Microdosing Psychedelics to Restore Synaptic Density in Schizophrenia
Abstract
1. Introduction
2. The Synaptic Hypothesis of Schizophrenia
2.1. Can Synaptic Loss Explain Cortical Thinning in Schizophrenia?
2.2. Genome-Wide Association Studies
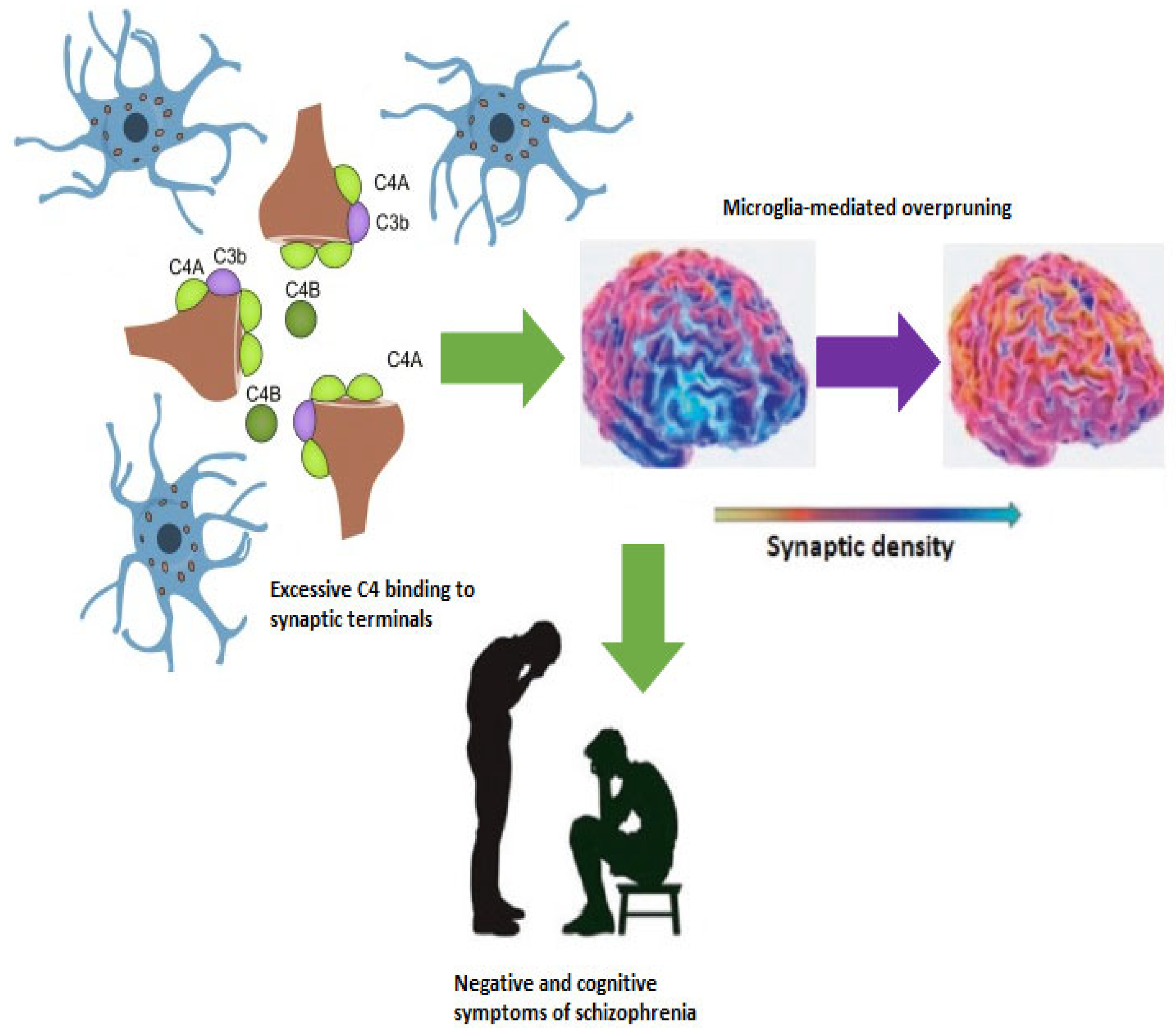
2.3. Complement-Dependent Pruning
3. Psychedelics Promote Neuroplasticity
4. Anti-Inflammatory Properties of Psychedelics
5. Could Psychedelics Be Used to Treat Schizophrenia?
5.1. Safety and Feasibility
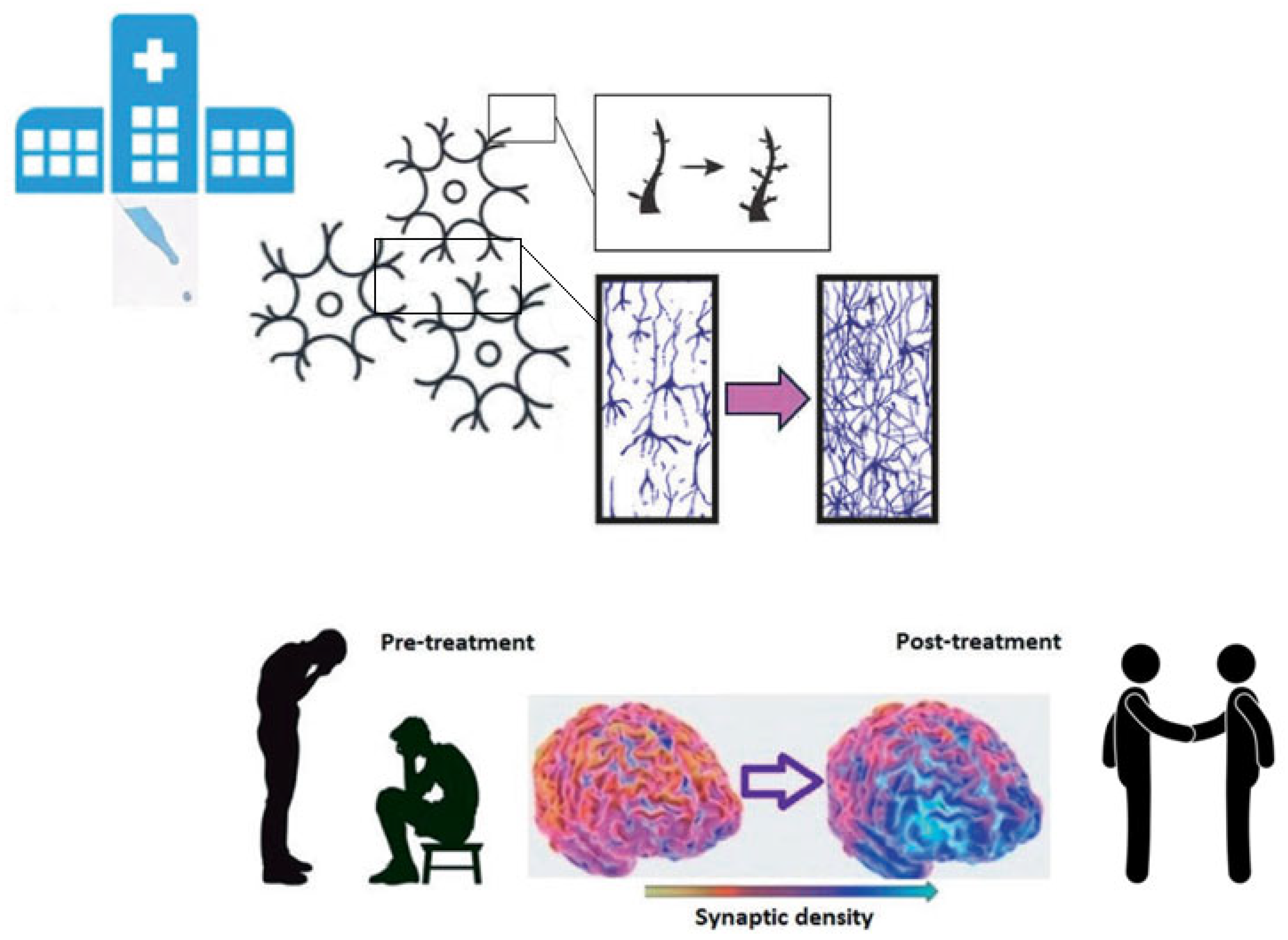
Microdosing
5.2. Possible Target Population and Treatment Protocol
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “Just the Facts” 4. Clinical Features and Conceptualization. Schizophr. Res. 2009, 110, 1–23. [Google Scholar] [CrossRef]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “Just the Facts” 5. Treatment and Prevention Past, Present, and Future. Schizophr. Res. 2010, 122, 1–23. [Google Scholar] [CrossRef]
- Bechi, M.; Bosia, M.; Spangaro, M.; Buonocore, M.; Cavedoni, S.; Agostoni, G.; Bianchi, L.; Cocchi, F.; Guglielmino, C.; Smeraldi, E.; et al. Exploring Functioning in Schizophrenia: Predictors of Functional Capacity and Real-World Behaviour. Psychiatry Res. 2017, 251, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sabe, M.; Chen, C.; Perez, N.; Solmi, M.; Mucci, A.; Galderisi, S.; Strauss, G.P.; Kaiser, S. Thirty Years of Research on Negative Symptoms of Schizophrenia: A Scientometric Analysis of Hotspots, Bursts, and Research Trends. Neurosci. Biobehav. Rev. 2023, 144, 104979. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia—An Overview. JAMA Psychiatry 2020, 77, 201–210, Erratum in JAMA Psychiatry 2021, 78, 224. [Google Scholar] [CrossRef]
- Calzavara Pinton, I.; Nibbio, G.; Bertoni, L.; Cicale, A.; Necchini, N.; Zardini, D.; Bosco Ubertino, U.; Cerati, C.; Deste, G.; Barlati, S.; et al. The Economic Burden of Schizophrenia Spectrum Disorders: Clinical and Functional Correlates and Predictors of Direct Costs. A Retrospective Longitudinal Study. Psychiatry Res. 2024, 342, 116240. [Google Scholar] [CrossRef]
- Sapienza, J.; Martini, F.; Comai, S.; Cavallaro, R.; Spangaro, M.; De Gregorio, D.; Bosia, M. Psychedelics and Schizophrenia: A Double-Edged Sword. Mol. Psychiatry 2025, 30, 679–692. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Galderisi, S. Deficit Schizophrenia: An Update. World Psychiatry 2008, 7, 143–147. [Google Scholar] [CrossRef]
- Kochunov, P.; Thompson, P.M.; Hong, L.E. Toward High Reproducibility and Accountable Heterogeneity in Schizophrenia Research. JAMA Psychiatry 2019, 76, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, M.S.; Tandon, R.; Boutros, N.N.; Nasrallah, H.A. Schizophrenia, “Just the Facts”: What We Know in 2008. Part 3: Neurobiology. Schizophr. Res. 2008, 106, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, J.L.; Tandon, N.; Haller, C.S.; Mathew, I.T.; Eack, S.M.; Clementz, B.A.; Pearlson, G.D.; Sweeney, J.A.; Tamminga, C.A.; Keshavan, M.S. Correlations Between Brain Structure and Symptom Dimensions of Psychosis in Schizophrenia, Schizoaffective, and Psychotic Bipolar i Disorders. Schizophr. Bull. 2015, 41, 154–162. [Google Scholar] [CrossRef]
- Hulshoff Pol, H.E.; Kahn, R.S. What Happens after the First Episode? A Review of Progressive Brain Changes in Chronically Ill Patients with Schizophrenia. Schizophr. Bull. 2008, 34, 354–366. [Google Scholar] [CrossRef]
