Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates
Abstract
:Introduction
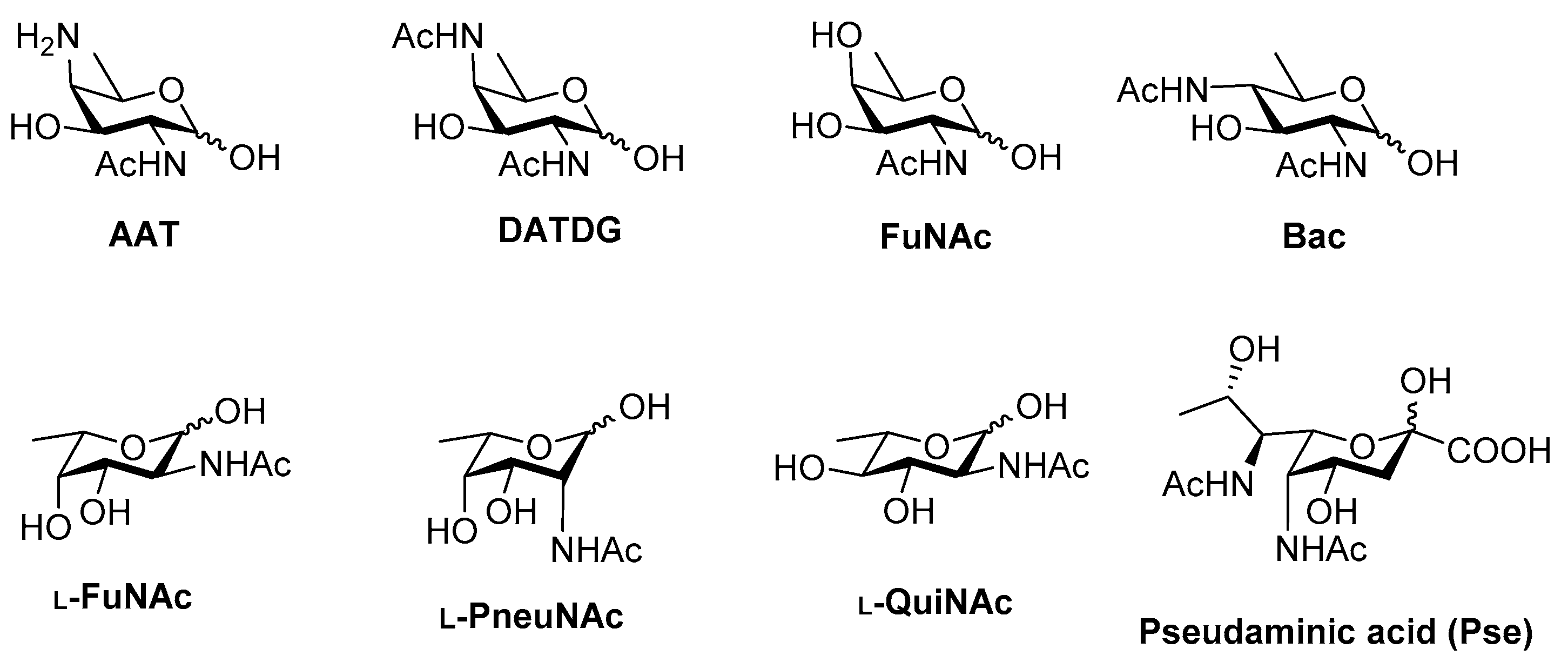
