Heavy Metals in Bioenergy Crop Production, Biomass Quality, and Biorefinery: Global Impacts and Sustainable Management Strategies
Abstract
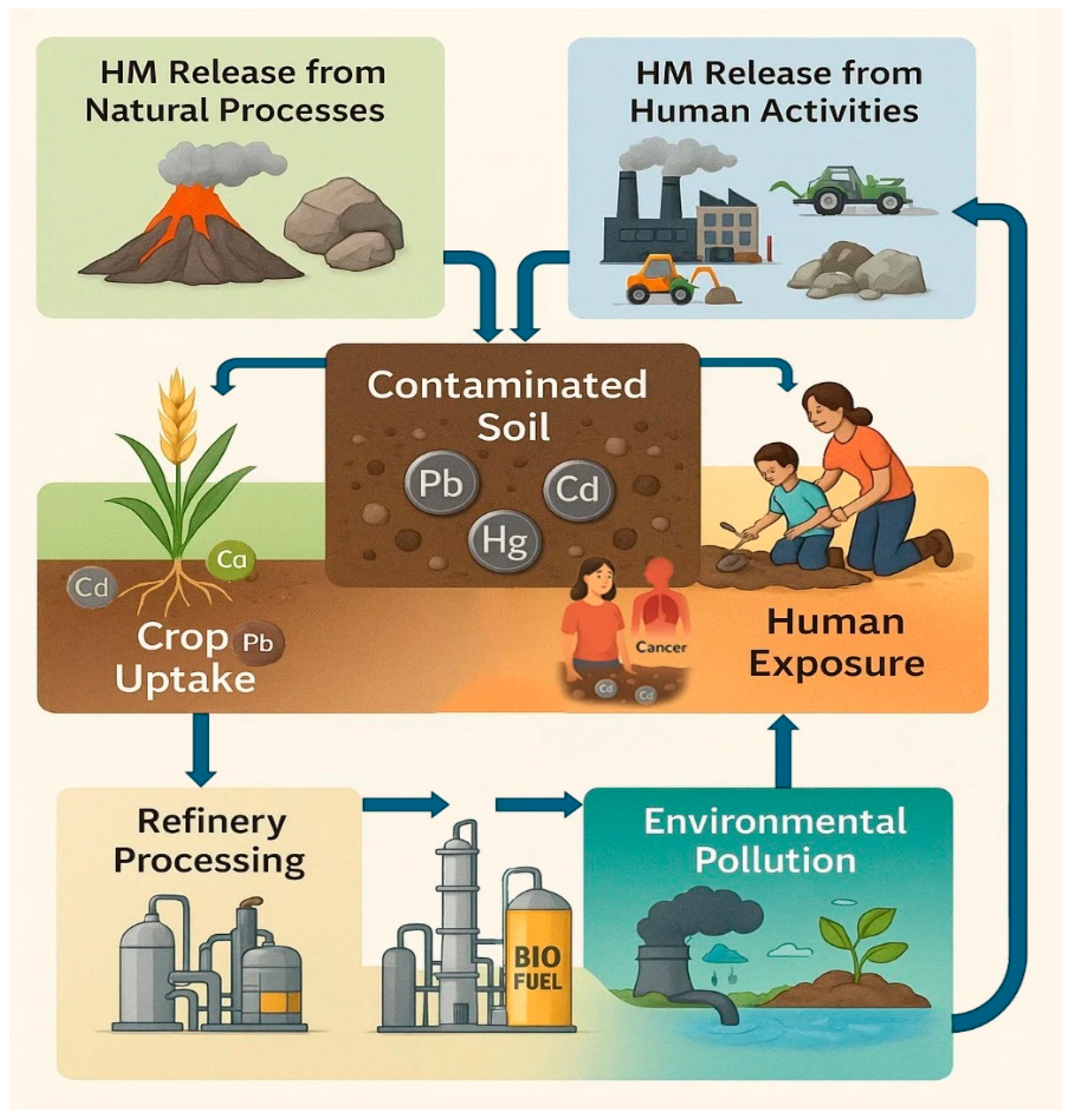
1. Introduction
2. Mechanisms of Heavy Metal Uptake and Translocation in Bioenergy Crops
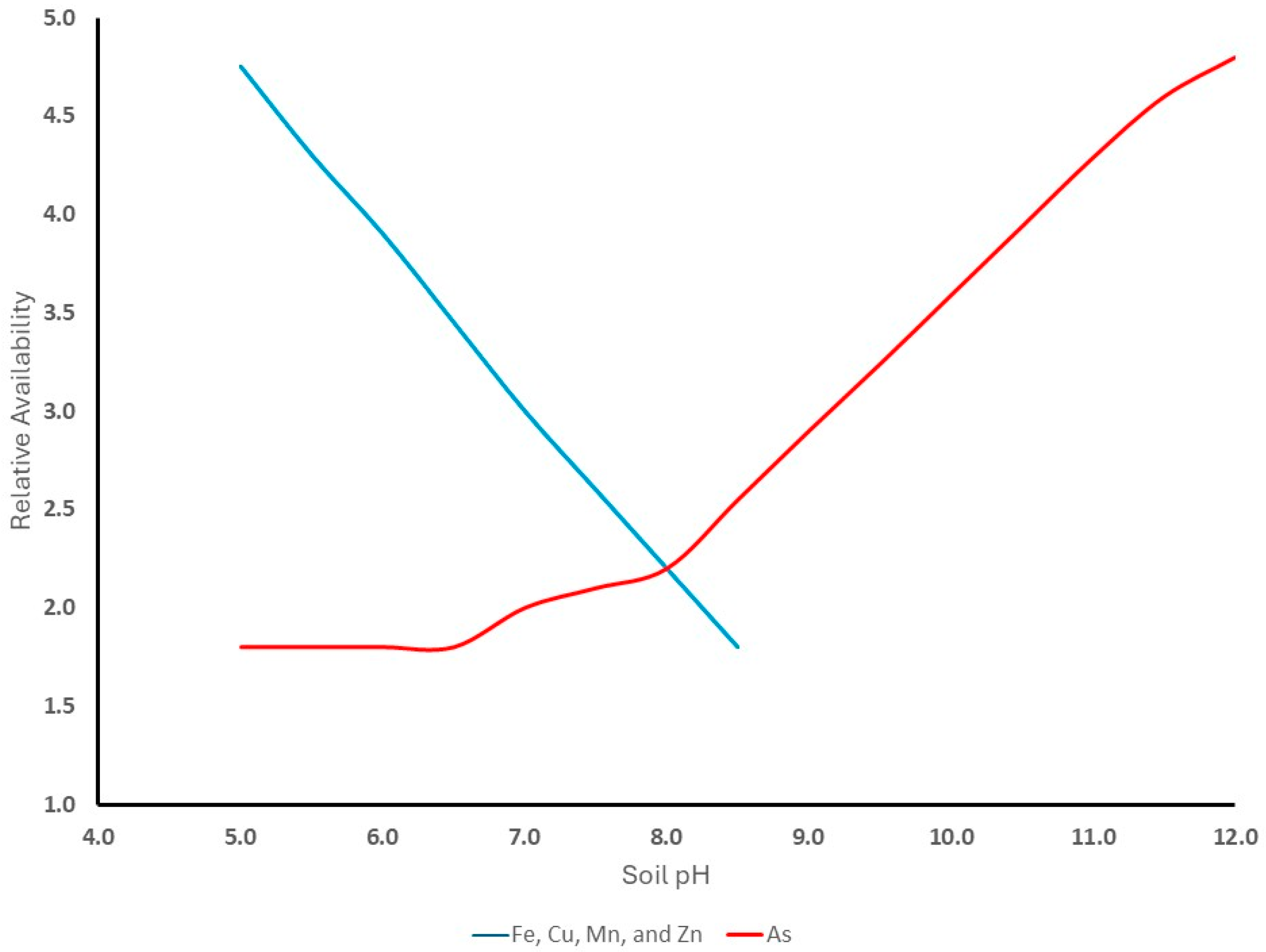
2.1. Soil–Plant Interactions
2.2. Uptake and Translocation Mechanisms
2.3. Physiological and Biochemical Responses
2.4. Species-Specific Responses
3. Impacts of Heavy Metals on Bioenergy Crop Production
3.1. Growth and Yield Reduction
3.2. Soil Health and Microbial Interactions
3.3. Global and Regional Impacts
3.4. Socioeconomic Implications
4. Impacts of Heavy Metals on Biomass Quality and Biorefineries
4.1. Lignocellulosic Composition
4.2. Contaminant Transfer to Biomass
4.3. Quality Standards and Safety
4.4. Impacts on Biorefinery Processes
4.4.1. Bioethanol Production
4.4.2. Biodiesel and Biogas Production
| Heavy Metal | Observed Impact (Biomass Yield, Quality and Biorefinery) | Energy Crop | Heavy Metal Concentration (mg/kg) | Effect Category | Country | Reference |
|---|---|---|---|---|---|---|
| Cd, Pb, Zn | Reduced macronutrient status, notably N and K drought-induced senescence. Compromised mineral nutrition and yield stability. | Miscanthus × giganteus, Spartina pectinata | Cd: 20.6–21.1 (M. × giganteus), 13.6–14.0 (S. pectinata); Pb: 532–535 (M. × giganteus), 363–373 (S. pectinata); Zn: elevated, strongly influenced by fertilization. | Negative | Poland | [95] |
| Cu, Zn, Pb, Cd | HMs reduced shoot and root biomass. | Sorghum, Maize | Cd: 1.8; Cu: 75; Zn: 50–75; Pb: 85–130. | Negative | Nigeria | [96] |
| Cd, Pb, Cu, Zn, Cr | HM-contaminated biomass reduced the quality of biorefinery products. | Bioenergy crops | Maize: Cu 327–338, Pb 340, Zn 365–384; Willow: Zn 822–4636, Cd 40–80, Cu 11–15, Pb 14–26; Arundo: Cd 5–16, Cu 5–6, Pb 17–27. | Negative | China or Global | [69,97] |
| As Pb, Sb | Significant reduction in biomass yield; compromised biomass quality and biorefinery efficiency. | Miscanthus × giganteus | As 1700–83,000; Pb < 500–15,200; Sb 18–27. Plant: roots 602–1285 As, 38–327 Pb, 18–27 Sb; shoots 4–17 As, 1–43 Pb, 0.2–1.1 Sb. | Negative | France | [98] |
| Cd, Pb, Zn | Significant increase in biomass when remediated with mycorrhizal fungi. | Miscanthus × giganteus | Cd 12–15, Pb 675–815, Zn 819–1081. Roots accumulated much more than shoots. | Positive | France | [99] |
| Cd, Pb, Zn, Cu, Ni | M. × giganteus and S. pectinata: high yield, unaffected by contamination; P. virgatum: low yield, high metal uptake. | Miscanthus × giganteus, Spartina pectinata, Panicum virgatum | Cd 23–27; Pb 621–720; Zn 2590–3312. | Negative to Neutral | Poland | [47,100] |
| As, B, Cd, Cr, Cu, Pb, Hg, Ni, Zn | Miscanthus and willow showed poor yields; switchgrass failed to establish, and reed canary grass grew well with consistent high yields. | Miscanthus sp., willow, switchgrass, and reed canary grass | As: 145; B: 54; Cd: 5.5; Cr: 117; Cu: 229; Pb: 701; Hg: 2.7; Ni: 52; Zn: 3890. | Negative and Positive | England | [101] |
| Pb | The presence of HMs reduced the efficiency of the biorefinery system. | Switchgrass | 120 (initial), reduced to 10 after 3.5 years | Negative | USA | [35] |
| Cd, Pb | Significant reduction in biomass yield. | Switchgrass | Cd: 30–110.46; Pb: 400–1204.6 | Negative | China | [44] |
| Cd, Cr, Cu, Ni, Zn | High Cd and Zn caused severe leaf chlorosis; Cd, Cu, and Ni caused significant biomass reduction; Cd stress reduced photosynthesis. | Jatropha curcas | Cd: 11.2; Cu: 6.35; Ni: 5.9; Zn: 654; Cr: 5.2. | Negative | Japan | [43] |
| Cd | Cd reduced total biomass, hastened leaf senescence, reduced root length, and increased root diameter. | Miscanthus sinensis | Cd: 62–86. | Negative | Italy | [102] |
| Cd, Zn, Pb | Phytoattenuation of maize resulted in minimal removal of Cd and Pb, while Zn removal was significant. | Maize | Cd: 2; Pb: 9–10; Zn: 450–550. | Negative | Belgium and The Netherlands | [103] |
| Cd, Hg | Slight reduction in yield; low Cd and Hg in biomass; suitable for phytostabilization and biofuel. | Miscanthus × giganteus | Cd: 6.76; Hg: 0.109. | Positive and Negative | Croatia | [104] |
| Cd | Increased lignin accumulation; reduced bioethanol production. | Miscanthus sp. | Cd: 100. | Negative | China | [66] |
| Cd, Cr, Cu, Mn, Ni, Pb, Zn | HMs have no negative impact on biomass yield, and their concentration in ash remains below threshold limits. | Arundo donax and Phragmites australis | Arundo: Cd: 0.06–1.76; Cr: 1.56–4.96; Cu: 25–121; Mn: 62–94; Ni: 5–11; Pb: 0.31–1.76; Zn: 24–180. Cd < 0.10; Cr 0.15–0.08; Cu 2.60–1.12; Mn 23.0–5.65; Ni 3.98–1.27; Pb 5.82–3.61; Zn 10.2–6.32. | Positive | Italy | [105,106] |
| Cd, Pb, Zn | Biomass retained thermal quality; Miscanthus and Spartina suited for combined phytoremediation and energy use. | Miscanthus × giganteus, Spartina pectinata, Sida hermaphrodita, Panicum virgatum | Not specified. | Positive | Poland | [107] |