- Cropley, V.L.; Klauser, P.; Lenroot, R.K.; Bruggemann, J.; Sundram, S.; Bousman, C.; Pereira, A.; Di Biase, M.A.; Weickert, T.W.; Weickert, C.S.; et al. Accelerated Gray and White Matter Deterioration with Age in Schizophrenia. Am. J. Psychiatry 2017, 174, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Ziermans, T.B.; Schothorst, P.F.; Schnack, H.G.; Koolschijn, P.C.M.P.; Kahn, R.S.; Van Engeland, H.; Durston, S. Progressive Structural Brain Changes During Development of Psychosis. Schizophr. Bull. 2012, 38, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.D.; Chung, Y.; He, G.; Sun, D.; Jacobson, A.; Van Erp, T.G.M.; McEwen, S.; Addington, J.; Bearden, C.E.; Cadenhead, K.; et al. Progressive Reduction in Cortical Thickness as Psychosis Develops: A Multisite Longitudinal Neuroimaging Study of Youth at Elevated Clinical Risk. Biol. Psychiatry 2015, 77, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Q.; Shah, C.; Li, Q.; Sweeney, J.A.; Li, F.; Gong, Q. Cortical Thickness Abnormalities at Different Stages of the Illness Course in Schizophrenia: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2022, 79, 560–570. [Google Scholar] [CrossRef]
- Vita, A.; De Peri, L.; Deste, G.; Sacchetti, E. Progressive Loss of Cortical Gray Matter in Schizophrenia: A Meta-Analysis and Meta-Regression of Longitudinal MRI Studies. Transl. Psychiatry 2012, 2, e190, Erratum in Transl. Psychiatry 2013, 3, e275. [Google Scholar] [CrossRef]
- Vita, A.; De Peri, L.; Deste, G.; Barlati, S.; Sacchetti, E. The Effect of Antipsychotic Treatment on Cortical Gray Matter Changes in Schizophrenia: Does the Class Matter? A Meta-Analysis and Meta-Regression of Longitudinal Magnetic Resonance Imaging Studies. Biol. Psychiatry 2015, 78, 403–412. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R. Inflammation and the Neural Diathesis-Stress Hypothesis of Schizophrenia: A Reconceptualization. Transl. Psychiatry 2017, 7, e1024. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, I. Schizophrenia: Caused by a Fault in Programmed Synaptic Elimination during Adolescence? J. Psychiatr. Res. 1982, 17, 319–334. [Google Scholar] [CrossRef]
- Hoffman, R.E.; Dobscha, S.K. Cortical Pruning and the Development of Schizophrenia: A Computer Model. Schizophr. Bull. 1989, 15, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, J.; Bosia, M.; Spangaro, M.; Martini, F.; Agostoni, G.; Cuoco, F.; Cocchi, F.; Cavallaro, R. Schizophrenia and Psychedelic State: Dysconnection Versus Hyper-Connection. A Perspective on Two Different Models of Psychosis Stemming from Dysfunctional Integration Processes. Mol. Psychiatry 2023, 28, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, J.; Pacchioni, F.; Spangaro, M.; Bosia, M. Dysconnection in Schizophrenia: Filling the Dots from Old to New Evidence. Clin. Neurophysiol. 2024, 162, 226–228. [Google Scholar] [CrossRef]
- Friston, K.; Brown, H.R.; Siemerkus, J.; Stephan, K.E. The Dysconnection Hypothesis (2016). Schizophr. Res. 2016, 176, 83–94. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Anderson, S.; Pettergrew, J.W. Is Schizophrenia Due to Excessive Synaptic Pruning in the Prefrontal Cortex? The Feinberg Hypothesis Revisited. J. Psychiatr. Res. 1994, 28, 239–265. [Google Scholar] [CrossRef]