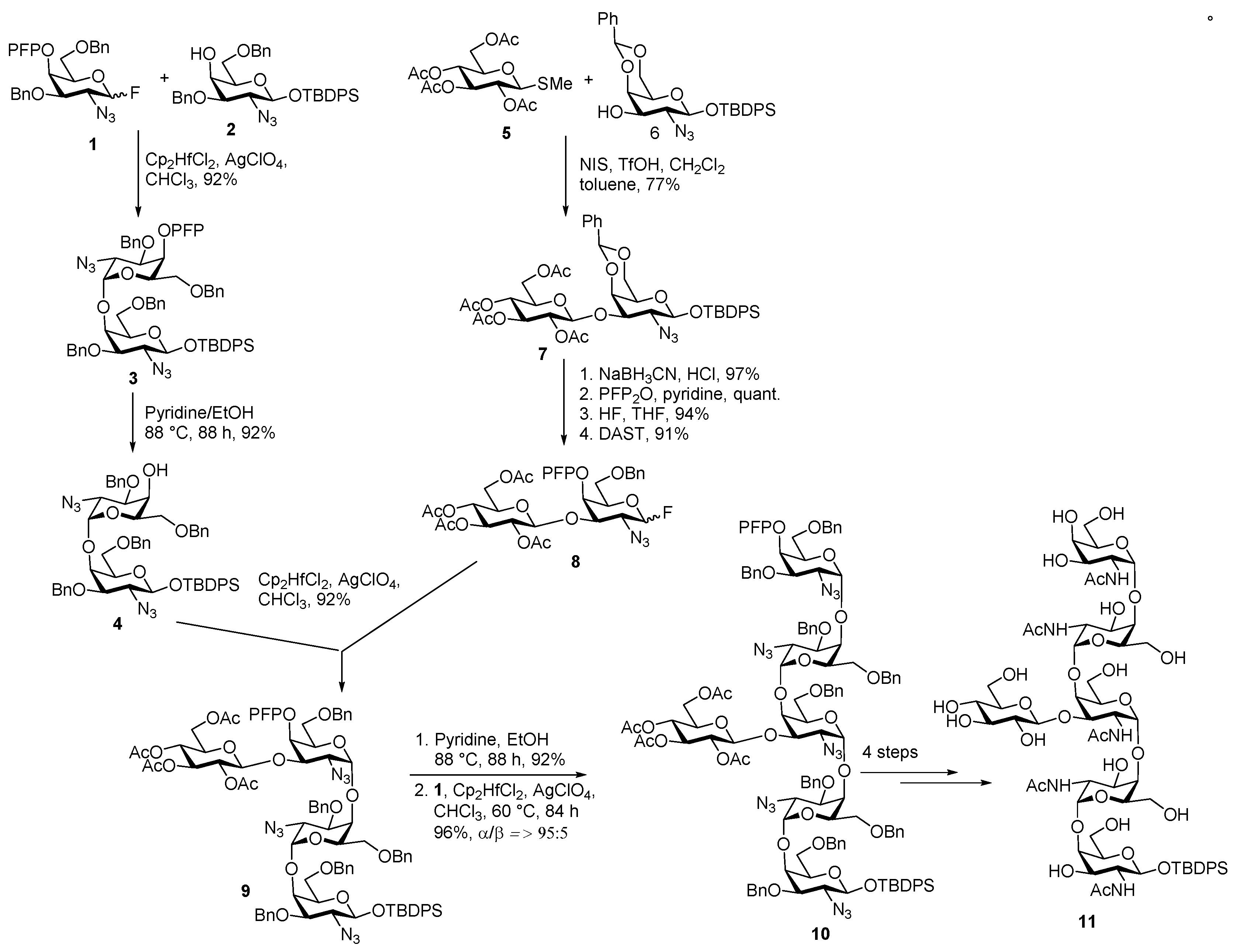
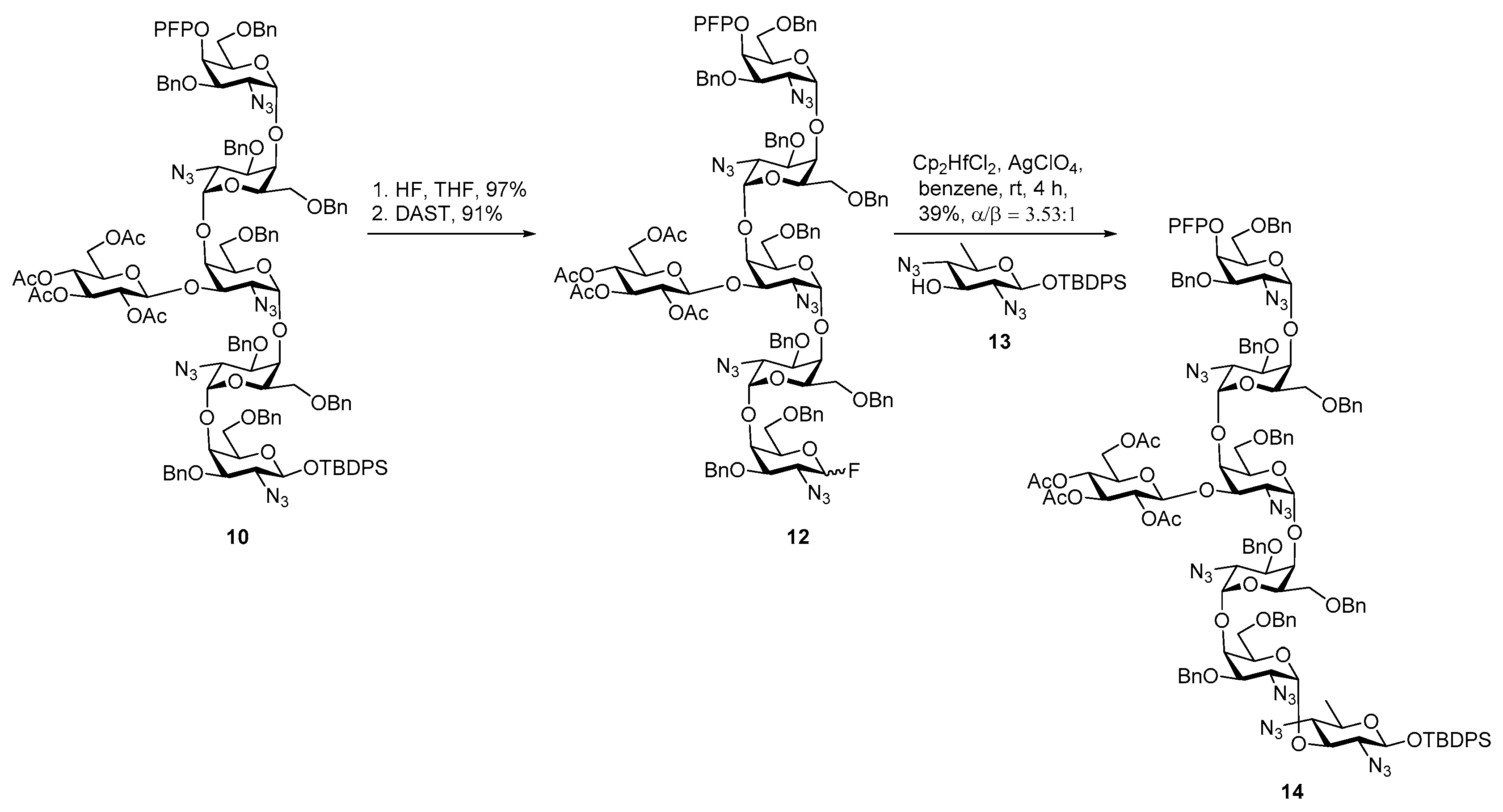
