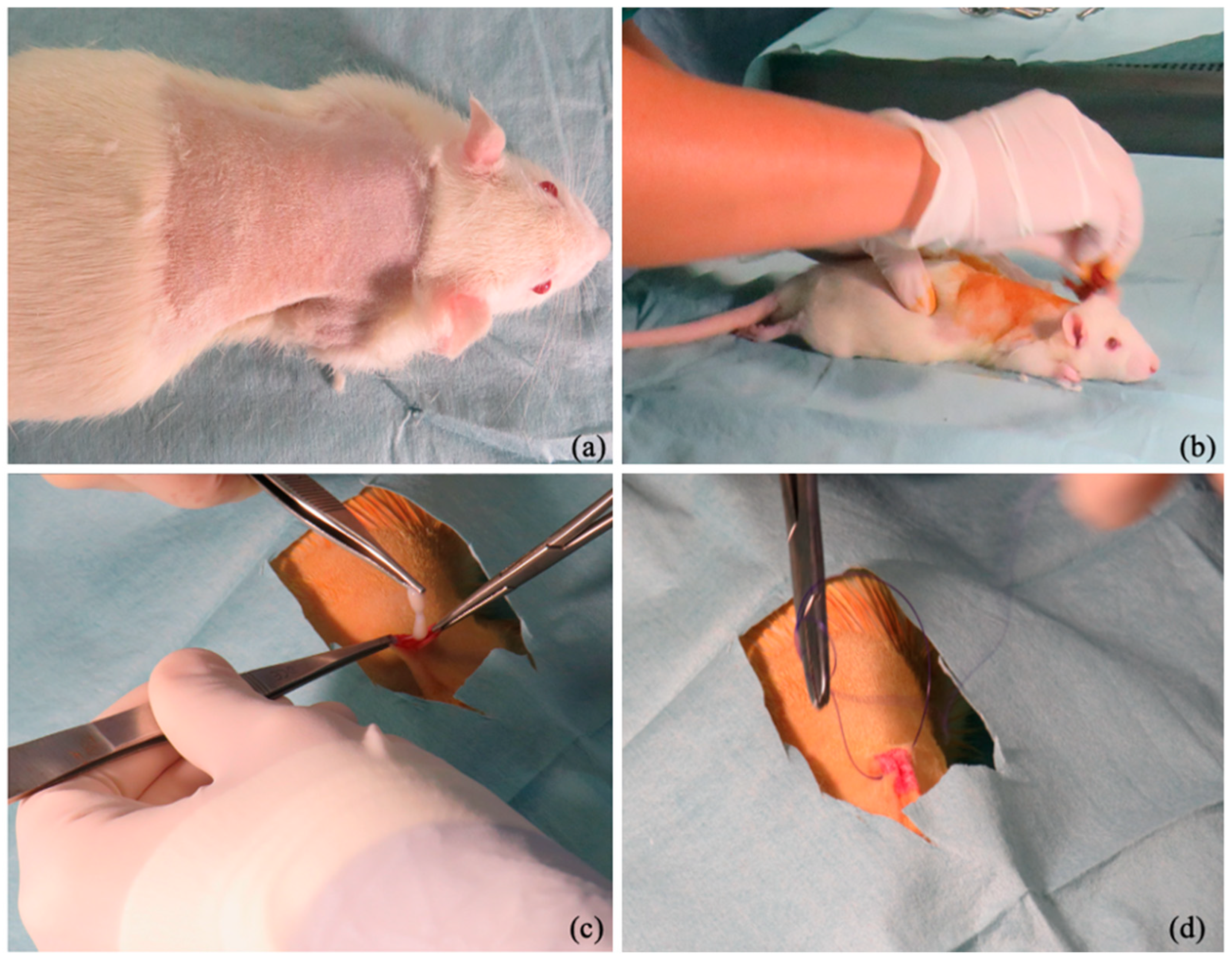
2.4. Surgical Procedure
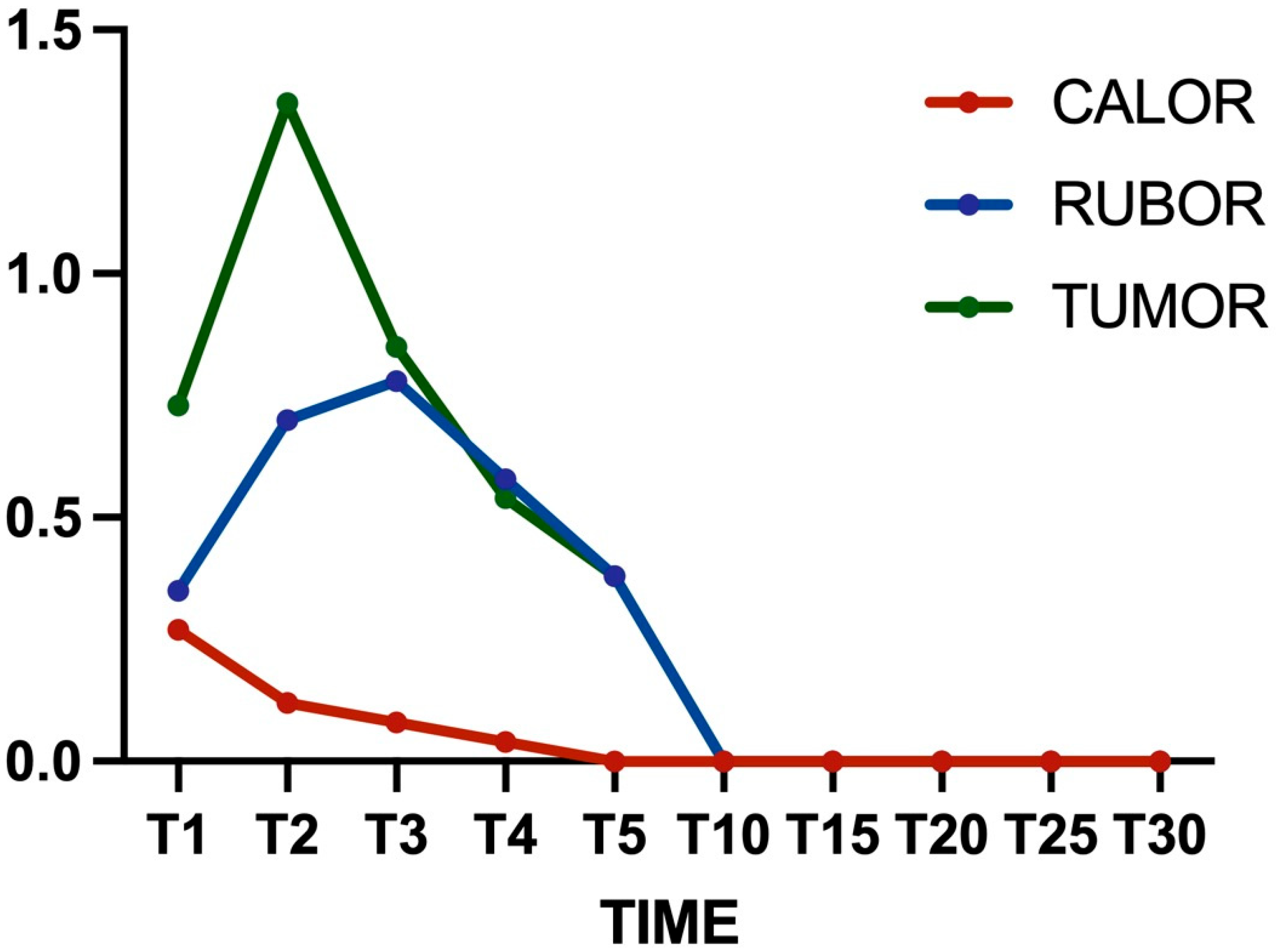
2.5. Clinical Evaluation
- Calor: a score of 0 indicated that the temperature of the infrascapular region is the same as the body skin, a 1 indicated a slight increase in temperature, a 2 indicated a moderate increase in temperature, and a 3 indicated a significant increase in temperature. The score was established to palpate these regions.
- Rubor: the absence of infrascapular skin redness was classified as 0, a slight reddening