- Li, W.; Lv, L.; Luo, X.J. In Vivo Study Sheds New Light on the Dendritic Spine Pathology Hypothesis of Schizophrenia. Mol. Psychiatry 2022, 27, 1866–1868. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Onwordi, E.C. The Synaptic Hypothesis of Schizophrenia Version III: A Master Mechanism. Mol. Psychiatry 2023, 28, 1843–1856. [Google Scholar] [CrossRef]
- Onwordi, E.C.; Whitehurst, T.; Shatalina, E.; Mansur, A.; Arumuham, A.; Osugo, M.; Marques, T.R.; Jauhar, S.; Gupta, S.; Mehrotra, R.; et al. Synaptic Terminal Density Early in the Course of Schizophrenia: An In Vivo UCB-J Positron Emission Tomographic Imaging Study of Synaptic Vesicle Glycoprotein 2A (SV2A). Biol. Psychiatry 2023, 95, 639–646. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Skosnik, P.D.; Ranganathan, M.; Naganawa, M.; Toyonaga, T.; Finnema, S.; Hillmer, A.T.; Esterlis, I.; Huang, Y.; Nabulsi, N.; et al. In Vivo Evidence of Lower Synaptic Vesicle Density in Schizophrenia. Mol. Psychiatry 2021, 26, 7690–7698. [Google Scholar] [CrossRef] [PubMed]
- Finnema, S.J.; Nabulsi, N.B.; Eid, T.; Detyniecki, K.; Lin, S.F.; Chen, M.K.; Dhaher, R.; Matuskey, D.; Baum, E.; Holden, D.; et al. Imaging Synaptic Density in the Living Human Brain. Sci. Transl. Med. 2016, 8, 348ra9. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Zhang, Z.; Mormino, E.; Davidzon, G.; Minzenberg, M.J.; Ballon, J.; Kalinowski, A.; Hardy, K.; Naganawa, M.; Carson, R.E.; et al. Reductions in Synaptic Marker SV2A in Early-Course Schizophrenia. J. Psychiatr. Res. 2023, 161, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Cummings, C.; Chapman, G.E.; Shatalina, E. Neuroimaging in Schizophrenia: An Overview of Findings and Their Implications for Synaptic Changes. Neuropsychopharmacology 2023, 48, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.S.; Lagopoulos, J.; Stait-Gardner, T.; Price, W.S.; Chohan, T.W.; Arnold, J.C.; Hatton, S.N.; Bennett, M.R. Stress-Induced Grey Matter Loss Determined by MRI Is Primarily Due to Loss of Dendrites and Their Synapses. Mol. Neurobiol. 2013, 47, 645–661. [Google Scholar] [CrossRef]
- Keifer, O.P.; Hurt, R.C.; Gutman, D.A.; Keilholz, S.D.; Gourley, S.L.; Ressler, K.J. Voxel-Based Morphometry Predicts Shifts in Dendritic Spine Density and Morphology with Auditory Fear Conditioning. Nat. Commun. 2015, 6, 7582. [Google Scholar] [CrossRef]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.R.; Farh, K.H.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H.; et al. Biological Insights from 108 Schizophrenia-Associated Genetic Loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- The Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium; Ripke, S.; Sanders, A.R.; Kendler, K.S.; Levinson, D.F.; Sklar, P.; Holmans, P.A.; Lin, D.-Y.; Duan, J.; Ophoff, R.A.; Andreassen, O.A.; et al. Genome-Wide Association Study Identifies Five New Schizophrenia Loci. Nat. Genet. 2011, 43, 969–976. [Google Scholar] [CrossRef]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping Genomic Loci Implicates Genes and Synaptic Biology in Schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Kim, M.; Haney, J.R.; Zhang, P.; Hernandez, L.M.; Wang, L.K.; Perez-Cano, L.; Loohuis, L.M.O.; de la Torre-Ubieta, L.; Gandal, M.J. Brain Gene Co-Expression Networks Link Complement Signaling with Convergent Synaptic Pathology in Schizophrenia. Nat. Neurosci. 2021, 24, 799–809. [Google Scholar] [CrossRef]
