Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice
Abstract
:1. Introduction
2. Results
2.1. Histological Observations of Spleen and Thymus
2.2. Body Weights and Immune Organ Index
2.3. Effect of Bergenin on Cytokine Levels in Serum of Cy-Treated Mice
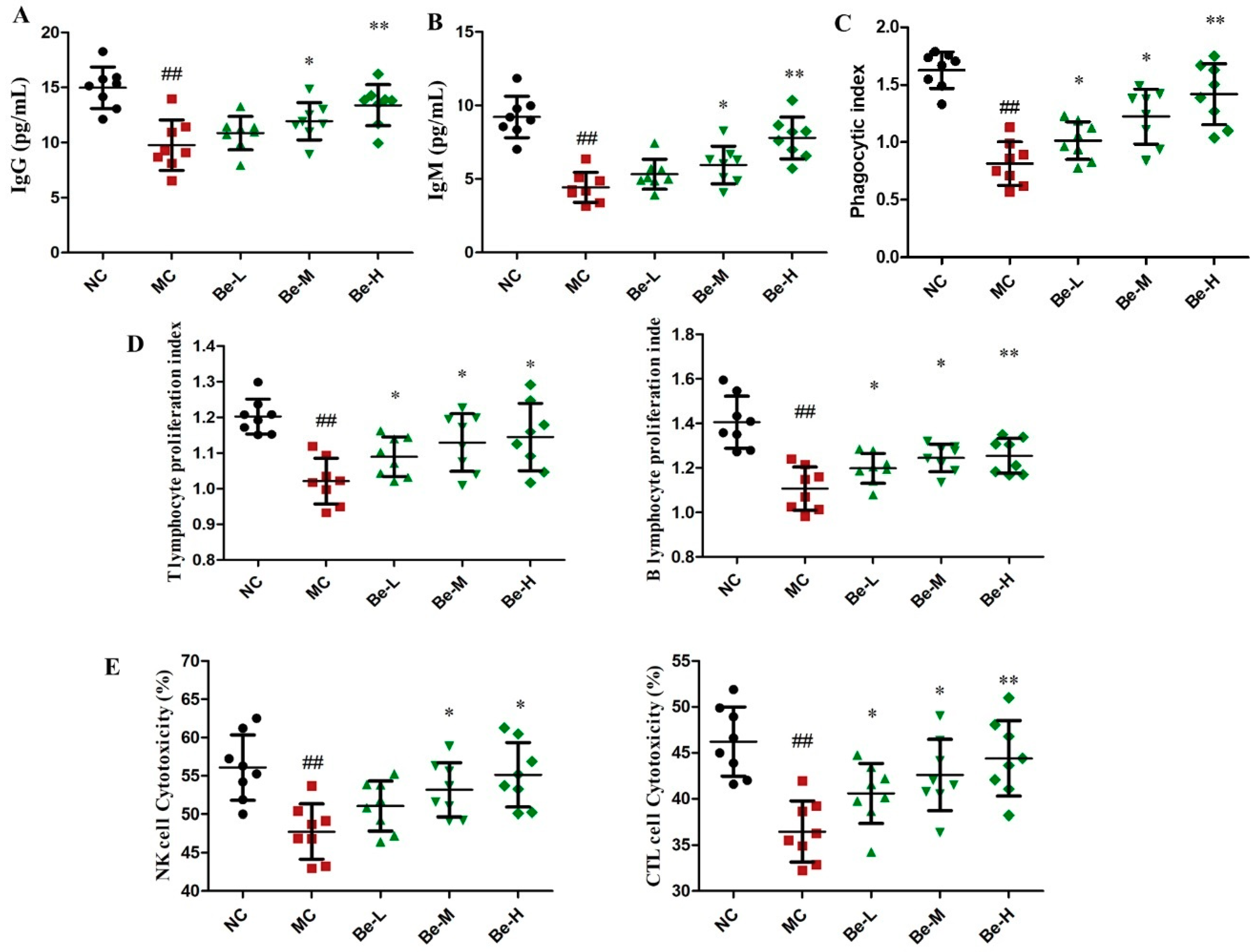
2.4. Effect of Bergenin on Immunoglobulin Levels in Serum
2.5. Effect of Bergein on Phagocytic Activity of Macrophages in Cy-Treated Mice
2.6. Effects of Bergenin on Splenocyte Proliferation in Cy-Treated Mice
2.7. Effects of Bergenin on Splenic NK and CTL Cytotoxicities in Cy-Treated Mice
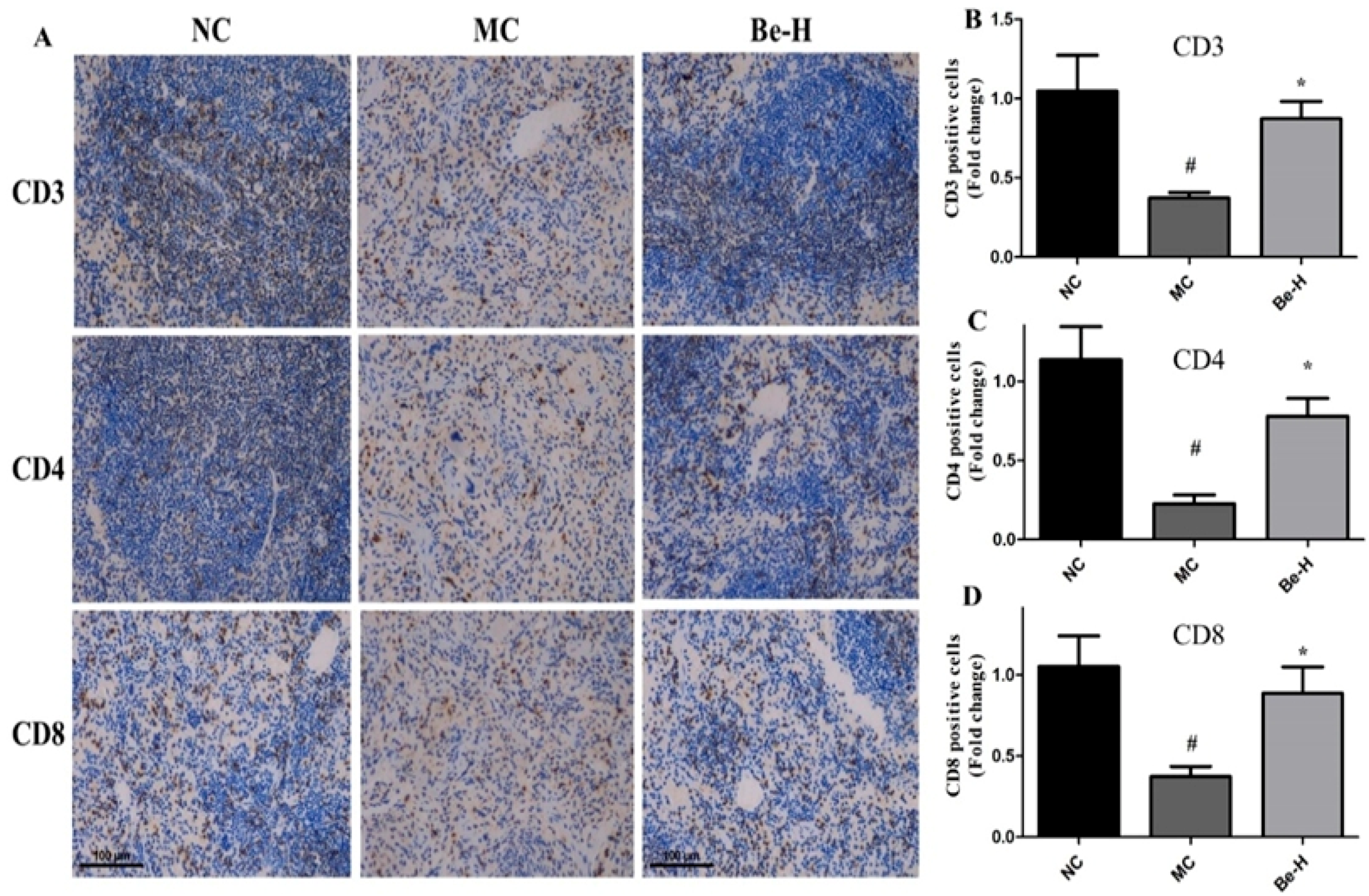
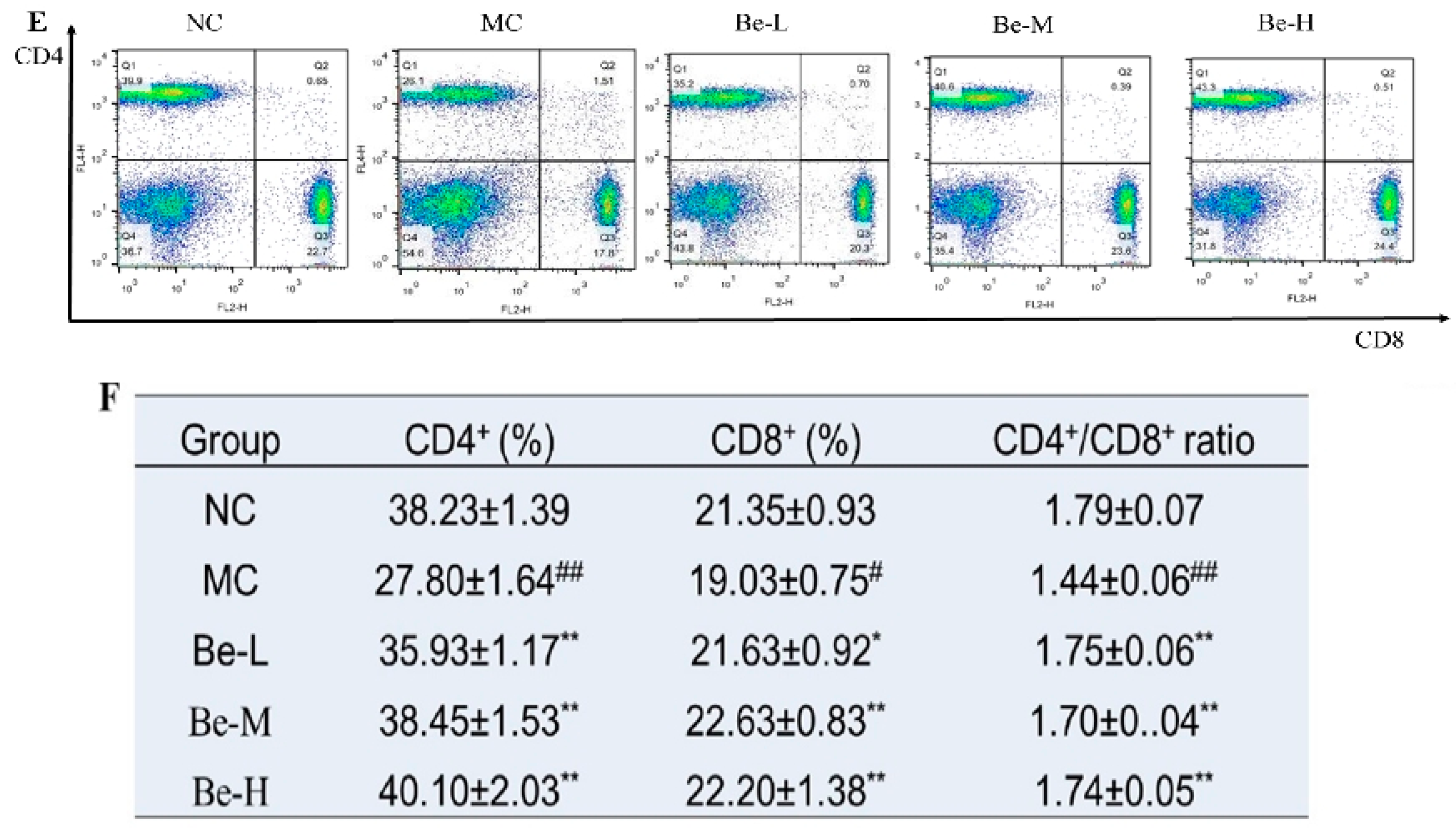
2.8. Effects of Bergenin on Splenic T Lymphocyte Subsets
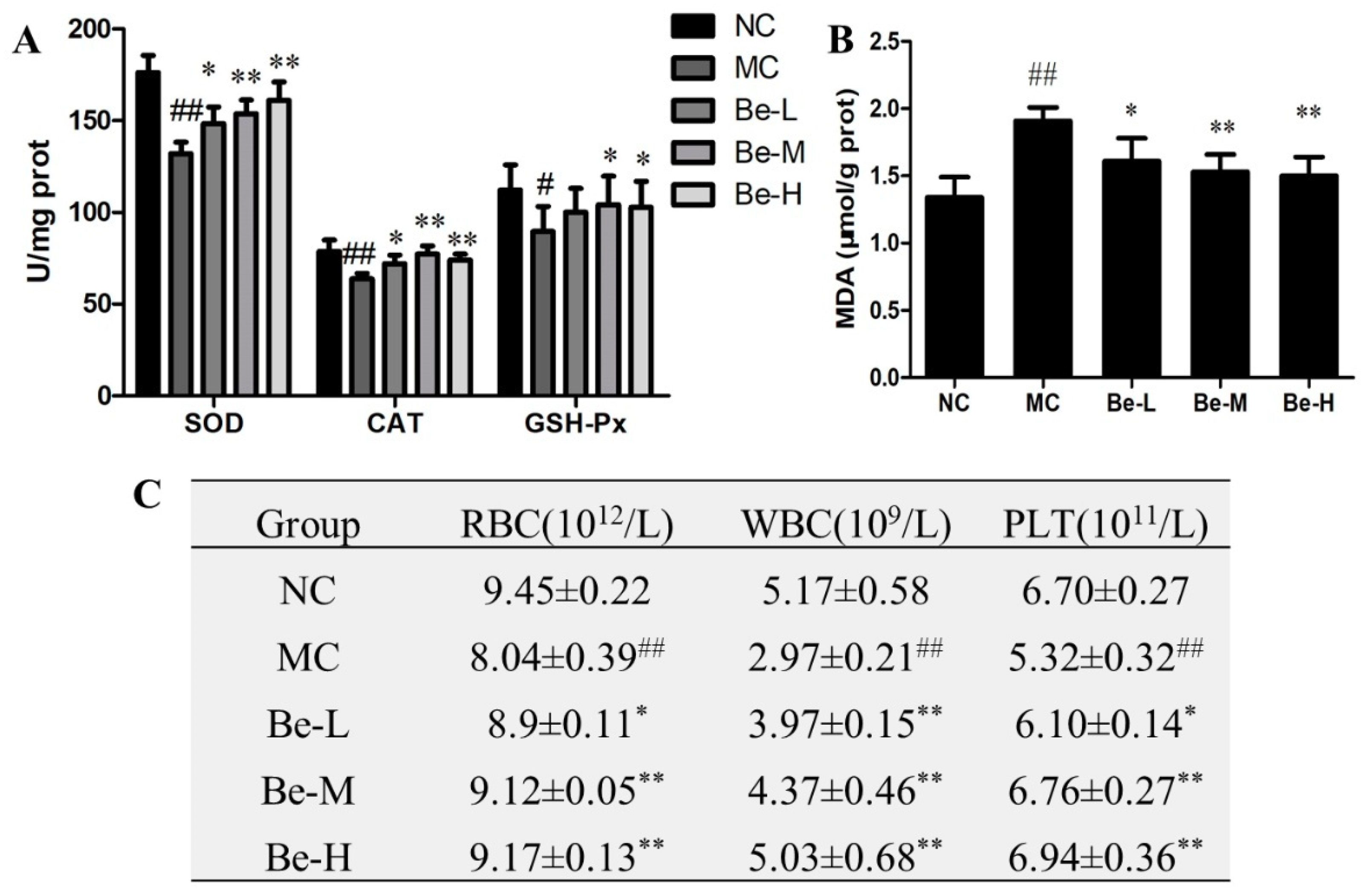
2.9. Effect of Bergenin on Redox Imbalance in Cy-Treated Mice
2.10. Effect of Bergenin on RBC, WBC and Platelets Count in Cy-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals and Experimental Design
4.3. HE Staining of Spleen and Thymus Tissues
4.4. Effect of Bergenin on Mouse Spleen and Thymus Indices
4.5. Determination of Immunoglobulin and Cytokines in Serum by ELISA
4.6. Phagocytosis of Peritoneal Macrophages
4.7. Measurement of Lymphocyte Proliferation
4.8. Splenic NK and CTL Cell Cytotoxicity Activity Assays
4.9. Immunohistochemical Analysis of CD3, CD4 and CD8 Expressions in Spleen Tissues
4.10. Flow Cytometry Analysis of Splenic T Lymphocyte Subpopulations
4.11. Measurements of Antioxidant Enzyme Activities and the MDA Level
4.12. Peripheral White Blood Cell, Red Blood Cell and Platelets Counts
4.13. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Solomon, G.F.; Amkraut, A.A.; Kasper, P. Immunity, emotions and stress. With special reference to the mechanisms of stress effects on the immune system. Ann. Clin. Res. 1974, 6, 313–322. [Google Scholar] [PubMed]