| Pb | Increased biomass under Pb stress by limiting translocation to shoots. | Salix matsudana | Pb: 9000–27,600. | Positive | China | [108] |
| Fe, As, Cr, Cu, Mn, Ni, Pb, Zn | Biomass unaffected by HM contamination under in vitro conditions. | Arundo donax | Cr: 100; Cd: 500 (lethal at 1000); Cu: 3000; Ni: 280; Pb: 270. | Neutral | Spain | [109] |
| Cd, Zn | High biomass production; suitable for bioenergy production. | Pennisetum purpureum, Arundo donax, Miscanthus sp., Panicum virgatum | Cd: 1.6–47; Zn: 2000. | Positive | China | [110] |
| Cd, Zn, Pb | Biomass is unaffected by HM contamination. | Miscanthus × giganteus | Cd: 1.8–1.9; Zn: 6.9–62.9; Pb: 0.6–10.6. | Neutral | Germany | [111] |
| Cd, Pb, Cu, Zn, Ni, As | Significant reduction in biomass yield. | Miscanthus × giganteus | Cd: 2.1–2.2; Ni: 2.9–3.2; Zn: 206–241; Pb: 4.7–4.9; Cu: 8.7–9.6; Cr: 4–5; As: 6.1–8.1. | Negative | Belgium | [112] |
| Zn | Biomass yield and tolerance varied by genotype; some were tolerant while others were sensitive. | Miscanthus sp., Arundo donax | Not specified. | Positive and Negative | Italy | [113] |
5. Environmental and Health Risks
5.1. Atmospheric Emissions
5.2. Ecological Impacts
5.3. Human Health Consequences
6. Sustainable Management Strategies
6.1. Phytoremediation
| Heavy Metals | Phytoremediation Potential | Heavy Metal Concentration (mg/kg) | Energy Crops | Country | Reference |
|---|---|---|---|---|---|
| Cd | High phytoextraction potential under well-drained conditions; phytostabilization under flooded conditions. | Cd: 63–159 | Salix sps. Willow | China | [146] |
| Zn, Cu, Cd | Removed Zn, Cu, and Cd; suitable for bioenergy. | Zn: 24.0–121.0; Cu: 4.4–11.0; Cd: 0.02–0.35. | Arundo donax | Italy | [105] |
| Cd, Al | Suitable for phytoremediation-accumulated Cd and Al in two years. | Al: 10.4–116; Cd: 0.55–8.9. | Populus sps. | Belgium | [147] |
| Cd, Zn, V, Pb, Cu, Ni, Sb, Mn, As, Th, Hg, Sn, Cr, Co, Al | Miscanthus giganteus: High biomass; multi-HM removal. Phalaris arundinacea: Moderate biomass; effective HM uptake. | Cd: 0.094–0.211; Pb: 1.06–1.81; Cu: 2.73–9.89; Zn: 36.9–61.9; Mn: 10.0–32.5; Cr: 1.21–1.82; Ni: 0.021–0.036; As: 0.003–0.005; Co: 0.0023–0.0058; Hg: 0.087–0.163. | Miscanthus giganteus Phalaris arundinacea | Ukraine | [148] |
| Cd, Cr, Cu, Pb, Hg, Ni, Zn | Arundo donax—Accumulates Cd, Cr, and Cu; strong phytoextraction and rhizofiltration. Miscanthus × giganteus—Zn accumulator; suited for marginal soils. Panicum virgatum-Cd removal; Cr phytoextraction. Pennisetum purpureum—Similar to P. virgatum; efficient in Cr rhizofiltration. Sida hermaphrodita—Strong accumulator of Cd, Ni, Pb, and Zn. Sorghum x drummondii—Mycorrhizal-assisted HM uptake. | Not specified. | Arundo donax, Miscanthus × giganteus, Panicum virgatum, Pennisetum purpureum, Sida hermaphrodita and Sorghum × drummondii | Croatia | [149] |
| Zn, Pb, Cr | Suitable for phytostabilization. | Not specified. | Miscanthus sps. and Arundo donax | Italy | [150] |
| Zn, Cr | High Zn and Cr tolerance and uptake. | Zn: 1105; Cr: 348. | Arundo donax, Miscanthus sacchariflorus | China | [151] |
| Cd, Ni, Zn | Year 1: high Cd, Zn, and Ni; declines in year 2; potential for phytoextraction. | Cd: 0.35; Ni: 5.0; Zn: 123. | Miscanthus × giganteus, Phalaris arundinacea | Poland | [152] |
| Cu, Ni, Zn | Zn stabilization or extraction; low Cu or Ni potential. | Cu: 43; Ni: 126; Zn: 1385. | Miscanthus × giganteus, Spartina pectinata | Poland | [153] |
| Cr, Zn, Cu, Ni | Phytoremediation potential of HMs: Cr > Zn > Cu > Ni. | Cr: 250; Zn: 1616; Cu: 223; Ni: 75. | Salix schwerinii | Finland | [154] |
| Cd, Zn, Cu | Salix clones showed higher potential than willow clones. | Willow: Cd: 6.8; Zn = 909; Cu = 17.7. | Clones of willow and poplar trees | Czech Republic | [155] |
| Populus: Cd: 2.06; Zn: 463, Cu: 11.8. | |||||
| Cd | Enhanced Cd phytoextraction through synergistic inoculation with Cd-tolerant Bacillus spp. and arbuscular mycorrhizae. | Cd: 2.3. | Arundo donax | India | [156] |
6.2. Microbial Bioremediation
6.3. Soil Amendments
6.4. Crop Selection and Genetic Engineering
6.5. Policy Framework for Heavy Metal Management in Bioenergy Systems
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashemi, M.; Sadeghpour, A. Establishment and production of switchgrass grown for combustion: A review. Int. J. Plant Biol. Res. 2013, 1, 1002. [Google Scholar]
- Sadeghpour, A.; Hashemi, M.; DaCosta, M.; Gorlitsky, L.E.; Jahanzad, E.; Herbert, S.J. Assessing winter cereals as cover crops for weed control in reduced-tillage switchgrass establishment. Ind. Crops Prod. 2014, 62, 522–525. [Google Scholar] [CrossRef]
- Gorlitsky, L.E.; Sadeghpour, A.; Hashemi, M.; Etemadi, F.; Herbert, S.J. Biomass vs. quality tradeoffs for switchgrass in response to fall harvesting period. Ind. Crops Prod. 2015, 63, 311–315. [Google Scholar] [CrossRef]