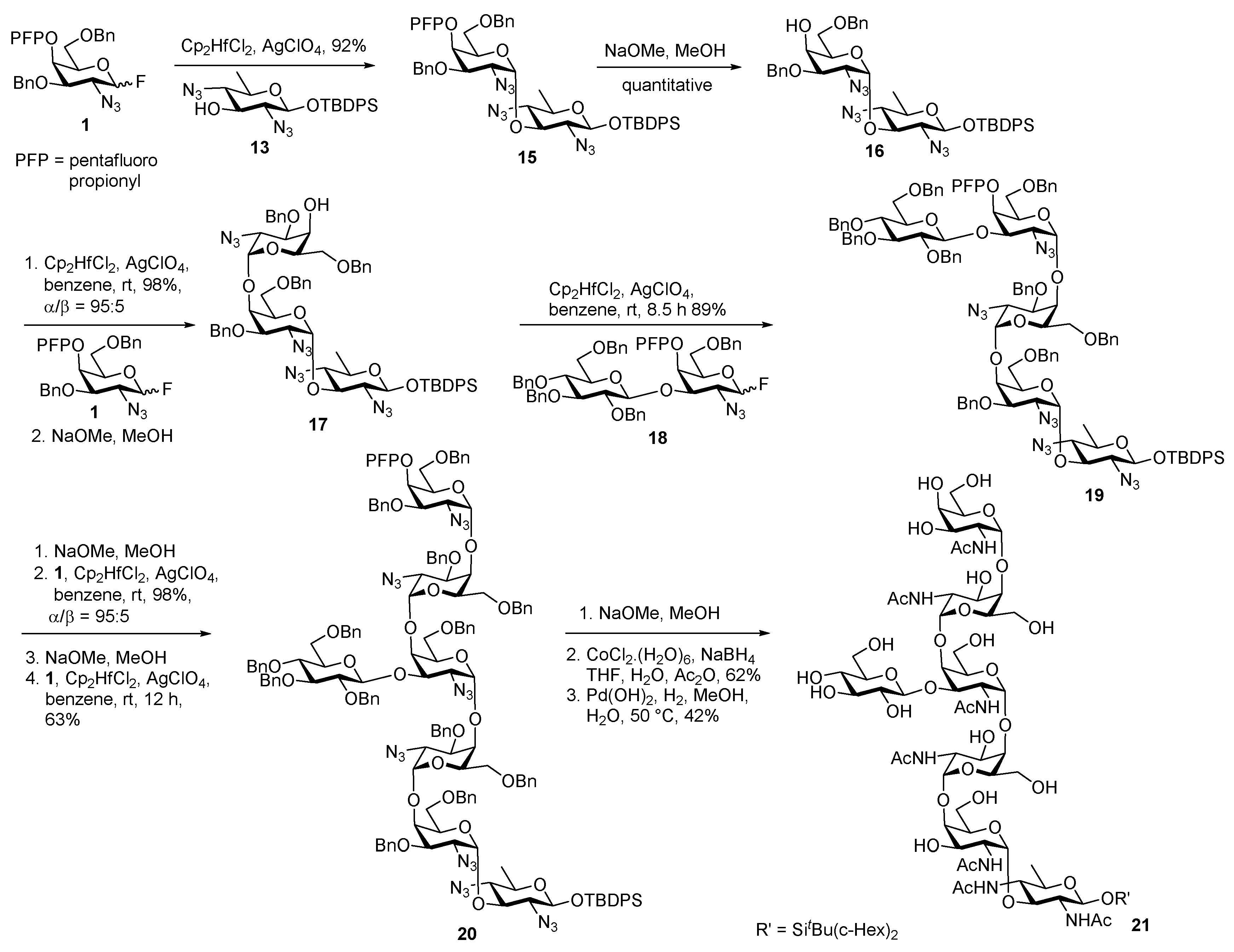
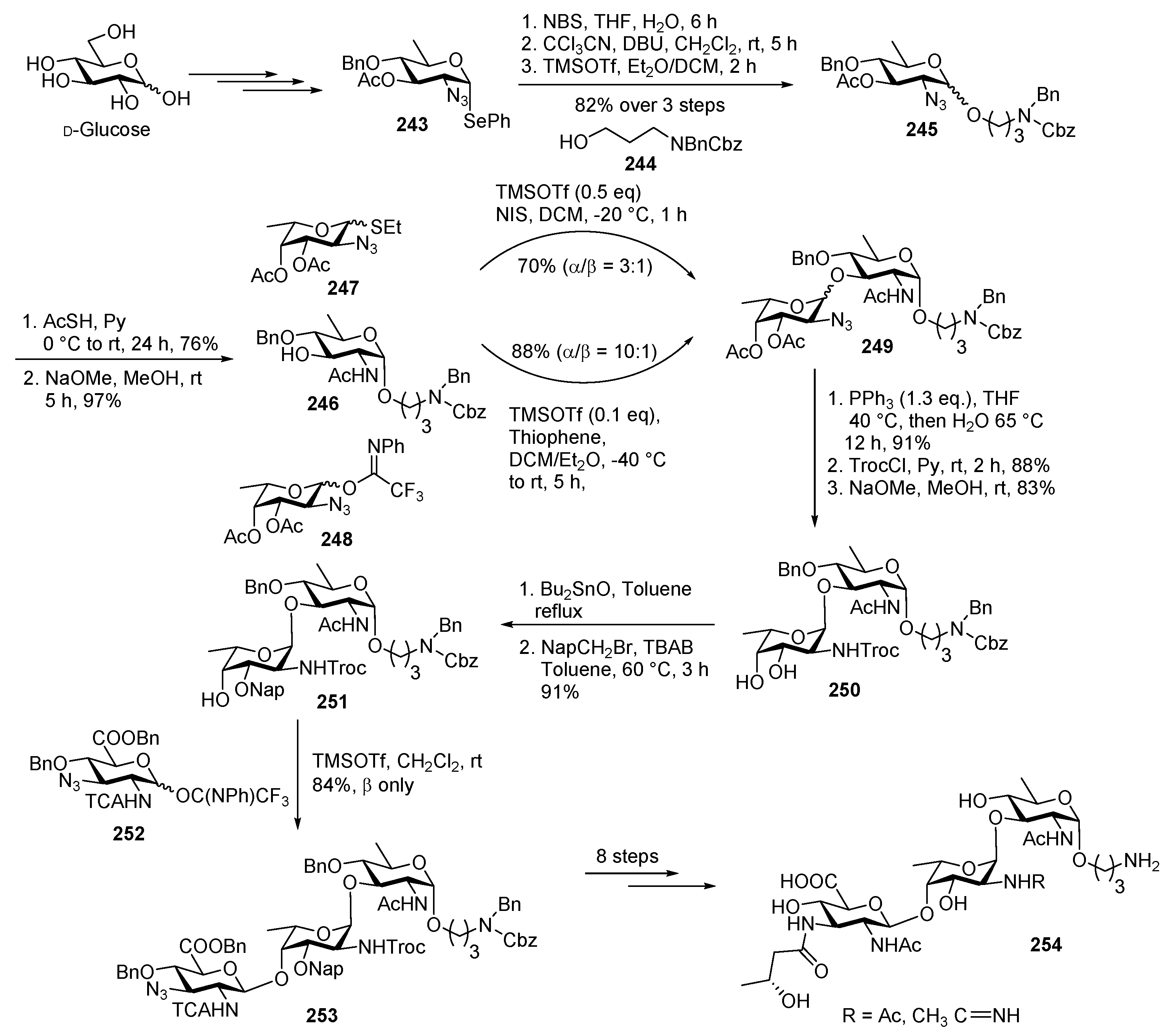