of the skin at the surgical site was classified as 1, reddening of the skin not exceeding two millimeters was classified as 2, and reddening of the skin at the surgical site exceeding two millimeters was classified as 3.
- Tumor: increased infrascapular skin thickness of less than 2 mm compared with the preoperative thickness was rated as 0, an increase between 2 and 4 mm was 1, an increase between 4 and 6 mm was 2, and an increase of more than 6 mm was 3. The skin measurement was performed using a manual caliper
2.6. Histological Analysis
2.7. Immunohistochemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinical Outcomes
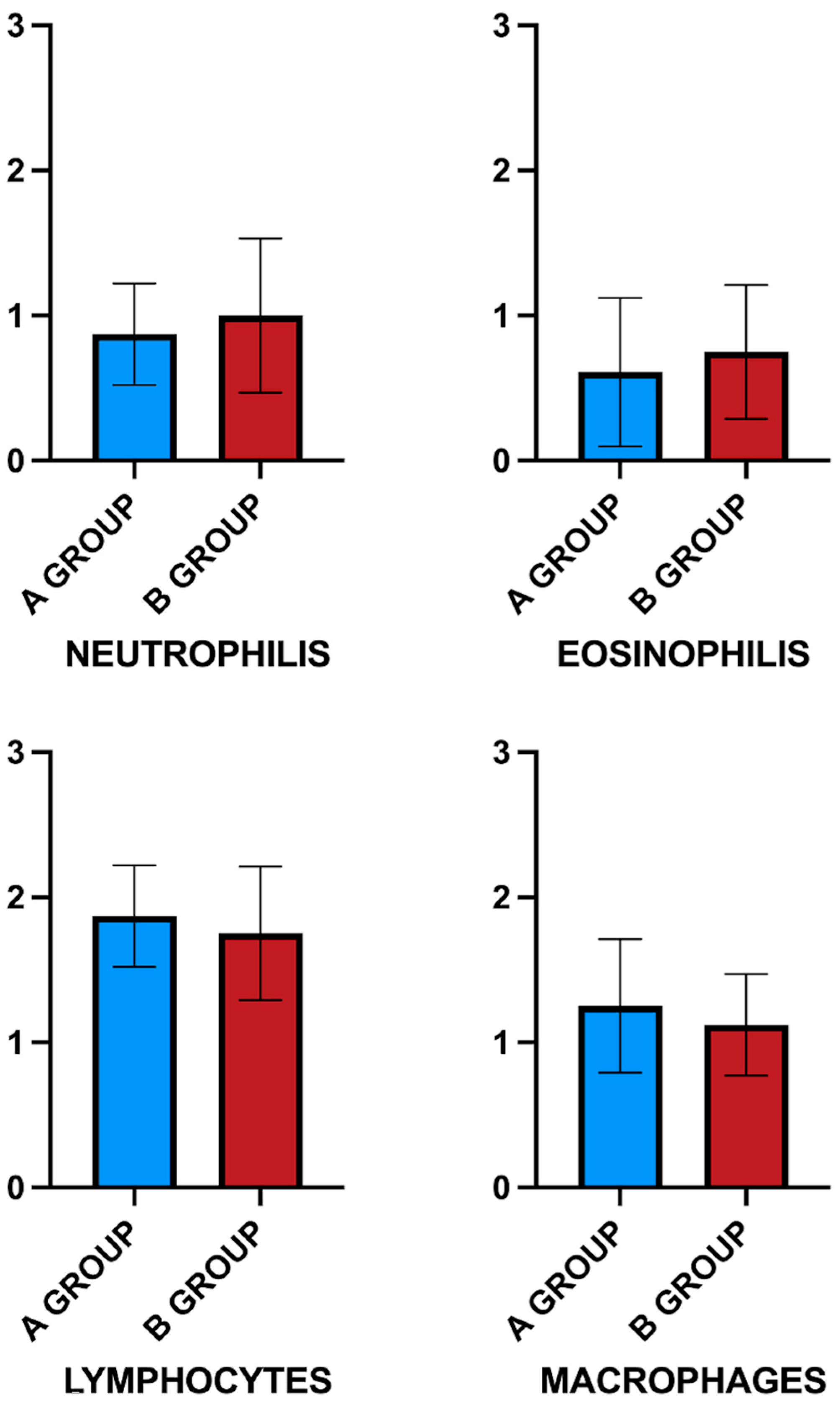
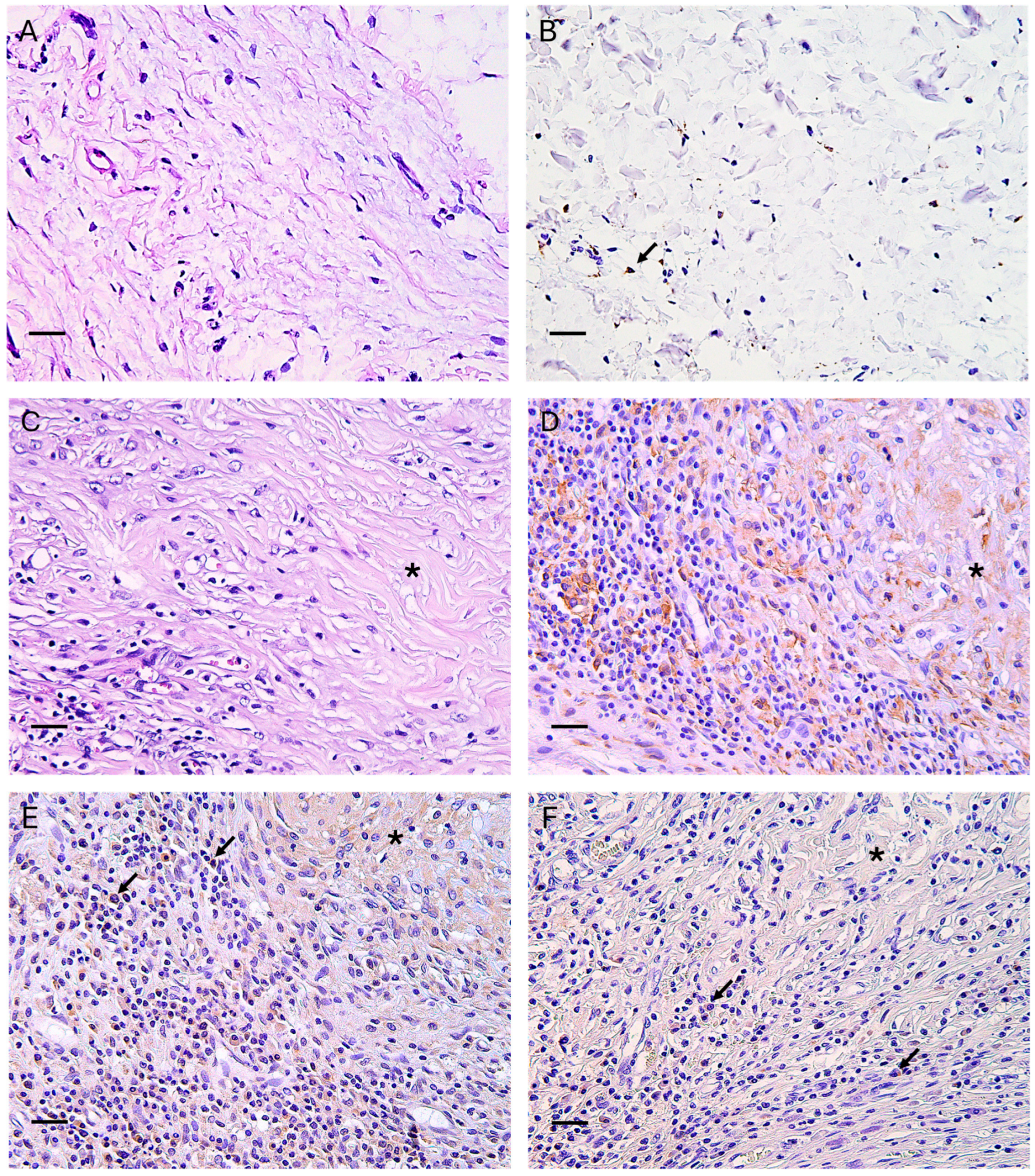
3.2. Host Inflammatory and Immune Response to Scaffold
3.3. Macrophage Polarization
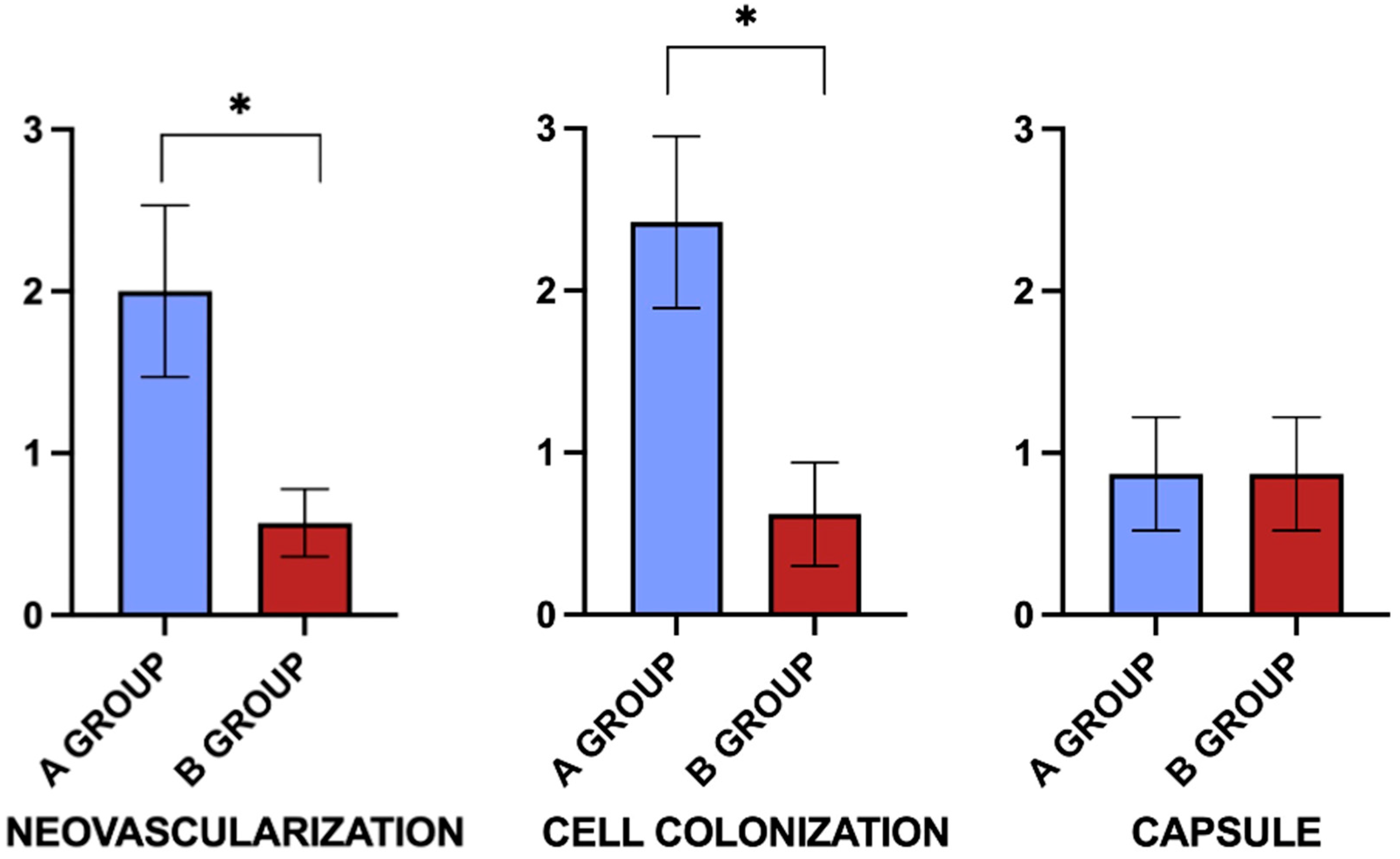
3.4. Neo-Angiogenesis, Fibroblastic Colonization, and Capsule Formation
4. Discussion
4.1. Acute Immune Response to Biomaterial Implant in the Rat
4.2. Chronic Immune Response to Biomaterial Implant in the Rat
4.3. Characterization of the In Vivo Host Remodeling Response
4.4. Animal Model
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, B.J.; Wamai, R.G.; Henebeng, E.B.; Tobian, A.A.; Klausner, J.D.; Banerjee, J.; Hankins, C.A. Estimation of Country-Specific and Global Prevalence of Male Circumcision. Popul. Health Metr. 2016, 14, 4. [Google Scholar] [CrossRef]
- Chapter 61-CIRCUMCISION-Creighton University. Available online: https://researchworks.creighton.edu/esploro/outputs/bookChapter/chapter-61---CIRCUMCISION/991005970696402656 (accessed on 15 October 2025).