- Ozturk, M.; Saba, N.; Altay, V.; Iqbal, R.; Hakeem, K.R.; Jawaid, M.; Ibrahim, F.H. Biomass and bioenergy: An overview of the development potential in Turkey and Malaysia. Renew. Sustain. Energy Rev. 2017, 79, 1285–1302. [Google Scholar] [CrossRef]
- Karp, A.; Shield, I. Bioenergy from plants and the sustainable yield challenge. New Phytol. 2008, 179, 15–32. [Google Scholar] [CrossRef]
- Battaglia, M.; Fike, J.; Fike, W.; Sadeghpour, A.; Diatta, A. Miscanthus × giganteus biomass yield and quality in the Virginia Piedmont. Grassl. Sci. 2019, 65, 233–240. [Google Scholar] [CrossRef]
- Ford, J.S.; Bale, C.S.E.; Taylor, P.G. The factors determining uptake of energy crop cultivation and woodland creation in England: Insights from farmers and landowners. Biomass Bioenergy 2024, 180, 107021. [Google Scholar] [CrossRef]
- Melebary, S.J. Heavy Metal Toxicity and Remediation in Human and Agricultural Systems: An Updated Review. Adv. Anim. Vet. Sci. 2023, 11, 679–694. [Google Scholar] [CrossRef]
- Deng, M.; Yang, X.; Dai, X.; Zhang, Q.; Malik, A.; Sadeghpour, A. Heavy metal pollution risk assessments and their transportation in sediment and overlay water for the typical Chinese reservoirs. Ecol. Indic. 2020, 112, 106166. [Google Scholar] [CrossRef]
- Deng, M.; Malik, A.; Zhang, Q.; Sadeghpour, A.; Zhu, Y.; Li, Q. Improving Cd risk managements of rice cropping system by integrating source-soil-rice-human chain for a typical intensive industrial and agricultural region. J. Clean. Prod. 2021, 313, 127883. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.G.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil—Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Brevik, E.C.; Cerdà, A.; Mataix-Solera, J.; Pereg, L.; Quinton, J.N.; Six, J.; Van Oost, K. The interdisciplinary nature of SOIL. SOIL 2015, 1, 117–129. [Google Scholar] [CrossRef]
- Brevik, E.C.; Burgess, L.C. Soils and Human Health; CRC Press: Boca Raton, FL, USA, 2013; p. 391. [Google Scholar] [CrossRef]
- Pogrzeba, M.; Krzyżak, J.; Rusinowski, S.; McCalmont, J.P.; Jensen, E. Energy Crop at Heavy Metal-Contaminated Arable Land as an Alternative for Food and Feed Production: Biomass Quantity and Quality. In Plant Metallomics and Functional Omics: A System-Wide Perspective; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–21. [Google Scholar] [CrossRef]
- Gomes, L.; Costa, J.; Moreira, J.; Cumbane, B.; Abias, M.; Santos, F.; Zanetti, F.; Monti, A.; Fernando, A.L. Switchgrass and Giant Reed Energy Potential when Cultivated in Heavy Metals Contaminated Soils. Energies 2022, 15, 5538. [Google Scholar] [CrossRef]
- European Commission. Renewable Energy Directive II (RED II). Off. J. Eur. Union 2018, L 328, 82–209. [Google Scholar]
- Khaledian, Y.; Pereira, P.; Brevik, E.C.; Pundyte, N.; Paliulis, D. The Influence of Organic Carbon and pH on Heavy Metals, Potassium, and Magnesium Levels in Lithuanian Podzols. Land Degrad. Dev. 2017, 28, 345–354. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Barbosa, B.; Costa, J.; Fernando, A.L. Production of Energy Crops in Heavy Metals Contaminated Land: Opportunities and Risks. In Land Allocation for Biomass Crops: Challenges and Opportunities with Changing Land Use; Springer: Cham, Switzerland, 2018; pp. 83–102. [Google Scholar]
- Manning, B.A.; Goldberg, S. Modeling Competitive Adsorption of Arsenate with Phosphate and Molybdate on Oxide Minerals. Soil Sci. Soc. Am. J. 1996, 60, 121–131. [Google Scholar] [CrossRef]
- Ning, X.; He, L.; Long, S.; Wang, S. Bioavailability, migration and driving factors of As, Cd and Pb in calcareous soil amended with organic fertilizer and manganese oxidizing bacteria in arid northwest China. J. Hazard. Mater. 2025, 489, 137528. [Google Scholar] [CrossRef]
- Poulsen, I.F.; Hansen, H.C.B. Soil sorption of nickel in presence of citrate or arginine. Water Air Soil Pollut. 2000, 120, 249–259. [Google Scholar] [CrossRef]
- Sun, S.Q.; He, M.; Cao, T.; Yusuyin, Y.; Han, W.; Li, J.L. Antioxidative responses related to H2O2 depletion in Hypnum plumaeforme under the combined stress induced by Pb and Ni. Environ. Monit. Assess. 2010, 163, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; Víllora, G.; Soriano, M.T.; Castilla, N.; Romero, L. Sulfur, chromium, and selenium accumulated in Chinese cabbage under direct covers. J. Environ. Manag. 2005, 74, 89–96. [Google Scholar] [CrossRef]
- Bachate, S.P.; Khapare, R.M.; Kodam, K.M. Oxidation of arsenite by two β-proteobacteria isolated from soil. Appl. Microbiol. Biotechnol. 2012, 93, 2135–2145. [Google Scholar] [CrossRef]
- Bolan, N.S.; Choppala, G.; Kunhikrishnan, A.; Park, J.; Naidu, R. Microbial transformation of trace elements in soils in relation to bioavailability and remediation. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2013; Volume 225, pp. 1–56. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Meena, N.L.; Singh, U. Zinc Transporter: Mechanism for Improving Zn Availability. In Biofortification of Food Crops; Springer: New Delhi, India, 2016; pp. 129–146. [Google Scholar] [CrossRef]