Campylobacter jejuni Heptasaccharide
Zwitterionic Polysaccharides
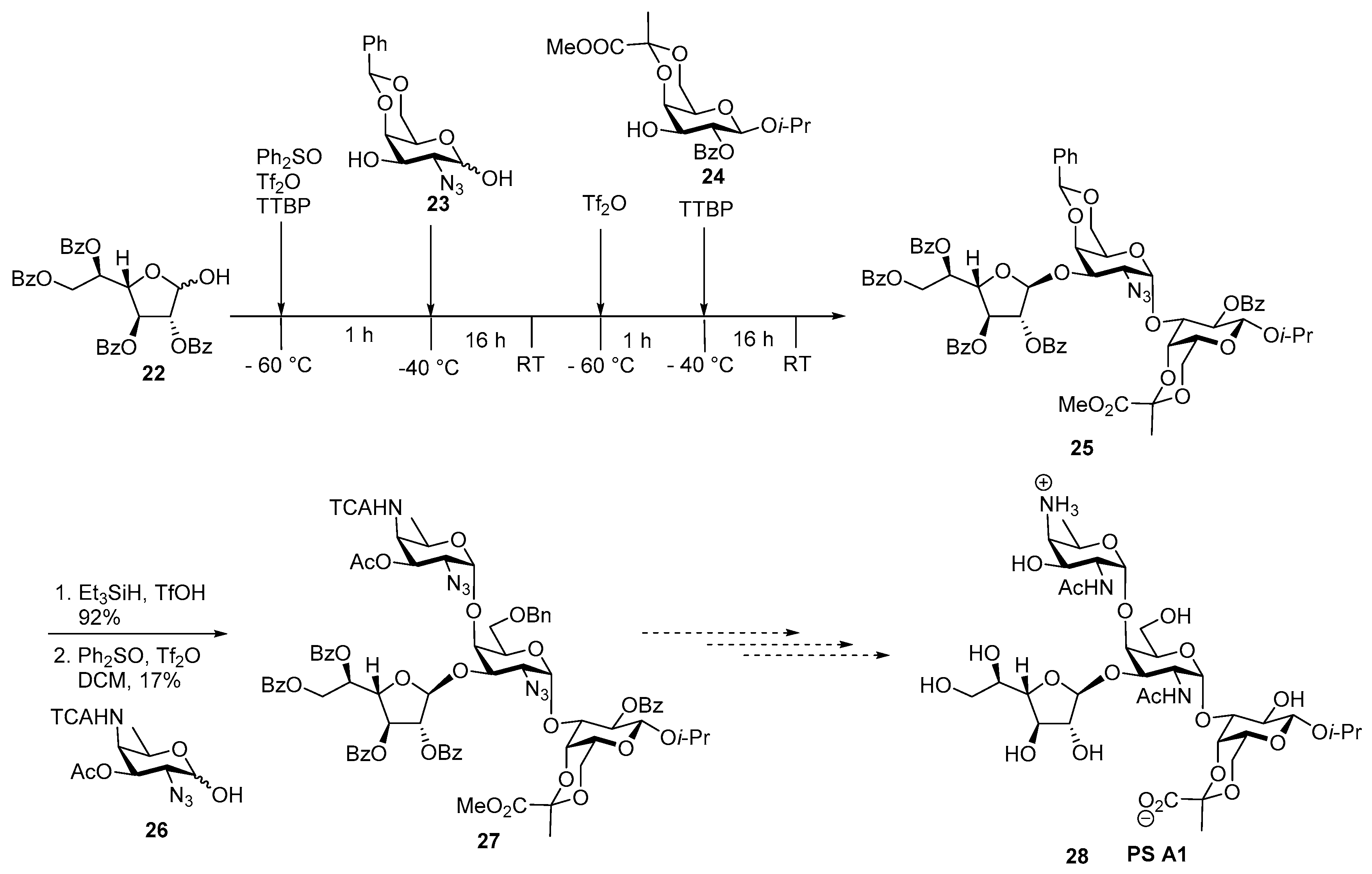