- Yang, M.-H.; Tsao, C.-W.; Wu, S.-T.; Chuang, F.-P.; Meng, E.; Tang, S.-H.; Sun, G.-H.; Yu, D.-S.; Chang, S.-Y.; Cha, T.-L. The Effect of Circumcision on Young Adult Sexual Function. Kaohsiung J. Med. Sci. 2014, 30, 305–309. [Google Scholar] [CrossRef]
- Boyle, G.J.; Goldman, R.; Svoboda, J.S.; Fernandez, E. Male Circumcision: Pain, Trauma and Psychosexual Sequelae. J. Health Psychol. 2002, 7, 329–343. [Google Scholar] [CrossRef]
- Friedman, B.; Khoury, J.; Petersiel, N.; Yahalomi, T.; Paul, M.; Neuberger, A. Pros and Cons of Circumcision: An Evidence-Based Overview. Clin. Microbiol. Infect. 2016, 22, 768–774. [Google Scholar] [CrossRef]
- Miani, A.; Bernardo, G.A.D.; Højgaard, A.D.; Earp, B.D.; Zak, P.J.; Landau, A.M.; Hoppe, J.; Winterdahl, M. Neonatal Male Circumcision Is Associated with Altered Adult Socio-Affective Processing. Heliyon 2020, 6, e05566. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, S.; Zhu, C.; Wan, Q.; Chen, Z. Erectile function evaluation after adult circumcision. Zhonghua Nan Ke Xue 2004, 10, 18–19. [Google Scholar] [PubMed]
- Kim, D.; Pang, M.-G. The Effect of Male Circumcision on Sexuality. BJU Int. 2007, 99, 619–622. [Google Scholar] [CrossRef]
- Masood, S.; Patel, H.R.H.; Himpson, R.C.; Palmer, J.H.; Mufti, G.R.; Sheriff, M.K.M. Penile Sensitivity and Sexual Satisfaction after Circumcision: Are We Informing Men Correctly? Urol. Int. 2005, 75, 62–66. [Google Scholar] [CrossRef]
- Del Giudice, M. Sex Differences in Attachment Styles. Curr. Opin. Psychol. 2019, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hodges, F.M. The Ideal Prepuce in Ancient Greece and Rome: Male Genital Aesthetics and Their Relation to Lipodermos, Circumcision, Foreskin Restoration, and the Kynodesme. Bull. Hist. Med. 2001, 75, 375–405. [Google Scholar] [CrossRef]
- Gupta, R.; Mehta, S.; Gupta, R. A Novel Procedure of Prepuce Reconstruction Customized to the Religious Needs of Some Individuals. Indian J. Plast. Surg. 2021, 54, 114–117. [Google Scholar] [CrossRef]
- Timmermans, F.W.; Mokken, S.E.; Poor Toulabi, S.C.Z.; Bouman, M.-B.; Özer, M. A Review on the History of and Treatment Options for Foreskin Reconstruction after Circumcision. Int. J. Impot. Res. 2022, 34, 424–433. [Google Scholar] [CrossRef]
- Collier, R. Whole Again: The Practice of Foreskin Restoration. Can. Med. Assoc. J. 2011, 183, 2092–2093. [Google Scholar] [CrossRef]
- Cold, C.J.; Taylor, J.R. The Prepuce. BJU Int. 1999, 83, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Novotna, O.; Novakova, Z.V.; Galfiova, P.; Lorencova, M.; Klein, M.; Žiaran, S.; Kuniakova, M. Decellularization Techniques of Human Foreskin for Tissue Engineering Application. Physiol. Res. 2023, 72, S287–S297. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Jalili, A.; Banitalebi Dehkordi, M.; Przedborski, M. An Effective Method for Decellularization of Human Foreskin: Implications for Skin Regeneration in Small Wounds. Cell J. 2022, 24, 506–514. [Google Scholar] [CrossRef]
- Purpura, V.; Bondioli, E.; Cunningham, E.J.; De Luca, G.; Capirossi, D.; Nigrisoli, E.; Drozd, T.; Serody, M.; Aiello, V.; Melandri, D. The Development of a Decellularized Extracellular Matrix–Based Biomaterial Scaffold Derived from Human Foreskin for the Purpose of Foreskin Reconstruction in Circumcised Males. J. Tissue Eng. 2018, 9, 2041731418812613. [Google Scholar] [CrossRef] [PubMed]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized Extracellular Matrix Biomaterials for Regenerative Therapies: Advances, Challenges and Clinical Prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular Matrix as an Inductive Scaffold for Functional Tissue Reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef]