- Fernando, A.L.; Barbosa, B.; Gomes, L.A.; Costa, J.; Papazoglou, E.G. Giant reed (Arundo donax L.)—A multi-purpose crop bridging phytoremediation with sustainable bioeconomy. In Bioremediation and Bioeconomy: A Circular Economy Approach, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 119–144. [Google Scholar] [CrossRef]
- McBride, M.B.; Martinez, C.E.; Kim, B. Zn, Cd, S and trace metal bioaccumulation in willow (Salix spp.) cultivars grown hydroponically. Int. J. Phytoremediation 2016, 18, 1178–1186. [Google Scholar] [CrossRef]
- P.P., S.; Puthur, J.T. Heavy Metal Phytoremediation by Bioenergy Plants and Associated Tolerance Mechanisms. Soil Sediment Contam. 2021, 30, 253–274. [Google Scholar] [CrossRef]
- Lynch, M.N.; Satrio, J.A. Utilization of Grassy Biomass Grown in Heavy-Metal Contaminated Soil as Feedstock for Bioenergy Production—An LCA Study. IOP Conf. Ser. Earth Environ. Sci. 2022, 1034, 012022. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Påhlsson, A.M.B. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants—A literature review. Water Air Soil Pollut. 1989, 47, 287–319. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and Molecular Mechanisms of Plant Responses to Copper Stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Marchand, L.; Douay, F.; Mench, M. Arundo donax L., a Candidate for Phytomanaging Water and Soils Contaminated by Trace Elements and Producing Plant-Based Feedstock. A Review. Int. J. Phytoremediation 2014, 16, 982–1017. [Google Scholar] [CrossRef]
- Yamada, M.; Malambane, G.; Yamada, S.; Suharsono, S.; Tsujimoto, H.; Moseki, B.; Akashi, K. Differential physiological responses and tolerance to potentially toxic elements in biodiesel tree Jatropha curcas. Sci. Rep. 2018, 8, 1635. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Cao, X.; Jiang, W.; Liu, X.; Liu, Q.; Chen, Z.; Zhou, W.; Cui, J.; Wang, Q. Phytoremediation of Cd and Pb interactive polluted soils by switchgrass (Panicum virgatum L.). Int. J. Phytoremediation 2019, 21, 1486–1496. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Esteban, E.; Peñalosa, J.M. The fate of arsenic in soil-plant systems. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2012; Volume 215, pp. 1–37. [Google Scholar] [CrossRef]
- Rusinowski, S.; Krzyżak, J.; Sitko, K.; Kalaji, H.M.; Jensen, E.; Pogrzeba, M. Cultivation of C4 perennial energy grasses on heavy metal contaminated arable land: Impact on soil, biomass, and photosynthetic traits. Environ. Pollut. 2019, 250, 300–311. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Zhang, H.; Fan, Q.; Guo, B.; Li, J. A global meta-analysis reveals effects of heavy metals on soil microorganisms. J. Hazard. Mater. 2025, 491, 138018. [Google Scholar] [CrossRef]
- Xiao, Z.; Duan, C.; Li, S.; Chen, J.; Peng, C.; Che, R.; Liu, C.; Huang, Y.; Mei, R.; Xu, L.; et al. The microbial mechanisms by which long-term heavy metal contamination affects soil organic carbon levels. Chemosphere 2023, 340, 139770. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil organic matter prevails over heavy metal pollution and vegetation as a factor shaping soil microbial communities at historical Zn–Pb mining sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef]
- Moreno, J.L.; Bastida, F.; Ros, M.; Hernández, T.; García, C. Soil organic carbon buffers heavy metal contamination on semiarid soils: Effects of different metal threshold levels on soil microbial activity. Eur. J. Soil Biol. 2009, 45, 220–228. [Google Scholar] [CrossRef]
- Weidhuner, A.; Hanauer, A.; Krausz, R.; Crittenden, S.J.; Gage, K.; Sadeghpour, A. Tillage impacts on soil aggregation and aggregate-associated carbon and nitrogen after 49 years. Soil Tillage Res. 2021, 208, 104878. [Google Scholar] [CrossRef]
- Liptzin, D.; Norris, C.E.; Cappellazzi, S.B.; Bean, G.M.; Cope, M.; Greub, K.L.H.; Rieke, E.L.; Tracy, P.W.; Aberle, E.; Ashworth, A.; et al. An evaluation of carbon indicators of soil health in long-term agricultural experiments. Soil Biol. Biochem. 2022, 172, 108708. [Google Scholar] [CrossRef]
- Rieke, E.L.; Bagnall, D.K.; Morgan, C.L.S.; Flynn, K.D.; Howe, J.A.; Greub, K.L.H.; Bean, G.M.; Cappellazzi, S.B.; Cope, M.; Liptzin, D.; et al. Evaluation of aggregate stability methods for soil health. Geoderma 2022, 428, 116156. [Google Scholar] [CrossRef]
- Friedlova, M. The influence of heavy metals on soil biological and chemical properties. Soil Water Res. 2010, 5, 21–27. [Google Scholar] [CrossRef]
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.G.; Hu, Q.; Zhao, F.J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection; Ministry of Land and Resources. Bulletin of National Soil Pollution Survey; People’s Republic of China; Cambridge University Press: Cambridge, UK, 2014; Available online: https://cwrrr.org/research-reports/national-soil-pollution-survey-report/ (accessed on 21 July 2025).