- Yilmaz, M.; Yalcin, E.; Presumey, J.; Aw, E.; Ma, M.; Whelan, C.W.; Stevens, B.; McCarroll, S.A.; Carroll, M.C. Overexpression of Schizophrenia Susceptibility Factor Human Complement C4A Promotes Excessive Synaptic Loss and Behavioral Changes in Mice. Nat. Neurosci. 2021, 24, 214–224. [Google Scholar] [CrossRef]
- Reis Marques, T.; Ashok, A.H.; Pillinger, T.; Veronese, M.; Turkheimer, F.E.; Dazzan, P.; Sommer, I.E.C.; Howes, O.D. Neuroinflammation in Schizophrenia: Meta-Analysis of In Vivo Microglial Imaging Studies. Psychol. Med. 2018, 13, 2186–2196. [Google Scholar] [CrossRef]
- Gober, R.; Ardalan, M.; Shiadeh, S.M.J.; Duque, L.; Garamszegi, S.P.; Ascona, M.; Barreda, A.; Sun, X.; Mallard, C.; Vontell, R.T. Microglia Activation in Postmortem Brains with Schizophrenia Demonstrates Distinct Morphological Changes Between Brain Regions. Brain Pathol. 2022, 32, e13003. [Google Scholar] [CrossRef]
- Da Silva, T.; Guma, E.; Hafizi, S.; Koppel, A.; Rusjan, P.; Kennedy, J.L.; Chakravarty, M.M.; Mizrahi, R. Genetically Predicted Brain C4A Expression Is Associated with TSPO and Hippocampal Morphology. Biol. Psychiatry 2021, 90, 652–660. [Google Scholar] [CrossRef]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased Synapse Elimination by Microglia in Schizophrenia Patient-Derived Models of Synaptic Pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef]
- Druart, M.; Nosten-Bertrand, M.; Poll, S.; Crux, S.; Nebeling, F.; Delhaye, C.; Dubois, Y.; Mittag, M.; Leboyer, M.; Tamouza, R.; et al. Elevated Expression of Complement C4 in the Mouse Prefrontal Cortex Causes Schizophrenia-Associated Phenotypes. Mol. Psychiatry 2021, 26, 3489–3501. [Google Scholar] [CrossRef]
- Comer, A.L.; Jinadasa, T.; Sriram, B.; Phadke, R.A.; Kretsge, L.N.; Nguyen, T.P.H.; Antognetti, G.; Gilbert, J.P.; Lee, J.; Newmark, E.R.; et al. Increased Expression of Schizophrenia-Associated Gene C4 Leads to Hypoconnectivity of Prefrontal Cortex and Reduced Social Interaction. PLoS Biol. 2020, 18, e3000604. [Google Scholar] [CrossRef]
- Gangadin, S.S.; Germann, M.; de Witte, L.D.; Gelderman, K.A.; Mandl, R.C.W.; Sommer, I.E.C. Complement Component 4A Protein Levels Are Negatively Related to Frontal Volumes in Patients with Schizophrenia Spectrum Disorders. Schizophr. Res. 2023, 261, 6–14. [Google Scholar] [CrossRef]
- Selvaraj, S.; Bloomfield, P.S.; Cao, B.; Veronese, M.; Turkheimer, F.; Howes, O.D. Brain TSPO Imaging and Gray Matter Volume in Schizophrenia Patients and in People at Ultra High Risk of Psychosis: An [11C]PBR28 Study. Schizophr. Res. 2018, 195, 206–214. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.S.; Sonderby, I.E.; Frei, O.; Van Der Meer, D.; Athanasiu, L.; Smeland, O.B.; Alnæs, D.; Kaufmann, T.; Westlye, L.T.; Steen, V.M.; et al. Association Between Complement Component 4A Expression, Cognitive Performance and Brain Imaging Measures in UK Biobank. Psychol. Med. 2022, 52, 3497–3507. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, G.; Holland, J.; Mothersill, D.; McCarthy-Jones, S.; Cosgrove, D.; Harold, D.; Richards, A.; Mantripragada, K.; Owen, M.J.; O’Donovan, M.C.; et al. Genetically Predicted Complement Component 4A Expression: Effects on Memory Function and Middle Temporal Lobe Activation. Psychol. Med. 2018, 48, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L. Serotonin, Psychedelics and Psychiatry. World Psychiatry 2018, 17, 358–359. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef]