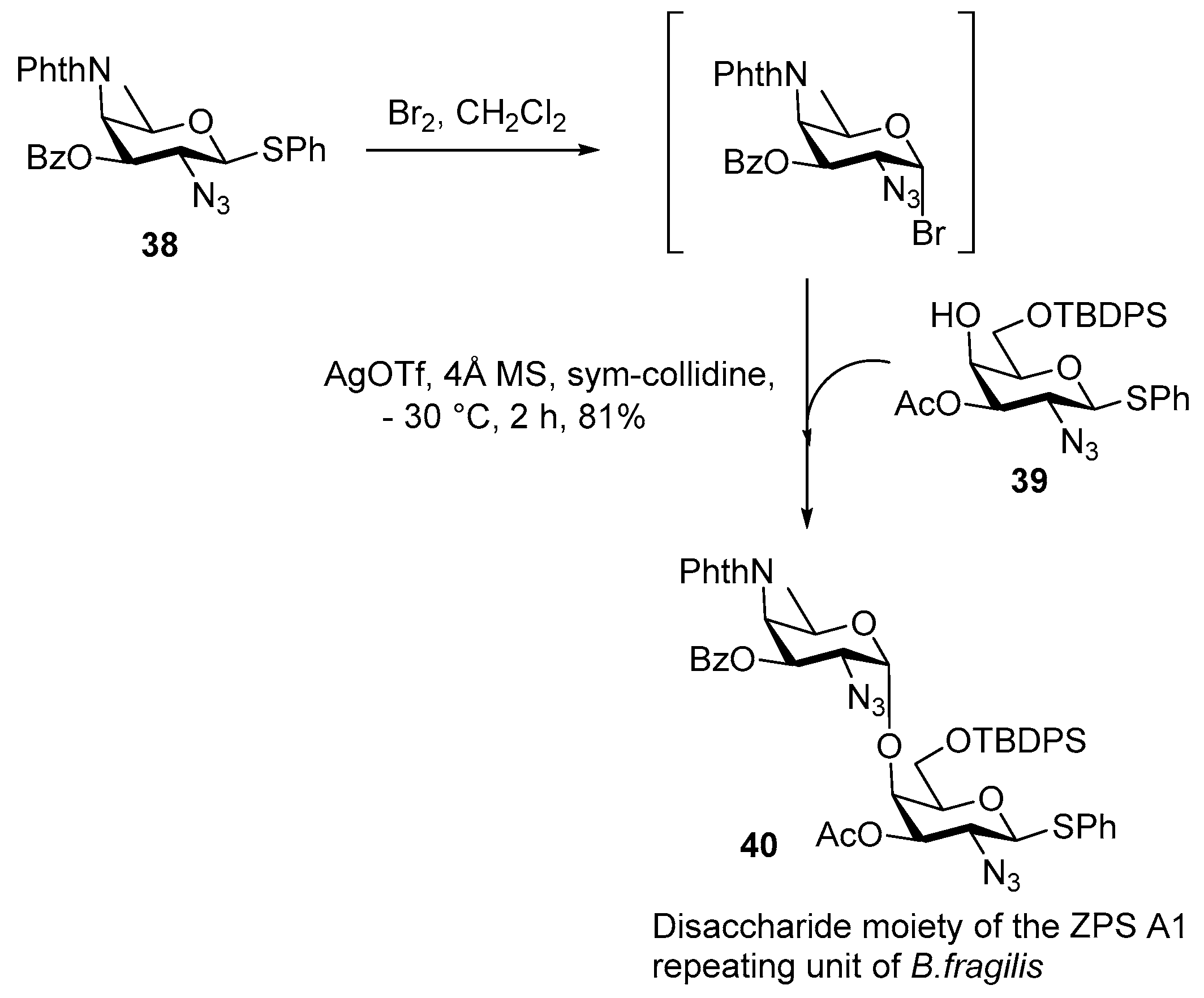
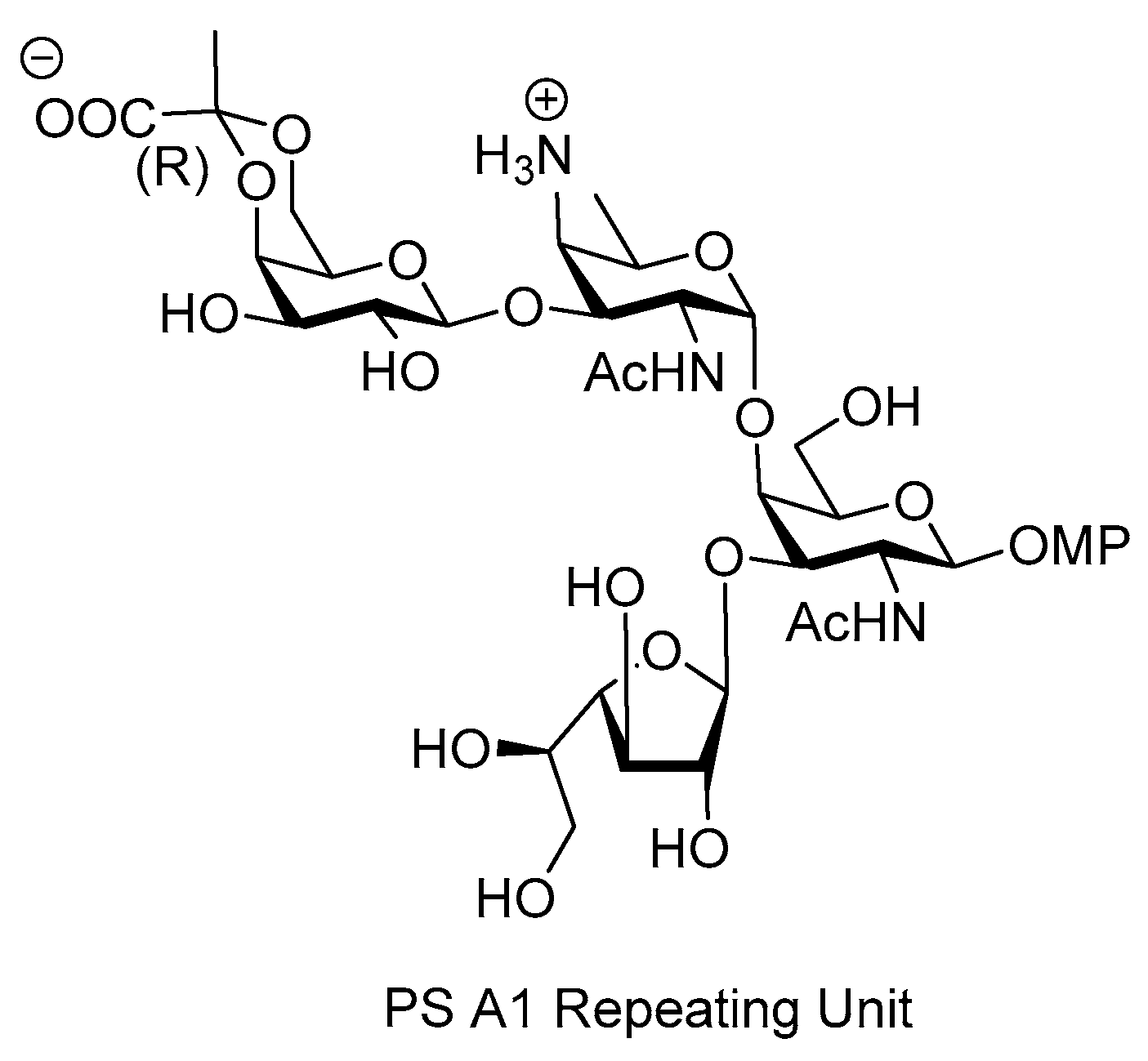