- Jensen, J.K.; Holm, P.E.; Nejrup, J.; Larsen, M.B.; Borggaard, O.K. The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environ. Pollut. 2009, 157, 931–937. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar] [CrossRef]
- Eijsackers, H.; Reinecke, A.; Reinecke, S.; Maboeta, M. Heavy Metal Threats to Plants and Soil Life in Southern Africa: Present Knowledge and Consequences for Ecological Risk Assessment. Rev. Environ. Contam. Toxicol. 2019, 249, 29–70. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Elbersen, H.W.; Cosentino, S.L.; Zatta, A.; Alexopoulou, E.; Monti, A. Agronomic aspects of future energy crops in Europe. Biofuels Bioprod. Biorefining 2010, 4, 674–691. [Google Scholar] [CrossRef]
- Gelaye, Y. Public health and economic burden of heavy metals in Ethiopia: Review. Heliyon 2024, 10, e39022. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Chang, J.; Xia, B. Using bioenergy crop cassava (Manihot esculenta) for reclamation of heavily metal-contaminated land. Int. J. Phytoremediation 2020, 22, 1313–1320. [Google Scholar] [CrossRef]
- Baker, K.; Koduru, S.; Babaei, S.; Adeyemi, O.; Williams, G.; Armstrong, S.; Margenot, A.J.; Sadeghpour, A. Precision planting effect on winter rye yield and quality for biofuel and forage production. Biomass Bioenergy 2024, 184, 107219. [Google Scholar] [CrossRef]
- Koduru, S.; Gorlitsky, L.; Hashemi, M.; Herbert, S.J.; Sadeghpour, A. Decreasing the cutting height increases young and mature switchgrass yield with no impact on quality and stand vigor at different harvesting seasons. Biomass Bioenergy 2024, 183, 107153. [Google Scholar] [CrossRef]
- Cheng, S.; Yu, H.; Hu, M.; Wu, Y.; Cheng, L.; Cai, Q.; Tu, Y.; Xia, T.; Peng, L. Miscanthus accessions distinctively accumulate cadmium for largely enhanced biomass enzymatic saccharification by increasing hemicellulose and pectin and reducing cellulose CrI and DP. Bioresour. Technol. 2018, 263, 67–74. [Google Scholar] [CrossRef]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant Sci. 2017, 8, 286473. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Hoson, T.; Masuda, H. The Modification of Cell Wall Properties Is Involved in the Growth Inhibition of Rice Coleoptiles Induced by Lead Stress. Life 2023, 13, 471. [Google Scholar] [CrossRef]
- Mehrez, K.; Fryda, L.; Visser, R.; Kane, A.; Leblanc, N.; Djelal, H. Hydrothermal processes of contaminated biomass: Fate of heavy metals and liquid effluent valorization. Biomass Conv. Bioref. 2025, 15, 11493–11508. [Google Scholar] [CrossRef]
- Dastyar, W.; Raheem, A.; He, J.; Zhao, M. Biofuel Production Using Thermochemical Conversion of Heavy Metal-Contaminated Biomass (HMCB) Harvested from Phytoextraction Process. Chem. Eng. J. 2019, 358, 759–785. [Google Scholar] [CrossRef]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Environ. Manag. 2019, 240, 266–272. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Li, Z.; Men, Y.; Wu, J. Impacts of Cellulase and Amylase on Enzymatic Hydrolysis and Methane Production in the Anaerobic Digestion of Corn Straw. Sustainability 2020, 12, 5453. [Google Scholar] [CrossRef]
- Chai, Y.; Bai, M.; Chen, A.; Peng, L.; Shao, J.; Shang, C.; Peng, C.; Zhang, J.; Zhou, Y. Thermochemical conversion of heavy metal contaminated biomass: Fate of the metals and their impact on products. Sci. Total Environ. 2022, 822, 153426. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Rolbiecki, D.; Korzeniewska, E.; Harnisz, M. Heavy Metal and Antimicrobial Residue Levels in Various Types of Digestate from Biogas Plants—A Review. Sustainability 2025, 17, 416. [Google Scholar] [CrossRef]
- US EPA. Metals. Available online: https://www.epa.gov/caddis/metals (accessed on 30 June 2025).
- Abdel-Shafy, H.I.; Mansour, M.S.M. Biogas production as affected by heavy metals in the anaerobic digestion of sludge. Egypt. J. Pet. 2014, 23, 409–417. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Energy Potential of Zea mays Grown in Cadmium-Contaminated Soil. Energies 2025, 18, 2402. [Google Scholar] [CrossRef]
- Carvalho, D.J.; Moretti, R.R.; Colodette, J.L.; Bizzo, W.A. Assessment of the self-sustained energy generation of an integrated first and second generation ethanol production from sugarcane through the characterization of the hydrolysis process residues. Energy Convers. Manag. 2020, 203, 112267. [Google Scholar] [CrossRef]
- Ko, D.; Lee, J.S.; Patel, H.A.; Jakobsen, M.H.; Hwang, Y.; Yavuz, C.T.; Hansen, H.C.B.; Andersen, H.R. Selective removal of heavy metal ions by disulfide linked polymer networks. J. Hazard. Mater. 2017, 332, 140–148. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Parhi, P.K.; Patra, J.K.; Panda, C.R.; Thatoi, H.N. Biodetoxification of Toxic Heavy Metals by Marine Metal Resistant Bacteria—A Novel Approach for Bioremediation of the Polluted Saline Environment. Microb. Biotechnol. 2017, 1, 343–376. [Google Scholar] [CrossRef]
- Ko, C.H.; Wang, Y.N.; Chang, F.C.; Chen, J.J.; Chen, W.H.; Hwang, W.S. Potentials of lignocellulosic bioethanols produced from hardwood in Taiwan. Energy 2012, 44, 329–334. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of Lignocellulosic Biomass to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- CKo, H.; Yu, F.C.; Chang, F.C.; Yang, B.Y.; Chen, W.H.; Hwang, W.S.; Tu, T.C. Bioethanol production from recovered napier grass with heavy metals. J. Environ. Manag. 2017, 203, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, Q.; Wu, M.; Meng, X.; Song, R.; Gao, M. Improved ethanol production in the presence of cadmium ions by a Saccharomyces cerevisiae transformed with a novel cadmium-resistance gene DvCRP1. Environ. Technol. 2016, 37, 2945–2952. [Google Scholar] [CrossRef]
- Skountzou, P.; Soupioni, M.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Marchant, R.; Banat, I.M. Lead(II) uptake during baker’s yeast production by aerobic fermentation of molasses. Process Biochem. 2003, 38, 1479–1482. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Scrano, L.; Siesto, G.; Romano, P. Yeast Starter as a Biotechnological Tool for Reducing Copper Content in Wine. Front. Microbiol. 2018, 8, 2632. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Liu, L.; Jia, B.; Zhao, F.; Huang, W.; Zhan, J. Copper Tolerance and Biosorption of Saccharomyces cerevisiae during Alcoholic Fermentation. PLoS ONE 2015, 10, e0128611. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Liu, X.; Wang, Y. The Fate of Heavy Metals and Risk Assessment of Heavy Metal in Pyrolysis Coupling with Acid Washing Treatment for Sewage Sludge. Toxics 2023, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S. Disruption of RIM15 confers an increased tolerance to heavy metals in Saccharomyces cerevisiae. Biotechnol. Lett. 2020, 42, 1193–1202. [Google Scholar] [CrossRef]
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef]
- Mudhoo, A.; Kumar, S. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int. J. Environ. Sci. Technol. 2013, 10, 1383–1398. [Google Scholar] [CrossRef]
- Lin, C.Y. Effect of heavy metals on volatile fatty acid degradation in anaerobic digestion. Water Res. 1992, 26, 177–183. [Google Scholar] [CrossRef]
- Tian, K.; Li, M.; Hu, W.; Fan, Y.; Huang, B.; Zhao, Y. Environmental capacity of heavy metals in intensive agricultural soils: Insights from geochemical baselines and source apportionment. Sci. Total Environ. 2022, 819, 153078. [Google Scholar] [CrossRef] [PubMed]
- Pogrzeba, M.; Krzyżak, J.; Rusinowski, S.; Werle, S.; Hebner, A.; Milandru, A. Case study on phytoremediation driven energy crop production using Sida hermaphrodita. Int. J. Phytoremediation 2018, 20, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Nzeako, A.C.; Abdulhamid, A. Effects of heavy metals (Cu, Zn, Cd and Pb) on growth of selected maize and sorghum cultivas. Biol. Environ. Sci. J. Trop. 2024, 21, 249–257. [Google Scholar]