- Inserra, A.; De Gregorio, D.; Gobbi, G. Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Pharmacol. Rev. 2021, 73, 202–277. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics Promote Neuroplasticity Through the Activation of Intracellular 5-HT2A Receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef]
- Savalia, N.K.; Shao, L.X.; Kwan, A.C. A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci. 2021, 44, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a Convergent Mechanism of Ketamine and Classical Psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Vargas, M.V.; Duim, W.C.; Grodzki, A.C.G.; Lein, P.J.; Olson, D.E. Transient Stimulation with Psychoplastogens Is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci. 2021, 4, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.E.; Hasler, G. Towards an Understanding of Psychedelic-Induced Neuroplasticity. Neuropsychopharmacology 2023, 48, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin Induces Rapid and Persistent Growth of Dendritic Spines in Frontal Cortex In Vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef]
- Olson, D.E. Toward Translatable Biomarkers of Psychedelic-Induced Neuroplasticity. Am. J. Psychiatry 2025, 182, 10–12. [Google Scholar] [CrossRef]
- Shahar, O.; Botvinnik, A.; Shwartz, A.; Lerer, E.; Golding, P.; Buko, A.; Hamid, E.; Kahn, D.; Guralnick, M.; Blakolmer, K.; et al. Effect of Chemically Synthesized Psilocybin and Psychedelic Mushroom Extract on Molecular and Metabolic Profiles in Mouse Brain. Mol. Psychiatry 2024, 29, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with Psychological Support for Treatment-Resistant Depression: An Open-Label Feasibility Study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin Versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Nau, F.; Yu, B.; Martin, D.; Nichols, C.D. Serotonin 5-HT2A Receptor Activation Blocks TNF-α Mediated Inflammation In Vivo. PLoS ONE 2013, 8, e75426. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Sebastian, M.N.; Battaglia, D.M.; Foster, T.P.; Maillet, E.L.; Nichols, C.D. Activation of 5-HT2 Receptors Reduces Inflammation in Vascular Tissue and Cholesterol Levels in High-Fat Diet-Fed Apolipoprotein E Knockout Mice. Sci. Rep. 2019, 9, 13444. [Google Scholar] [CrossRef]
- Yu, B.; Becnel, J.; Zerfaoui, M.; Rohatgi, R.; Boulares, A.H.; Nichols, C.D. Serotonin 5-Hydroxytryptamine(2A) Receptor Activation Suppresses Tumor Necrosis Factor-Alpha-Induced Inflammation with Extraordinary Potency. J. Pharmacol. Exp. Ther. 2008, 327, 316–323. [Google Scholar] [CrossRef]
- de Deus, J.L.; Maia, J.M.; Soriano, R.N.; Amorim, M.R.; Branco, L.G.S. Psychedelics in Neuroinflammation: Mechanisms and Therapeutic Potential. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 137, 111278. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as Anti-Inflammatory Agents. Int. Rev. Psychiatry 2018, 30, 363–375. [Google Scholar] [CrossRef]