- Kumar, S.; Sharma, G.; Sidiq, T.; Khajuria, A.; Jain, M.; Bhagwat, D.; Dhar, K.L.L. Immunomodulatory potential of a bioactive fraction from the leaves of Phyllostachys bambusoides (bamboo) in BALB/c mice. EXCLI J. 2014, 13, 137–150. [Google Scholar] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA: Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, W.; Yu, G.; Li, Y.; Hu, Y.; Lu, J.; Jin, L. Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Gupta, R.K.; Shau, H.; Ray, P.K. Effect of ASTA-Z 7575 (INN Maphosphamide) on human lymphokine-activated killer cell induction. Immunopharmacol. Immunotoxicol. 1993, 15, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhong, Y.F.; Wu, Y.P.; Luo, Z.; Sun, Y.M.; Wang, G.E.; Kurihara, H.; Li, Y.F.; He, R.R. Carnosine attenuates cyclophosphamide-induced bone marrow suppression by reducing oxidative DNA damage. Redox Biol. 2018, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yang, J.; Pan, L.; Guo, N.; Li, B.; Yao, J.; Wang, M.; Qi, C.; Zhang, G.; Liu, Z. Improvement of icaritin on hematopoietic function in cyclophosphamide-induced myelosuppression mice. Immunopharmacol. Immunotoxicol. 2018, 40, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Manente, F.A.; Quinello, C.; Ferreira, L.S.; de Andrade, C.R.; Jellmayer, J.A.; Portuondo, D.L.; Batista-Duharte, A.; Carlos, I.Z. Experimental sporotrichosis in a cyclophosphamide-induced immunosuppressed mice model. Med. Mycol. 2018, 56, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Kuttan, G. Ameliorative action of Vernonia cinerea L. on cyclophosphamide-induced immunosuppression and oxidative stress in mice. Inflammopharmacology 2010, 18, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, A.; Sorensen, O.; Suarez-Pinzon, W.L.; Power, R.F.; Rajotte, R.V.; Bleackley, R.C. Analysis of cytokine mRNA expression in syngeneic islet grafts of NOD mice: Interleukin 2 and interferon gamma mRNA expression correlate with graft rejection and interleukin 10 with graft survival. Diabetologia 1994, 37, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Huang, D.F.; Li, W.J.; Xie, M.Y. Molecular mechanism underlying chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2015, 15, 52–60. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Krifa, M.; Mensi, R.; Bhouri, W.; Ghedira, K.; Chekir-Ghedira, L. In vitro and in vivo immunomodulatory and anti-ulcerogenic activities of Teucrium ramosissimum extracts. J. Immunotoxicol. 2011, 8, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Koo, S.J.; Hwang, J.K. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscorea batatas). J. Ethnopharmacol. 2004, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pr. 2012, 2012, 282570. [Google Scholar] [CrossRef] [PubMed]
- Taixiang, W.; Munro, A.J.; Guanjian, L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst. Rev. 2005, 1, Cd004540. [Google Scholar]
- Uniyal, S.K.; Singh, K.N.; Jamwal, P.; Lal, B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomedicine 2006, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Piacente, S.; Pizza, C.; De Tommasi, N.; Mahmood, N. Constituents of Ardisia japonica and their in vitro anti-HIV activity. J. Nat. Prod. 1996, 59, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kosaka, M.; Watanabe, Y.; Nakade, K.; Fukuyama, Y. Synthesis and neuroprotective activity of bergenin derivatives with antioxidant activity. Bioorg. Med. Chem. 2003, 11, 1781–1788. [Google Scholar] [CrossRef]
- Yun, J.; Lee, Y.; Yun, K.; Oh, S. Bergenin decreases the morphine-induced physical dependence via antioxidative activity in mice. Arch. Pharmacal Res. 2015, 38, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Patel, D.K.; Prasad, S.K.; Laloo, D.; Krishnamurthy, S.; Hemalatha, S. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia 2012, 83, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.L.; Huang, X.; Zhao, J.H.; Hong, A. Bergenin is the antiarrhythmic principle of Fluggea virosa. Planta Medica 2002, 68, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Kim, H.S.; Chung, M.W.; Kim, Y.C. Protective effects of bergenin, the major constituent of Mallotus japonicus, on d-galactosamine-intoxicated rat hepatocytes. J. Ethnopharmacol. 2000, 70, 69–72. [Google Scholar] [CrossRef]