- Raikova, S.; Piccini, M.; Surman, M.K.; Allen, M.J.; Chuck, C.J. Making light work of heavy metal contamination: The potential for coupling bioremediation with bioenergy production. J. Chem. Technol. Biotechnol. 2019, 94, 3064–3072. [Google Scholar] [CrossRef]
- Wanat, N.; Austruy, A.; Joussein, E.; Soubrand, M.; Hitmi, A.; Gauthier-Moussard, C.; Lenain, J.F.; Vernay, P.; Munch, J.C.; Pichon, M. Potentials of Miscanthus × giganteus grown on highly contaminated Technosols. J. Geochem. Explor. 2013, 126–127, 78–84. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidi, S.; Fontaine, J.; Muchembled, J.; Sahraoui, A.L.-H.; Fourrier, H.; et al. Metal accumulation and shoot yield of Miscanthus × giganteus growing in contaminated agricultural soils: Insights into agronomic practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Kocoń, A.; Jurga, B. The evaluation of growth and phytoextraction potential of Miscanthus × giganteus and Sida hermaphrodita on soil contaminated simultaneously with Cd, Cu, Ni, Pb, and Zn. Environ. Sci. Pollut. Res. 2017, 24, 4990–5000. [Google Scholar] [CrossRef]
- Lord, R.A. Reed canarygrass (Phalaris arundinacea) outperforms Miscanthus or willow on marginal soils, brownfield and non-agricultural sites for local, sustainable energy crop production. Biomass Bioenergy 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Arduini, I.; Masoni, A.; Mariotti, M.; Ercoli, L. Low cadmium application increase miscanthus growth and cadmium translocation. Environ. Exp. Bot. 2004, 52, 89–100. [Google Scholar] [CrossRef]
- Meers, E.; Van Slycken, S.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Laing, G.D.; Witters, N.; Thewys, T.; Tack, F.M.G. The use of bio-energy crops (Zea mays) for ‘phytoattenuation’ of heavy metals on moderately contaminated soils: A field experiment. Chemosphere 2010, 78, 35–41. [Google Scholar] [CrossRef]
- Zgorelec, Z.; Bilandzija, N.; Knez, K.; Galic, M.; Zuzul, S. Cadmium and Mercury phytostabilization from soil using Miscanthus × giganteus. Sci. Rep. 2020, 10, 6685. [Google Scholar] [CrossRef]
- Danelli, T.; Sepulcri, A.; Masetti, G.; Colombo, F.; Sangiorgio, S.; Cassani, E.; Anelli, S.; Adani, F.; Pilu, R. Arundo donax L. Biomass Production in a Polluted Area: Effects of Two Harvest Timings on Heavy Metals Uptake. Appl. Sci. 2021, 11, 1147. [Google Scholar] [CrossRef]
- Bonanno, G.; Cirelli, G.L.; Toscano, A.; Giudice, R.L.; Pavone, P. Heavy metal content in ash of energy crops growing in sewage-contaminated natural wetlands: Potential applications in agriculture and forestry? Sci. Total Environ. 2013, 452–453, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Werle, S.; Bisorca, D.; Katelbach-Woźniak, A.; Pogrzeba, M.; Krzyżak, J.; Ratman-Kłosińska, I.; Burnete, D. Phytoremediation as an effective method to remove heavy metals from contaminated area—TG/FT-IR analysis results of the gasification of heavy metal contaminated energy crops. J. Energy Inst. 2017, 90, 408–417. [Google Scholar] [CrossRef]
- Tang, C.; Song, J.; Hu, X.; Hu, X.; Zhao, Y.; Li, B.; Ou, D.; Peng, L. Exogenous spermidine enhanced Pb tolerance in Salix matsudana by promoting Pb accumulation in roots and spermidine, nitric oxide, and antioxidant system levels in leaves. Ecol. Eng. 2017, 107, 41–48. [Google Scholar] [CrossRef]
- Cano-Ruiz, J.; Galea, M.R.; Amorós, M.C.; Alonso, J.; Mauri, P.V.; Lobo, M.C. Assessing Arundo donax L. in vitro-tolerance for phytoremediation purposes. Chemosphere 2020, 252, 126576. [Google Scholar] [CrossRef]
- Hou, X.; Teng, W.; Hu, Y.; Yang, Z.; Li, C.; Scullion, J.; Guo, Q.; Zheng, R. Potential phytoremediation of soil cadmium and zinc by diverse ornamental and energy grasses. Bioresources 2019, 15, 616–640. [Google Scholar] [CrossRef]
- Zadel, U.; Nesme, J.; Michalke, B.; Vestergaard, G.; Płaza, G.A.; Schröder, P.; Radl, V.; Schloter, M. Changes induced by heavy metals in the plant-associated microbiome of Miscanthus × giganteus. Sci. Total Environ. 2020, 711, 134433. [Google Scholar] [CrossRef]
- Lutts, S.; Zhou, M.X.; Flores-Bavestrello, A.; Hainaut, P.; Dailly, H.; Debouche, G.; Foucart, G. Season-dependent physiological behavior of Miscanthus × giganteus growing on heavy-metal contaminated areas in relation to soil properties. Heliyon 2024, 10, e25943. [Google Scholar] [CrossRef]
- Islam, M.; Ferrarini, A.; Ali, A.; Kam, J.; Trindade, L.M.; Clifton-Brown, J.; Amaducci, S. Assessment of Drought and Zinc Stress Tolerance of Novel Miscanthus Hybrids and Arundo donax Clones Using Physiological, Biochemical, and Morphological Traits. Biology 2023, 12, 1525. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, Y.; Hu, K.; Xia, R.; Zhou, J.; Zhou, J. Impacts of atmospheric deposition on the heavy metal mobilization and bioavailability in soils amended by lime. Sci. Total Environ. 2024, 914, 170082. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.B.; Wu, H.T.; Huang, C.K. Evaluation on speciation and removal efficiencies of mercury from municipal solid waste incinerators in Taiwan. Sci. Total Environ. 2000, 246, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Z.; Huang, S.; Zhang, P.; Peng, Y.; Wu, P.; Gu, J.; Dutkiewicz, S.; Zhang, H.; Wu, S.; et al. Global health effects of future atmospheric mercury emissions. Nat. Commun. 2021, 12, 3035. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline for Clinical Management of Exposure to Lead. 2020. Available online: https://www.who.int/publications/i/item/9789240037045 (accessed on 18 July 2025).
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S.P. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wen, Y.H.; Feng, D.; El-Mekkawy, R.M.; Kong, M.; Qin, X.; Lu, Q.; Dan, X.; Zhu, Q.; Tang, S.; et al. Cadmium inhibits carbon and nitrogen cycling through soil microbial biomass and reduces soil nitrogen availability. J. Hazard. Mater. 2025, 489, 137524. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Pereg, L.; Steffan, J.J.; Gedeon, C.; Thomas, P.; Brevik, E.C. Medical Geology of Soil Ecology. In Practical Applications of Medical Geology; Springer International Publishing: Cham, Switzerland, 2021; pp. 343–401. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; KC, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Nahmani, J.; Hodson, M.E.; Black, S. A review of studies performed to assess metal uptake by earthworms. Environ. Pollut. 2007, 145, 402–424. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Nathanail, P.; Tian, L.; Ma, Y. Integrated GIS and multivariate statistical analysis for regional scale assessment of heavy metal soil contamination: A critical review. Environ. Pollut. 2017, 231, 1188–1200. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of Environmental Methylmercury on the Health of Wild Birds, Mammals, and Fish. AMBIO 2007, 36, 12–19. [Google Scholar] [CrossRef]
- Permana, R.; Akbarsyah, N. Phytoplankton susceptibility towards toxic heavy metal cadmium: Mechanism and its recent updates. World News Nat. Sci. 2021, 38, 83–97. [Google Scholar]
- Lal, R.; Bouma, J.; Brevik, E.; Dawson, L.; Field, D.J.; Glaser, B.; Hatano, R.; Hartemink, A.E.; Kosaki, T.; Lascelles, B.; et al. Soils and sustainable development goals of the United Nations: An International Union of Soil Sciences perspective. Geoderma Reg. 2021, 25, e00398. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and Human Health: Current Status and Future Needs. Air Soil Water Res. 2020, 13, 1178622120934441. [Google Scholar] [CrossRef]
- Mansourri, G.; Madani, M.; Mansourri, G.; Madani, M. Examination of the Level of Heavy Metals in Wastewater of Bandar Abbas Wastewater Treatment Plant. Open J. Ecol. 2016, 6, 55–61. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, 3310. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy Metal Pollution in the Environment and Its Impact on Health: Exploring Green Technology for Remediation. Environ. Health Insights 2023, 2023, 17. [Google Scholar] [CrossRef]
- Environmental Science and Technology Briefs for Citizens Human Health Effects of Heavy Metals. Available online: www.engg.ksu.edu/CHSR/ (accessed on 16 August 2025).