- Holloway, T.; González-Maeso, J. Epigenetic Mechanisms of Serotonin Signaling. ACS Chem. Neurosci. 2015, 6, 1099–1109. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N,N-Dimethyltryptamine and 5-Methoxy-N,N-Dimethyltryptamine Modulate Innate and Adaptive Inflammatory Responses Through the Sigma-1 Receptor of Human Monocyte-Derived Dendritic Cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Schwarcz, R.; Erhardt, S. Neuroactive Kynurenines as Pharmacological Targets: New Experimental Tools and Exciting Therapeutic Opportunities. Pharmacol. Rev. 2024, 76, 978–1008. [Google Scholar] [CrossRef]
- Campanale, A.; Inserra, A.; Comai, S. Therapeutic Modulation of the Kynurenine Pathway in Severe Mental Illness and Comorbidities: A Potential Role for Serotonergic Psychedelics. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 134, 111058. [Google Scholar] [CrossRef]
- La Torre, J.T.; Gallo, J.; Mahammadli, M.; Zalewa, D.; Williams, M.T. Experiences of Psychedelic Drug Use Among People with Psychotic Symptoms and Disorders: Personal Growth and Mystical Experiences. J. Psychedelic Stud. 2024, 8, 357–367. [Google Scholar] [CrossRef]
- La Torre, J.T.; Mahammadli, M.; Faber, S.C.; Greenway, K.T.; Williams, M.T. Expert Opinion on Psychedelic-Assisted Psychotherapy for People with Psychopathological Psychotic Experiences and Psychotic Disorders. Int. J. Ment. Health Addict. 2024, 22, 913–937. [Google Scholar] [CrossRef]
- Simonsson, O.; Mosing, M.A.; Osika, W.; Ullén, F.; Larsson, H.; Lu, Y.; Wesseldijk, L.W. Adolescent Psychedelic Use and Psychotic or Manic Symptoms. JAMA Psychiatry 2024, 81, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Simonsson, O.; Goldberg, S.B.; Osika, W.; Stenfors, C.U.D.; Chaturvedi, S.; Swords, C.M.; Narayanan, J.; Hendricks, P.S. Longitudinal Associations of Naturalistic Psychedelic Use with Psychotic and Manic Symptoms. Psychol. Med. 2025, 55, e99. [Google Scholar] [CrossRef] [PubMed]
- Honk, L.; Stenfors, C.U.D.; Goldberg, S.B.; Hendricks, P.S.; Osika, W.; Dourron, H.M.; Lebedev, A.; Petrovic, P.; Simonsson, O. Longitudinal Associations Between Psychedelic Use and Psychotic Symptoms in the United States and the United Kingdom. J. Affect. Disord. 2024, 351, 194–201. [Google Scholar] [CrossRef]
- Sabé, M.; Sulstarova, A.; Glangetas, A.; De Pieri, M.; Mallet, L.; Curtis, L.; Richard-Lepouriel, H.; Penzenstadler, L.; Seragnoli, F.; Thorens, G.; et al. Reconsidering Evidence for Psychedelic-Induced Psychosis: An Overview of Reviews, a Systematic Review, and Meta-Analysis of Human Studies. Mol. Psychiatry 2024, 30, 1223–1255. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Manzanares-Teson, N.; Barnes, N.M. Cytokine Function in Medication-Naive First Episode Psychosis: A Systematic Review and Meta-Analysis. Schizophr. Res. 2014, 155, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and Immunity in Schizophrenia: Implications for Pathophysiology and Treatment. Lancet Psychiatry 2015, 2, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Bender, L.; Goldschmidt, L.; Sankar, D.V. Treatment of Autistic Schizophrenic Children with LSD-25 and UML-491. Recent. Adv. Biol. Psychiatry 1961, 4, 170–179. [Google Scholar] [CrossRef]
- Bender, L. D-Lysergic Acid in the Treatment of the Biological Features of Childhood Schizophrenia. Dis. Nerv. Syst. 1966, 7, 43–46. [Google Scholar]