- Gierek, M.; Łabuś, W.; Kitala, D.; Lorek, A.; Ochała-Gierek, G.; Zagórska, K.M.; Waniczek, D.; Szyluk, K.; Niemiec, P. Human Acellular Dermal Matrix in Reconstructive Surgery—A Review. Biomedicines 2022, 10, 2870. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Rotili, D.; Mai, A.; Steegborn, C.; Xu, S.; Jin, Z.G. SIRT6 Protects Against Lipopolysaccharide-Induced Inflammation in Human Pulmonary Lung Microvascular Endothelial Cells. Inflammation 2024, 47, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, L.; Li, X.; Ge, C.; Pei, W.; Zhang, M.; Zhong, M.; Zhu, X.; Lv, K. M2 Macrophage Exosome-Derived lncRNA AK083884 Protects Mice from CVB3-Induced Viral Myocarditis through Regulating PKM2/HIF-1α Axis Mediated Metabolic Reprogramming of Macrophages. Redox Biol. 2023, 69, 103016. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Gao, S.; He, S.; Liu, W.; Liu, Q.; Lan, S.; Chen, J.; Li, F.; Ruan, R.; Zhang, J.; et al. 3D-Printed Scaffold with Biomimetic Gradient Structure for Promoting Bone Regeneration through Inhibiting Inflammation and Facilitating in-Situ Biomineralization. Biomater. Adv. 2026, 178, 214467. [Google Scholar] [CrossRef]
- Chávez, J.C.P.; Dobricic, M.; McGrath, M.; O’Connor, C.; McGuire, T.K.; Maughan, J.; Dervan, A.; Dixon, J.E.; Kearney, C.J.; Browne, S.; et al. Scaffold-Mediated miRNA-155 Inhibition Promotes Regenerative Macrophage Polarisation Leading to Anti-Inflammatory, Angiogenic and Neurogenic Responses for Wound Healing. bioRxiv 2025. [Google Scholar] [CrossRef]
- Dilek, Ö.F.; Sevim, K.Z.; Dilek, O.N. Acellular Dermal Matrices in Reconstructive Surgery; History, Current Implications and Future Perspectives for Surgeons. World J. Clin. Cases 2024, 12, 6791–6807. [Google Scholar] [CrossRef]
- Aodi, J.; Ying, L.; Chengyang, S.; Hongfeng, Z. Acellular Dermal Matrix in Urethral Reconstruction. Front. Pediatr. 2024, 12, 1342906. [Google Scholar] [CrossRef]
- Mohammadyari, F.; Parvin, S.; Khorvash, M.; Amini, A.; Behzadi, A.; HajEbrahimi, R.; Kasaei, F.; Olangian-Tehrani, S. Acellular Dermal Matrix in Reconstructive Surgery: Applications, Benefits, and Cost. Front. Transplant. 2023, 2, 1133806. [Google Scholar] [CrossRef]
- Acellular Extracellular Matrix Scaffolds in Regenerative Medicine: Advances in Decellularization and Clinical Applications. Available online: https://www.mdpi.com/2079-4983/16/10/383 (accessed on 24 October 2025).
- Fu, L.; Perez, A.V.; Maqsood, S.; Kopsachilis, N.; Foti, R.; D’Esposito, F.; Musa, M.; Tognetto, D.; Gagliano, C.; Zeppieri, M. Artificial and Bioengineered Therapeutic Options for Corneal Endothelial Disease. Bioengineering 2025, 12, 1064. [Google Scholar] [CrossRef]
- dos Santos, A.C.; de Andrade, L.M.B.; Candelária, R.A.Q.; de Carvalho, J.C.; Valbão, M.C.M.; da Barreto, R.S.N.; de Faria, M.D.; Buchaim, R.L.; Buchaim, D.V.; Miglino, M.A. From Cartilage to Matrix: Protocols for the Decellularization of Porcine Auricular Cartilage. Bioengineering 2025, 12, 52. [Google Scholar] [CrossRef]
- Lorenzo-Betancor, O.; Galosi, L.; Bonfili, L.; Eleuteri, A.M.; Cecarini, V.; Verin, R.; Dini, F.; Attili, A.-R.; Berardi, S.; Biagini, L.; et al. Homozygous CADPS2 Mutations Cause Neurodegenerative Disease with Lewy Body-like Pathology in Parrots. Mov. Disord. 2022, 37, 2345–2354. [Google Scholar] [CrossRef]
- Prudente, A.; Fávaro, W.J.; Latuf Filho, P.; Riccetto, C.L.Z. Host Inflammatory Response to Polypropylene Implants: Insights from a Quantitative Immunohistochemical and Birefringence Analysis in a Rat Subcutaneous Model. Int. Braz J. Urol. 2016, 42, 585–593. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Immunogenicity of Decellularized Extracellular Matrix Scaffolds: A Bottleneck in Tissue Engineering and Regenerative Medicine|Biomaterials Research. Available online: https://spj.science.org/doi/full/10.1186/s40824-023-00348-z (accessed on 15 October 2025).