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Qureshi, Y. Impact of Heavy Metals Consumption on Human Health: A Literature Review. J. Pharm. Res. Int. 2021, 33, 412–421. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Hashemi, M.; DaCosta, M.; Gorlitsky, L.E.; Jahanzad, E.; Herbert, S.J. Switchgrass Establishment and Biomass Yield Response to Seeding Date and Herbicide Application. Agron. J. 2015, 107, 142–148. [Google Scholar] [CrossRef]
- Khanna, M.; Chen, L.; Basso, B.; Cai, X.; Field, J.L.; Guan, K.; Jiang, C.; Lark, T.J.; Richard, T.L.; Spawn-Lee, S.A.; et al. Redefining marginal land for bioenergy crop production. GCB Bioenergy 2021, 13, 1590–1609. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Hashemi, M.; Jahanzad, E.; Herbert, S.J. Switchgrass Stand Density and Yield as Influenced by Seedbed Preparation Methods in a Sandy Loam Soil. Bioenergy Res. 2015, 8, 1840–1846. [Google Scholar] [CrossRef]
- Cumplido-Marin, L.; Graves, A.R.; Burgess, P.J.; Morhart, C.; Paris, P.; Jablonowsk, N.D.; Facciotto, G.; Bury, M.; Martens, R.; Nahm, M. Two Novel Energy Crops: Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L.—State of Knowledge. Agronomy 2020, 10, 928. [Google Scholar] [CrossRef]
- Sauer, T.J.; Wacha, K.M.; Brevik, E.C.; Zamora, D. Eastern red cedar effects on carbon sequestration and soil quality in the Great Plains. Soil Sci. Soc. Am. J. 2023, 87, 932–947. [Google Scholar] [CrossRef]
- Wijekoon, W.; Priyashantha, H.; Gajanayake, P.; Manage, P.; Liyanage, C.; Jayarathna, S.; Kumarasinghe, U. Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation. Sustainability 2025, 17, 822. [Google Scholar] [CrossRef]
- Sarath, N.G.; Puthur, J.T. Heavy metal pollution assessment in a mangrove ecosystem scheduled as a community reserve. Wetl. Ecol. Manag. 2021, 29, 719–730. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Y.; Ding, Z.; Yang, X.; Zhao, F.; Zhu, Z. Uptake and accumulation of cadmium in flooded versus non-flooded Salix genotypes: Implications for phytoremediation. Ecol. Eng. 2019, 136, 79–88. [Google Scholar] [CrossRef]
- Laureysens, I.; De Temmerman, L.; Hastir, T.; Van Gysel, M.; Ceulemans, R. Clonal variation in heavy metal accumulation and biomass production in a poplar coppice culture. II. Vertical distribution and phytoextraction potential. Environ. Pollut. 2005, 133, 541–551. [Google Scholar] [CrossRef]
- Romanchuk, L.; Matviichuk, N.; Abramova, I.; Matviichuk, B.; Tryboi, O. Removal of heavy metals by energy crops when grown on technologically contaminated soils. Ecol. Eng. Environ. Technol. 2025, 26, 92–102. [Google Scholar] [CrossRef]
- Prelac, M.; Bilandzija, N.; Zgorelec, Z. The phytoremediation potential of heavy metals from soil using Poaceae energy crops: A review. J. Cent. Eur. Agric. 2016, 17, 901–916. [Google Scholar] [CrossRef][Green Version]
- Nafziger, E.D. Nitrogen Rates for Corn Managing Nitrogen. 2021. Available online: https://extension.illinois.edu/sites/default/files/iah_-_nitrogen_management_for_corn_v4.pdf (accessed on 22 July 2025).
- Li, C.; Xiao, B.; Wang, Q.H.; Yao, S.H.; Wu, J.Y. Phytoremediation of Zn- and Cr-Contaminated Soil Using Two Promising Energy Grasses. Water Air Soil Pollut. 2014, 225, 2027. [Google Scholar] [CrossRef]
- Rosikon, K.; Fijalkowski, K.; Kacprzak, M. Phytoremediation Potential of Selected Energetic Plants (Miscanthus giganteus L. and Phalaris arundinacea L.) in Dependence on Fertilization. J. Environ. Sci. Eng. A 2015, 4, 587–595. [Google Scholar]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Phytoremediation potential of Miscanthus × giganteus and Spartina pectinata in soil contaminated with heavy metals. Environ. Sci. Pollut. Res. 2015, 22, 11648–11657. [Google Scholar] [CrossRef]
- Salam, M.M.A.; Kaipiainen, E.; Mohsin, M.; Villa, A.; Kuittinen, S.; Pulkkinen, P.; Pelkonen, P.; Mehtätalo, L.; Pappinen, A. Effects of contaminated soil on the growth performance of young Salix (Salix schwerinii E. L. Wolf) and the potential for phytoremediation of heavy metals. J. Environ. Manag. 2016, 183, 467–477. [Google Scholar] [CrossRef]
- Kacálková, L.; Tlustoš, P.; Száková, J. Phytoextraction of Risk Elements by Willow and Poplar Trees. Int. J. Phytoremediation 2015, 17, 414–421. [Google Scholar] [CrossRef]
- Sarathambal, C.; Khankhane, P.J.; Gharde, Y.; Kumar, B.; Varun, M.; Arun, S. The effect of plant growth-promoting rhizobacteria on the growth, physiology, and Cd uptake of Arundo donax L. Int. J. Phytoremediation 2017, 19, 360–370. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Chakraborty, S.; Yadav, Y.; Sharma, B.; Singh, S.; Arya, M. Bioremediation strategies and mechanisms of bacteria for resistance against heavy metals: A review. Bioremediation J. 2024, 1–33. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Darwesh, M.; Elbeltagy, H.; Abo-alhamd, F.G.; Amer, A.A.; Elsegaiy, M.A.; Khattab, I.A.; Elsharawy, E.A.; Ebehiry, F.; El-Ramady, H.; et al. Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: A review. Environ. Monit. Assess. 2021, 193, 449. [Google Scholar] [CrossRef] [PubMed]
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for heavy metal removal: A review. SN Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P.; Gorby, Y.A.; Landa, E.R. Microbial reduction of uranium. Nature 1991, 350, 413–416. [Google Scholar] [CrossRef]
- Fang, L.; Huang, Q.; Wei, X.; Liang, W.; Rong, X.; Chen, W.; Cai, P. Microcalorimetric and potentiometric titration studies on the adsorption of copper by extracellular polymeric substances (EPS), minerals and their composites. Bioresour. Technol. 2010, 101, 5774–5779. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Adhami, Z.; Shahmoradi, A.; Mousavi, S.N.; Sangi, M.R. Multivariate optimization of Cd(II) biosorption onto Ulmus tree leaves from aqueous waste. Environ. Toxicol. Chem. 2010, 92, 1461–1470. [Google Scholar] [CrossRef]
- Dinakarkumar, Y.; Ramakrishnan, G.; Gujjula, K.R.; Vasu, V.; Balamurugan, P.; Murali, G. Fungal bioremediation: An overview of the mechanisms, applications and future perspectives. Environ. Chem. Ecotoxicol. 2024, 6, 293–302. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015. [Google Scholar]
- Afshar, R.K.; Hashemi, M.; DaCosta, M.; Spargo, J.; Sadeghpour, A. Biochar Application and Drought Stress Effects on Physiological Characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Cole, E.J.; Barker, A.V.; Zandvakili, O.R.; Sadeghpour, A.; Xing, B.; Hashemi, M.; Allan-Perkins, E.; Jung, G. Soil nutrient and nematode community changes in response to hardwood charcoal application. Commun. Soil Sci. Plant Anal. 2021, 52, 917–925. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Jung, G.A.; Shaffer, J.A.; Stout, W.L. Switchgrass and Big Bluestem Responses to Amendments on Strongly Acid Soil. Agron. J 1988, 80, 669–676. [Google Scholar] [CrossRef]
- Reed, R.L.; Sanderson, M.A.; Allen, V.G.; Zartman, R.E. Cadmium application and pH effects on growth and cadmium accumulation in switchgrass. Commun. Soil Sci. Plant Anal. 2002, 33, 1187–1203. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Ketterings, Q.M.; Godwin, G.S.; Czymmek, K.J. Nitrogen- vs. Phosphorus-based manure and compost management of corn. Agron. J. 2016, 108, 185–195. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Ketterings, Q.M.; Godwin, G.S.; Czymmek, K.J. Shifting from N-based to P-based manure management maintains soil test phosphorus dynamics in a long-term corn and alfalfa rotation. Agron. Sustain. Dev. 2017, 37, 8. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Afshar, R.K. Livestock manure: From waste to resource in a circular economy. J. Agric. Food Res. 2024, 17, 101255. [Google Scholar] [CrossRef]
- Brevik, E.C. Soil, food security and human health. In Soils, Plant Growth and Crop Production; Verheye, W.H., Ed.; EOLSS Publishers: Oxford, UK, 2010; Volume III. [Google Scholar]