- Freedman, A.M.; Ebin, E.V.; Wilson, E.A. Autistic Schizophrenic Children. An Experiment in the Use of d-Lysergic Acid Diethylamide (LSD-25). Arch. Gen. Psychiatry 1962, 6, 203–213. [Google Scholar] [CrossRef]
- Cheeck, F.E.; Holstein, C.M. LSD-25 Dosage Levels, Group Differences, and Social Interaction. J. Nerv. Ment. Dis. 1971, 153, 133–147. [Google Scholar] [CrossRef]
- Busch, A.K.; Johnson, W.C. L.S.D. 25 as an Aid in Psychotherapy; Preliminary Report of a New Drug. Dis. Nerv. Syst. 1950, 11, 241–243. [Google Scholar]
- Abramson, H.A.; Hewitt, M.P.; Lennard, H.; Turner, W.J.; O’neill, F.J.; Merlis, S. The Stablemate Concept of Therapy as Affected by LSD in Schizophrenia. J. Psychol. Interdiscip. Appl. 1958, 45, 75–84. [Google Scholar] [CrossRef]
- Nardou, R.; Sawyer, E.; Song, Y.J.; Wilkinson, M.; Padovan-Hernandez, Y.; de Deus, J.L.; Wright, N.; Lama, C.; Faltin, S.; Goff, L.A.; et al. Psychedelics Reopen the Social Reward Learning Critical Period. Nature 2023, 618, 790–798. [Google Scholar] [CrossRef]
- de Gregorio, D.; Popic, J.; Enns, J.P.; Inserra, A.; Skalecka, A.; Markopoulos, A.; Posa, L.; Lopez-Canul, M.; Qianzi, H.; Lafferty, C.K.; et al. Lysergic Acid Diethylamide (LSD) Promotes Social Behavior Through MTORC1 in the Excitatory Neurotransmission. Proc. Natl. Acad. Sci. USA 2021, 118, e2020705118. [Google Scholar] [CrossRef]
- Tuck, J.R.; Dunlap, L.E.; Khatib, Y.A.; Hatzipantelis, C.J.; Novak, S.W.; Rahn, R.M.; Davis, A.R.; Mosswood, A.; Vernier, A.M.M.; Fenton, E.M.; et al. Molecular Design of a Therapeutic LSD Analogue with Reduced Hallucinogenic Potential. Proc. Natl. Acad. Sci. USA 2025, 122, e2416106122. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; Comai, S.; Posa, L.; Gobbi, G. D-Lysergic Acid Diethylamide (LSD) as a Model of Psychosis: Mechanism of Action and Pharmacology. Int. J. Mol. Sci. 2016, 17, 1953. [Google Scholar] [CrossRef]
- Steeds, H.; Carhart-Harris, R.L.; Stone, J.M. Drug Models of Schizophrenia. Ther. Adv. Psychopharmacol. 2015, 5, 43–58. [Google Scholar] [CrossRef]
- Schmid, Y.; Enzler, F.; Gasser, P.; Grouzmann, E.; Preller, K.H.; Vollenweider, F.X.; Brenneisen, R.; Müller, F.; Borgwardt, S.; Liechti, M.E. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol. Psychiatry 2015, 78, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.E. Modern Clinical Research on LSD. Neuropsychopharmacology 2017, 42, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Polito, V.; Liknaitzky, P. The Emerging Science of Microdosing: A Systematic Review of Research on Low Dose Psychedelics (1955–2021) and Recommendations for the Field. Neurosci. Biobehav. Rev. 2022, 139, 104706. [Google Scholar] [CrossRef]
- Denber, H.C.B.; Merlis, S. Studies on Mescaline. VI. Therapeutic Aspects of the Mescaline-Chlorpromazine Combination. J. Nerv. Ment. Dis. 1955, 122, 463–469. [Google Scholar] [CrossRef]
- Cholden, L.S.; Kurland, A.; Savage, C. Clinical Reactions and Tolerance to Lsd in Chronic Schizophrenia. J. Nerv. Ment. Dis. 1955, 122, 211–221. [Google Scholar] [CrossRef]
- Hoch, P.H.; Cattell, J.P.; Pennes, H.H. Effects of Mescaline and Lysergic Acid (d-LSD-25). Am. J. Psychiatry 1952, 108, 579–584. [Google Scholar] [CrossRef]
- Bleuler, M. Address to the Washington Psychiatric Society. 1950. [Google Scholar]