- Jiang, Z.; Schatzmayr, G.; Mohnl, M.; Applegate, T.J. Net Effect of an Acute Phase Response—Partial Alleviation with Probiotic Supplementation. Poult. Sci. 2010, 89, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, S.T.; Brown, B.N. Chapter 4-Host Response to Naturally Derived Biomaterials. In Host Response to Biomaterials; Badylak, S.F., Ed.; Academic Press: Oxford, UK, 2015; pp. 53–79. ISBN 978-0-12-800196-7. [Google Scholar]
- Jones, K.S. Effects of Biomaterial-Induced Inflammation on Fibrosis and Rejection. Semin. Immunol. 2008, 20, 130–136. [Google Scholar] [CrossRef]
- Macleod, T.M.; Williams, G.; Sanders, R.; Green, C.J. Histological Evaluation of PermacolTM as a Subcutaneous Implant over a 20-Week Period in the Rat Model. Br. J. Plast. Surg. 2005, 58, 518–532. [Google Scholar] [CrossRef]
- Lucke, S.; Hoene, A.; Walschus, U.; Kob, A.; Pissarek, J.-W.; Schlosser, M. Acute and Chronic Local Inflammatory Reaction after Implantation of Different Extracellular Porcine Dermis Collagen Matrices in Rats. BioMed Res. Int. 2015, 2015, 938059. [Google Scholar] [CrossRef]
- Suliman, S.; Sun, Y.; Pedersen, T.O.; Xue, Y.; Nickel, J.; Waag, T.; Finne-Wistrand, A.; Steinmüller-Nethl, D.; Krueger, A.; Costea, D.E.; et al. In Vivo Host Response and Degradation of Copolymer Scaffolds Functionalized with Nanodiamonds and Bone Morphogenetic Protein 2. Adv. Healthc. Mater. 2016, 5, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between Biomaterials and the Immune System: Challenges and Opportunities in Regenerative Medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, V.; Hassanabad, A.F.; Belke, D.; Teng, G.; Isidoro, C.A.; Dutta, D.; Turnbull, J.; Deniset, J.F.; Fedak, P.W.M. Micronized Acellular Matrix Biomaterial Leverages Eosinophils for Postinfarct Cardiac Repair. JACC Basic. Transl. Sci. 2023, 8, 939–954. [Google Scholar] [CrossRef]
- Pan, D.; Hunter, D.A.; Schellhardt, L.; Fuchs, A.; Halevi, A.E.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. T Cells Modulate IL-4 Expression by Eosinophil Recruitment within Decellularized Scaffolds to Repair Nerve Defects. Acta Biomater. 2020, 112, 149–163. [Google Scholar] [CrossRef]
- DeStefano, S.; Hartigan, D.R.; Josyula, A.; Faust, M.; Fertil, D.; Lokwani, R.; Ngo, T.B.; Sadtler, K. Conserved and Tissue-Specific Immune Responses to Biologic Scaffold Implantation. Acta Biomater. 2024, 184, 68–80. [Google Scholar] [CrossRef]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.-Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of Immune-Inflammatory Responses through Surface Modifications of Biomaterials to Promote Bone Healing and Regeneration. J. Tissue Eng. 2021, 12, 20417314211041428. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.H.; Stamer, D.K.; Kyriakides, T.R. The Host Response to Naturally-Derived Extracellular Matrix Biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Saleh, T.; Xu, M. Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells. Cells 2021, 10, 1787. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Lu, J.; Hernigou, P.; Jin, F. The Role of Macrophage Polarization in Tendon Healing and Therapeutic Strategies: Insights from Animal Models. Front. Bioeng. Biotechnol. 2024, 12, 1366398. [Google Scholar] [CrossRef]
- Mills, C. M1 and M2 Macrophages: Oracles of Health and Disease. CRI 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Jiang, S.; Li, J.; Wei, F.; Li, X.; Ding, Y.; Yang, Z.; Sun, Z.; Zha, K.; Wang, F.; et al. Cell-Free Decellularized Cartilage Extracellular Matrix Scaffolds Combined with Interleukin 4 Promote Osteochondral Repair through Immunomodulatory Macrophages: In Vitro and in Vivo Preclinical Study. Acta Biomater. 2021, 127, 131–145. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, S.; Zhang, H.; Zhu, B.; Su, Y.; Zheng, C.; Tian, R.; Wang, M.; Kuang, H.; Zhao, X.; et al. Mesenchymal Stem Cells and Extracellular Matrix Scaffold Promote Muscle Regeneration by Synergistically Regulating Macrophage Polarization toward the M2 Phenotype. Stem Cell Res. Ther. 2018, 9, 88. [Google Scholar] [CrossRef]
- Batten, P.; Sarathchandra, P.; Antoniw, J.W.; Tay, S.S.; Lowdell, M.W.; Taylor, P.M.; Yacoub, M.H. Human Mesenchymal Stem Cells Induce T Cell Anergy and Downregulate T Cell Allo-Responses via the TH2 Pathway: Relevance to Tissue Engineering Human Heart Valves. Tissue Eng. 2006, 12, 2263–2273. [Google Scholar] [CrossRef]