- Patel, D.K.; Patel, K.; Kumar, R.; Gadewar, M.; Tahilyani, V. Pharmacological and analytical aspects of bergenin: a concise report. Asian Pacific J. Tropical Disease 2012, 2, 163–167. [Google Scholar] [CrossRef]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Taneja, S.C.; Ahmad, S.F.; Bani, S.; Qazi, G.N. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—A flow cytometric study. J. Ethnopharmacol. 2007, 112, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, R.C.S.; Oliveira, V.G.; Da Silva, S.L.; Nunomura, S.M. Characterization of bergenin in Endopleura uchi bark and its anti-inflammatory activity. J. Braz. Chem. Soc. 2009, 20, 1060–1064. [Google Scholar] [CrossRef]
- Prahalad, S. Atopy, autoimmunity, and the TH1/TH2 balance. J. Pediatr. 2000, 137, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wu, J.; Li, S.T. Immuno-enhancement effects of Lycium ruthenicum Murr. polysaccharide on cyclophosphamide-induced immunosuppression in mice. Int. J. Clin. Exp. Med. 2015, 8, 20631–20637. [Google Scholar] [PubMed]
- Wang, Y.; Qi, Q.; Li, A.; Yang, M.; Huang, W.; Xu, H.; Zhao, Z.; Li, S. Immuno-enhancement effects of Yifei Tongluo Granules on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2016, 194, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Pass, G.J.; Carrie, D.; Boylan, M.; Lorimore, S.; Wright, E.; Houston, B.; Henderson, C.J.; Wolf, C.R. Role of hepatic cytochrome P450s in the pharmacokinetics and toxicity of cyclophosphamide: Studies with the hepatic cytochrome P450 reductase null mouse. Cancer Res. 2005, 65, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Li, J.; Wang, H.L.; Cheng, W.M.; Zhang, L.; Ge, J.F. Immunomodulating