- Broager, B.; Hertz, H. Klinische Erfahrungen an Geisteskranken Mit Lysergsäure—Diäthylamid. Acta Psychiatr. Scand. 1949, 24, 9–32. [Google Scholar] [CrossRef]
- Giacomo, U. De Catatonie Toxique Expérimental. Acta Neurol. 1951, 1, 5–10. [Google Scholar]
- Hock, P.H. Comments. Am. J. Psychiatry 1955, 111, 787–791. [Google Scholar] [CrossRef]
- An Der Heiden, W.; Könnecke, R.; Maurer, K.; Ropeter, D.; Häfner, H. Depression in the Long-Term Course of Schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 255, 174–184. [Google Scholar] [CrossRef]
- an der Heiden, W.; Leber, A.; Häfner, H. Negative Symptoms and Their Association with Depressive Symptoms in the Long-Term Course of Schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Tewari, T.; Mukherjee, S. Microdosing: Concept, Application and Relevance. Perspect. Clin. Res. 2010, 1, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, A.; Calder, A.E.; Hasler, G. Microdosing Psychedelics and the Risk of Cardiac Fibrosis and Valvulopathy: Comparison to Known Cardiotoxins. J. Psychopharmacol. 2024, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, J. The Key Role of Intracellular 5-HT2A Receptors: A Turning Point in Psychedelic Research? Psychoactives 2023, 2, 287–293. [Google Scholar] [CrossRef]
- Sarparast, A.; Thomas, K.; Malcolm, B.; Stauffer, C.S. Drug-Drug Interactions Between Psychiatric Medications and MDMA or Psilocybin: A Systematic Review. Psychopharmacology 2022, 239, 1945–1976. [Google Scholar] [CrossRef]
- Halman, A.; Kong, G.; Sarris, J.; Perkins, D. Drug-Drug Interactions Involving Classic Psychedelics: A Systematic Review. J. Psychopharmacol. 2024, 38, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.E. Biochemical Mechanisms Underlying Psychedelic-Induced Neuroplasticity. Biochemistry 2022, 61, 127–136. [Google Scholar] [CrossRef]
- Moreno, J.L.; Holloway, T.; Albizu, L.; Sealfon, S.C.; González-Maeso, J. Metabotropic Glutamate MGlu2 Receptor is Necessary for the Pharmacological and Behavioral Effects Induced by Hallucinogenic 5-HT2A Receptor Agonists. Neurosci. Lett. 2011, 493, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics Promote Plasticity by Directly Binding to BDNF Receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapienza, J.; Spangaro, M.; Comai, S.; Sabé, M.; La Torre, J.; Buonarroti, M.; Cavallaro, R.; Bosia, M. Microdosing Psychedelics to Restore Synaptic Density in Schizophrenia. Int. J. Mol. Sci. 2025, 26, 8949. https://doi.org/10.3390/ijms26188949
Sapienza J, Spangaro M, Comai S, Sabé M, La Torre J, Buonarroti M, Cavallaro R, Bosia M. Microdosing Psychedelics to Restore Synaptic Density in Schizophrenia. International Journal of Molecular Sciences. 2025; 26(18):8949. https://doi.org/10.3390/ijms26188949
Chicago/Turabian StyleSapienza, Jacopo, Marco Spangaro, Stefano Comai, Michel Sabé, Joseph La Torre, Matteo Buonarroti, Roberto Cavallaro, and Marta Bosia. 2025. "Microdosing Psychedelics to Restore Synaptic Density in Schizophrenia" International Journal of Molecular Sciences 26, no. 18: 8949. https://doi.org/10.3390/ijms26188949
APA StyleSapienza, J., Spangaro, M., Comai, S., Sabé, M., La Torre, J., Buonarroti, M., Cavallaro, R., & Bosia, M. (2025). Microdosing Psychedelics to Restore Synaptic Density in Schizophrenia. International Journal of Molecular Sciences, 26(18), 8949. https://doi.org/10.3390/ijms26188949