- McInnes, A.D.; Moser, M.A.J.; Chen, X. Preparation and Use of Decellularized Extracellular Matrix for Tissue Engineering. J. Funct. Biomater. 2022, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.C.; Chan, R.W.; Weinberger, D.G.; Efune, G.; Pawlowski, K.S. A Bovine Acellular Scaffold for Vocal Fold Reconstruction in a Rat Model. J. Biomed. Mater. Res. Part A 2010, 92A, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Boháč, M.; Danišovič, Ľ.; Koller, J.; Dragúňová, J.; Varga, I. What Happens to an Acellular Dermal Matrix after Implantation in the Human Body? A Histological and Electron Microscopic Study. Eur. J. Histochem. 2018, 62, 2873. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sukarto, A.; Deegan, D.; Fan, F. Characterization of Inflammatory and Fibrotic Aspects of Tissue Remodeling of Acellular Dermal Matrix in a Nonhuman Primate Model. Plast. Reconstr. Surg.–Glob. Open 2021, 9, e3420. [Google Scholar] [CrossRef]
- Waqanivavalagi, S.W.F.R.; Ground, M.B.; Alarcon, C.; Milsom, P.; Cornish, J. Subcutaneous Surgical Rat Models for the Evaluation of Decellularised Heart Valve Immunogenicity: A Systematic Review. Materialia 2022, 21, 101298. [Google Scholar] [CrossRef]
- Khorramirouz, R.; Go, J.L.; Noble, C.; Jana, S.; Maxson, E.; Lerman, A.; Young, M.D. A Novel Surgical Technique for a Rat Subcutaneous Implantation of a Tissue Engineered Scaffold. Acta Histochem. 2018, 120, 282–291. [Google Scholar] [CrossRef]
- Zang, M.; Zhang, Q.; Chang, E.I.; Mathur, A.B.; Yu, P. Decellularized Tracheal Matrix Scaffold for Tissue Engineering. Plast. Reconstr. Surg. 2012, 130, 532–540. [Google Scholar] [CrossRef]



| T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|
| Calor | 0.27 ± 0.10 | 0.12 ± 0.05 | 0.08 ± 0.01 * | 0.04 ± 0.01 *# | 0 *# |
| Rubor | 0.35 ± 0.18 | 0.70 ± 0.30 * | 0.78 ± 0.25 * | 0.58 ± 0.14 | 0.38 ± 0.19 #° |
| Tumor | 0.73 ± 0.23 | 1.35 ± 0.44 * | 0.85 ± 0.30 # | 0.54 ± 0.12 # | 0.38 ± 0.09 *#° |
| A Group | B Group | C Group | |
|---|---|---|---|
| Neutrophils | 0.87 ± 0.35 # | 1 ± 0.53 # | 0.2 ± 0.44 |
| Eosinophils | 0.61 ± 0.51 # | 0.75 ± 0.46 # | 0 |
| Lymphocytes | 1.87 ± 0.35 # | 1.75 ± 0.46 # | 0.2 ± 0.44 |
| Macrophages | 1.25 ± 0.46 # | 1.12 ± 0.35 # | 0.2 ± 0.44 |
| A Group | B Group | C Group | |
|---|---|---|---|
| Neovascularization | 2 ± 0.53 *# | 0.57 ± 0.21 # | 0.2 ± 0.44 |
| Cell colonization | 2.42 ± 0.53 *# | 0.62 ± 0.32 # | 0 |
| Capsule | 0.87 ± 0.35 # | 0.87 ± 0.35 # | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennasilico, L.; Galosi, M.; Biagini, L.; Riccio, V.; Di Bella, C.; Serino, F.; Sassaroli, S.; Ciabocco, F.; Bondioli, E.; Rossi, G.; et al. Immunogenicity and Integration of a Decellularized Extracellular Matrix-Based Scaffold for the Reconstruction of Human Foreskin: A Preclinical Animal Study. Bioengineering 2025, 12, 1186. https://doi.org/10.3390/bioengineering12111186
Pennasilico L, Galosi M, Biagini L, Riccio V, Di Bella C, Serino F, Sassaroli S, Ciabocco F, Bondioli E, Rossi G, et al. Immunogenicity and Integration of a Decellularized Extracellular Matrix-Based Scaffold for the Reconstruction of Human Foreskin: A Preclinical Animal Study. Bioengineering. 2025; 12(11):1186. https://doi.org/10.3390/bioengineering12111186
Chicago/Turabian StylePennasilico, Luca, Margherita Galosi, Lucia Biagini, Valentina Riccio, Caterina Di Bella, Federica Serino, Sara Sassaroli, Felice Ciabocco, Elena Bondioli, Giacomo Rossi, and et al. 2025. "Immunogenicity and Integration of a Decellularized Extracellular Matrix-Based Scaffold for the Reconstruction of Human Foreskin: A Preclinical Animal Study" Bioengineering 12, no. 11: 1186. https://doi.org/10.3390/bioengineering12111186
APA StylePennasilico, L., Galosi, M., Biagini, L., Riccio, V., Di Bella, C., Serino, F., Sassaroli, S., Ciabocco, F., Bondioli, E., Rossi, G., Aiello, V., Pratesi, A., & Palumbo Piccionello, A. (2025). Immunogenicity and Integration of a Decellularized Extracellular Matrix-Based Scaffold for the Reconstruction of Human Foreskin: A Preclinical Animal Study. Bioengineering, 12(11), 1186. https://doi.org/10.3390/bioengineering12111186