- Keshavarzi, A.; Kumar, V. Ecological risk assessment and source apportionment of heavy metal contamination in agricultural soils of Northeastern Iran. Int. J. Environ. Health Res. 2019, 29, 544–560. [Google Scholar] [CrossRef]
- Walker, D.J.; Clemente, R.; Bernal, M.P. Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 2004, 57, 215–224. [Google Scholar] [CrossRef]
- Saha, G.C.; Akhter, S.R.; Ali, M.A. Mobilization of arsenic from paddy field soil through adsorption-desorption and reductive dissolution processes. J. Civ. Eng. 2006, 34, 25–41. [Google Scholar]
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J. 2013, 1, 91–104. [Google Scholar] [CrossRef]
- Tsunematsu, S.; Uematsu, E.; Saito, K.; Tamura, H. Immobilization of arsenic in natural soils by gypsum powder, mechanistic interpretations. Trans. Jap. Soc. Irr. Drai. Rur. Eng. 2012, 80, 141–150. [Google Scholar]
- Vink, J.P.M.; Harmsen, J.; Rijnaarts, H. Delayed immobilization of heavy metals in soils and sediments under reducing and anaerobic conditions; consequences for flooding and storage. J. Soils Sediments 2010, 10, 1633–1645. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Gurajala, H.K.; Rashid, M.S.; He, Z.; Yang, X. Efficiency of lime, biochar, Fe containing biochar and composite amendments for Cd and Pb immobilization in a co-contaminated alluvial soil. Environ. Pollut. 2020, 257, 113609. [Google Scholar] [CrossRef]
- Vaughn, K.; Adeyemi, O.; Zandvakili, O.; Hunter, D.; Nair, J.; Still, S.; Sadeghpour, A. Winter rye cover crop biomass, nutrient uptake, and quality in response to fall and spring N fertilization. Cogent Food Agric. 2022, 8, 2132843. [Google Scholar] [CrossRef]
- Burkett, G.; Babaei, S.; Adeyemi, O.; Afshar, R.K.; Kula, C.; Vaughn, K.; Sadeghpour, A. Influence of manure injection versus surface application on corn for silage and winter rye yield, quality, phosphorus balance and soil test phosphorus. J. Agric. Food Res. 2024, 16, 101044. [Google Scholar] [CrossRef]
- Belimov, A.A.; Safronova, V.I.; Tsyganov, V.E.; Borisov, A.Y.; Kozhemyakov, A.P.; Stepanok, V.V.; Martenson, A.M.; Gianinazzi-Pearson, V.; Tikhonovich, I.A. Genetic variability in tolerance to cadmium and accumulation of heavy metals in pea (Pisum sativum L.). Euphytica 2003, 131, 25–35. [Google Scholar] [CrossRef]
- Naz, M.; Benavides-Mendoza, A.; Tariq, M.; Zhou, J.; Wang, J.; Qi, S.; Dai, Z.; Du, D. CRISPR/Cas9 technology as an innovative approach to enhancing the phytoremediation: Concepts and implications. J. Environ. Manag. 2022, 323, 116296. [Google Scholar] [CrossRef]
- FAO and UNEP. Global Assessment of Soil Pollution: Report; FAO and UNEP: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- European Commission. Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, when Sewage Sludge is Used in Agriculture; European Commission: Brussels, Belgium, 1986; Available online: https://eur-lex.europa.eu/eli/dir/1986/278/oj/eng (accessed on 15 August 2025).
- GB 15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment: Beijing, China, 2018. Available online: https://www.chinesestandard.net/PDF.aspx/GB15618-2018 (accessed on 15 August 2025).
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Tian, T.; Yu, L.; Feng, R.; Yao, C.; Gong, L.; Xiao, H.; Liu, L.; Li, F. Unveiling the combined effects of water management and lime on remediation of Cd-contaminated soils with improved soil quality. J. Environ. Chem. Eng. 2024, 12, 114778. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, J.; Sharma, N.R.; Natt, S.K.; Mohan, A.; Malik, T.; Girdhar, M. Heavy metal contamination in wastewater-irrigated vegetables: Assessing food safety challenges in developing Asian countries. Environ. Sci. Process. Impacts 2025, 27, 1747–1767. [Google Scholar] [CrossRef]
- Inobeme, A.; Mathew, J.T.; Jatto, E.; Inobeme, J.; Adetunji, C.O.; Muniratu, M.; Onyeachu, B.I.; Adekoya, M.A.; Ajai, A.I.; Mann, A.; et al. Recent advances in instrumental techniques for heavy metal quantification. Environ. Monit. Assess. 2023, 195, 452. [Google Scholar] [CrossRef]
- Huang, F.; Peng, S.; Yang, H.; Cao, H.; Ma, N.; Ma, L. Development of a novel and fast XRF instrument for large area heavy metal detection integrated with UAV. Environ. Res. 2022, 214, 113841. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, S.; Wang, Q.; Chen, Y.; Zhang, D. Using deep learning in an embedded system for real-time target detection based on images from an unmanned aerial vehicle: Vehicle detection as a case study. Int. J. Digit. Earth 2023, 16, 910–936. [Google Scholar] [CrossRef]
- Nogueira, P.; Silva, M.; Roseiro, J.; Potes, M.; Rodrigues, G. Mapping the Mine: Combining Portable X-ray Fluorescence, Spectroradiometry, UAV, and Sentinel-2 Images to Identify Contaminated Soils—Application to the Mostardeira Mine (Portugal). Remote Sens. 2023, 15, 5295. [Google Scholar] [CrossRef]
- CAP and the Environment—European Commission. An Environmentally Sustainable CAP; European Commission: Brussels, Belgium, 2023; Available online: https://agriculture.ec.europa.eu/cap-my-country/sustainability/environmental-sustainability/cap-and-environment_en (accessed on 15 August 2025).
| Metal | Growth Medium/Tissue | Deficient/Minimum Adequate | Adequate/Normal Range | Excess/Toxic Threshold | Remarks | Reference |
|---|---|---|---|---|---|---|
| Zn | Leaf tissue (µg g−1 dry weight) | <20–30 | 30–100 | 200–300 | Zn is the least toxic HM; essential for enzymes and auxin metabolism | [40] |
| Soil (mg kg−1) | ~6–20 | 20–80 | >100 | Soil adsorption is moderate; tolerance higher than that to Cu | ||
| Cu | Leaf tissue (µg g−1 dry weight) | <5 | 5–20 | >20–30 | Narrower range than Zn; deficiency more common | [41] |
| Soil (mg kg−1) | ~6–20 | 20–80 | 20–100 | Cu is highly bioavailable; toxicity impacts roots/photosynthesis |
| Heavy Metal | Major Health Effects | Reference |
|---|---|---|
| Mercury (Hg) | Skin lesions, hyperkeratosis, cancers, cardiovascular disease, diabetes. | [135] |
| Cadmium (Cd) | Respiratory damage, lung cancer, bone disorders, metabolic diseases. | [118,135,136] |
| Copper (Cu) | Gastrointestinal distress, liver and kidney problems, neurogenerative disorders. | [137] |
| Zinc (Zn) | Immune dysfunction, impaired copper absorption, neurological issues. | [137] |
| Nickel (Ni) | Dermatitis, respiratory cancers, cardiovascular effects. | [136] |
| Lead (Pb) | Neurotoxicity, hypertension, cancer, reproductive toxicity. | [133,136] |
| Chromium (Cr) | Lung cancer, nasal perforation, skin irritation, DNA damage. | [135] |
| Aluminum (Al) | Neurotoxicity, bone disorders, lung fibrosis, dialysis encephalopathy. | [138] |
| Cobalt (Co) | Cardiomyopathy, lung disease, thyroid dysfunction, neurotoxicity. | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghpour, A.; Javid, M.; Koduru, S.; Babaei, S.; Brevik, E.C. Heavy Metals in Bioenergy Crop Production, Biomass Quality, and Biorefinery: Global Impacts and Sustainable Management Strategies. Bioresour. Bioprod. 2025, 1, 2. https://doi.org/10.3390/bioresourbioprod1010002
Sadeghpour A, Javid M, Koduru S, Babaei S, Brevik EC. Heavy Metals in Bioenergy Crop Production, Biomass Quality, and Biorefinery: Global Impacts and Sustainable Management Strategies. Bioresources and Bioproducts. 2025; 1(1):2. https://doi.org/10.3390/bioresourbioprod1010002
Chicago/Turabian StyleSadeghpour, Amir, Moein Javid, Sowmya Koduru, Sirwan Babaei, and Eric C. Brevik. 2025. "Heavy Metals in Bioenergy Crop Production, Biomass Quality, and Biorefinery: Global Impacts and Sustainable Management Strategies" Bioresources and Bioproducts 1, no. 1: 2. https://doi.org/10.3390/bioresourbioprod1010002
APA StyleSadeghpour, A., Javid, M., Koduru, S., Babaei, S., & Brevik, E. C. (2025). Heavy Metals in Bioenergy Crop Production, Biomass Quality, and Biorefinery: Global Impacts and Sustainable Management Strategies. Bioresources and Bioproducts, 1(1), 2. https://doi.org/10.3390/bioresourbioprod1010002