effects of fractioned polysaccharides isolated from Yu-Ping-Feng-Powder in cyclophosphamide-treated mice. Am. J. Chin. Med. 2006, 34, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Sekiguchi, N.; Matsuda, K.; Matsumoto, A.; Ito, T.; Nakazawa, H.; Ishida, F. A screening method with lymphocyte percentage and proportion of granular lymphocytes in the peripheral blood for large granular lymphocyte (LGL) leukemia. Int. J. Hematol. 2017, 105, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, T.; Wang, L.; Su, Y.; Xian, C.J. Dioscorea bulbifera polysaccharide and cyclophosphamide combination enhances anti-cervical cancer effect and attenuates immunosuppression and oxidative stress in mice. Sci. Rep. 2016, 5, 19185. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lu, Y.; Wang, D.; Liu, J.; Song, X.; Zhang, W.; Zhao, X.; Nguyen, T.L.; Hu, Y. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell. Immunol. 2013, 281, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, C.; Fan, Y.; Guo, L.; Si, H.; Wang, Y.; Shi, Z.; Zhang, H. Immuno-enhancement effects of ethanol extract from Cyrtomium macrophyllum (Makino) Tagawa on cyclophosphamide-induced immunosuppression in BALB/c mice. J. Ethnopharmacol. 2014, 155, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Duggina, P.; Kalla, C.M.; Varikasuvu, S.R.; Bukke, S.; Tartte, V. Protective effect of centella triterpene saponins against cyclophosphamide-induced immune and hepatic system dysfunction in rats: Its possible mechanisms of action. J. Physiol. Biochem. 2015, 71, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tong, X.; Li, P.; Cao, H.; Su, W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2012, 139, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Chen, A.F.; Lin, Z.B. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fuster, F.; Vargas, J.I.; Jensen, D.; Sarmiento, V.; Acuña, P.; Peirano, F.; Fuster, F.; Arab, J.P.; Martínez, F. CD4/CD8 ratio as a predictor of the response to HBV vaccination in HIV-positive patients: A prospective cohort study. Vaccine 2016, 34, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, E.; Sugiura, T.; Zeki, K.; Yoshida, Y.; Yamashita, U. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice. J. Immunol. 2000, 164, 2386–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Zhang, X.; Zheng, L.; Han, Z.; Li, P.; Xiao, Y.; Zhang, Q.; Wang, F. The in vivo immunomodulatory and synergistic anti-tumor activity of thymosin α1-thymopentin fusion peptide and its binding to TLR2. Cancer Lett. 2013, 337, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gate, L.; Paul, J.; Ba, G.N.; Tew, K.D.; Tapiero, H. Oxidative stress induced in pathologies: the role of antioxidants. Biomed. Pharmacother. 1999, 53, 169–180. [Google Scholar] [CrossRef]
- Cigremis, Y.; Turel, H.; Adiguzel, K.; Akgoz, M.; Kart, A.; Karaman, M.; Ozen, H. The effects of acute acetaminophen toxicity on hepatic mRNA expression of SOD, CAT, GSH-Px, and levels of peroxynitrite, nitric oxide, reduced glutathione, and malondialdehyde in rabbit. Mol. Cell. Biochem. 2009, 323, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, H.T.; Zhang, Y.J.; Yang, C.R. A carbon-carbon-coupled dimeric bergenin derivative biotransformed by Pleurotus ostreatus. Bioorg. Med. Chem. Lett. 2005, 15, 4073–4075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Z.; Wang, Z.; Yu, S.; Long, T.; Zhou, X.; Bao, Y. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci. Rep. 2017, 7, 44822. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.J.; Guo, M.Y.; Zhang, Z.C.; Wang, T.C.; Cao, Y.G.; Zhang, N.S. Bergenin Plays an Anti-Inflammatory Role via the Modulation of MAPK and NF-κB Signaling Pathways in a Mouse Model of LPS-Induced Mastitis. Inflammation 2015, 38, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.P.; Bhattacharya, D.; Yadav, V.; Singh, D.K.; Kumar, S.; Singh, M.; Ojha, D.; Ranganathan, A.; Van Kaer, L.; Chattopadhyay, D.; et al. The Phytochemical Bergenin Enhances T Helper 1 Responses and Anti-Mycobacterial Immunity by Activating the MAP Kinase Pathway in Macrophages. Front. Cell. Infect. Microbiol. 2017, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.A.; Keisler, A.S.; Myrvik, Q.N.; Warinner, J.E. Differential uptake of neutral red by macrophages from three species of estuarine fish. Dev. Comp. Immunol. 1987, 11, 117–124. [Google Scholar] [CrossRef]
- Chen, J.R.; Yang, Z.Q.; Hu, T.J.; Yan, Z.T.; Niu, T.X.; Wang, L.; Cui, D.A.; Wang, M. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia 2010, 81, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Won, S.J. Effects of tramadol on T lymphocyte proliferation and natural killer cell activity in rats with sciatic constriction injury. Pain 2001, 92, 63–69. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds bergenin are available from the authors. |


| Group | Treatment (i.p.) | |
|---|---|---|
| Days 1–3 and 9 | Days 4–10 | |
| NC | Normal saline | Normal saline |
| MC | Cy (80 mg/kg/day) | Normal saline |
| Be-L | Cy (80 mg/kg/day) | Bergenin (5 mg/kg/day) |
| Be-M | Cy (80 mg/kg/day) | Bergenin (10 mg/kg/day) |
| Be-H | Cy (80 mg/kg/day) | Bergenin (20 mg/kg/day) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules 2018, 23, 2668. https://doi.org/10.3390/molecules23102668
Qi Q, Dong Z, Sun Y, Li S, Zhao Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules. 2018; 23(10):2668. https://doi.org/10.3390/molecules23102668
Chicago/Turabian StyleQi, Qiuchen, Zhonghua Dong, Yueyue Sun, Siying Li, and Zhongxi Zhao. 2018. "Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice" Molecules 23, no. 10: 2668. https://doi.org/10.3390/molecules23102668
APA StyleQi, Q., Dong, Z., Sun, Y., Li, S., & Zhao, Z. (2018). Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules, 23(10), 2668. https://doi.org/10.3390/molecules23102668