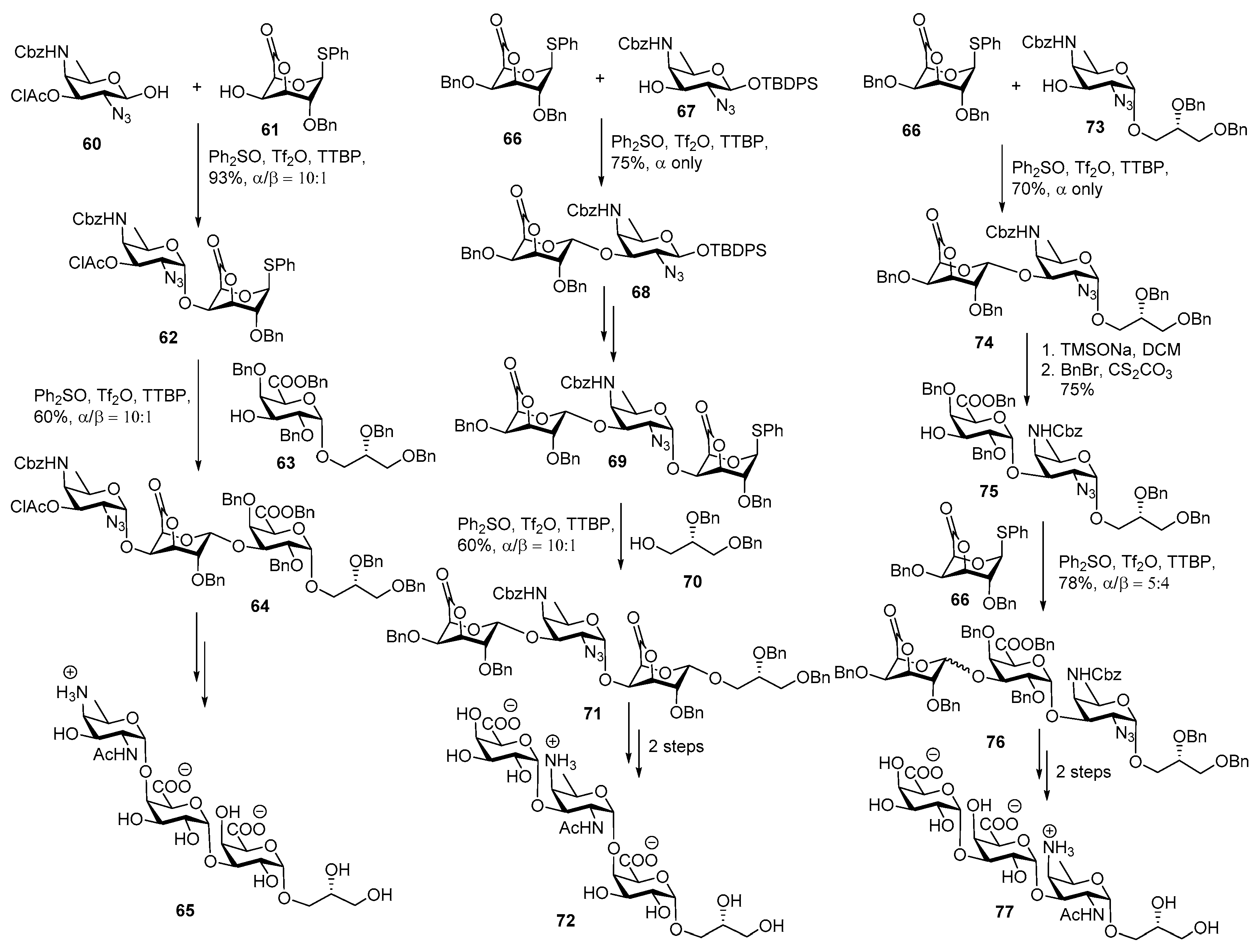
ZPS of Bacteroides fragilis
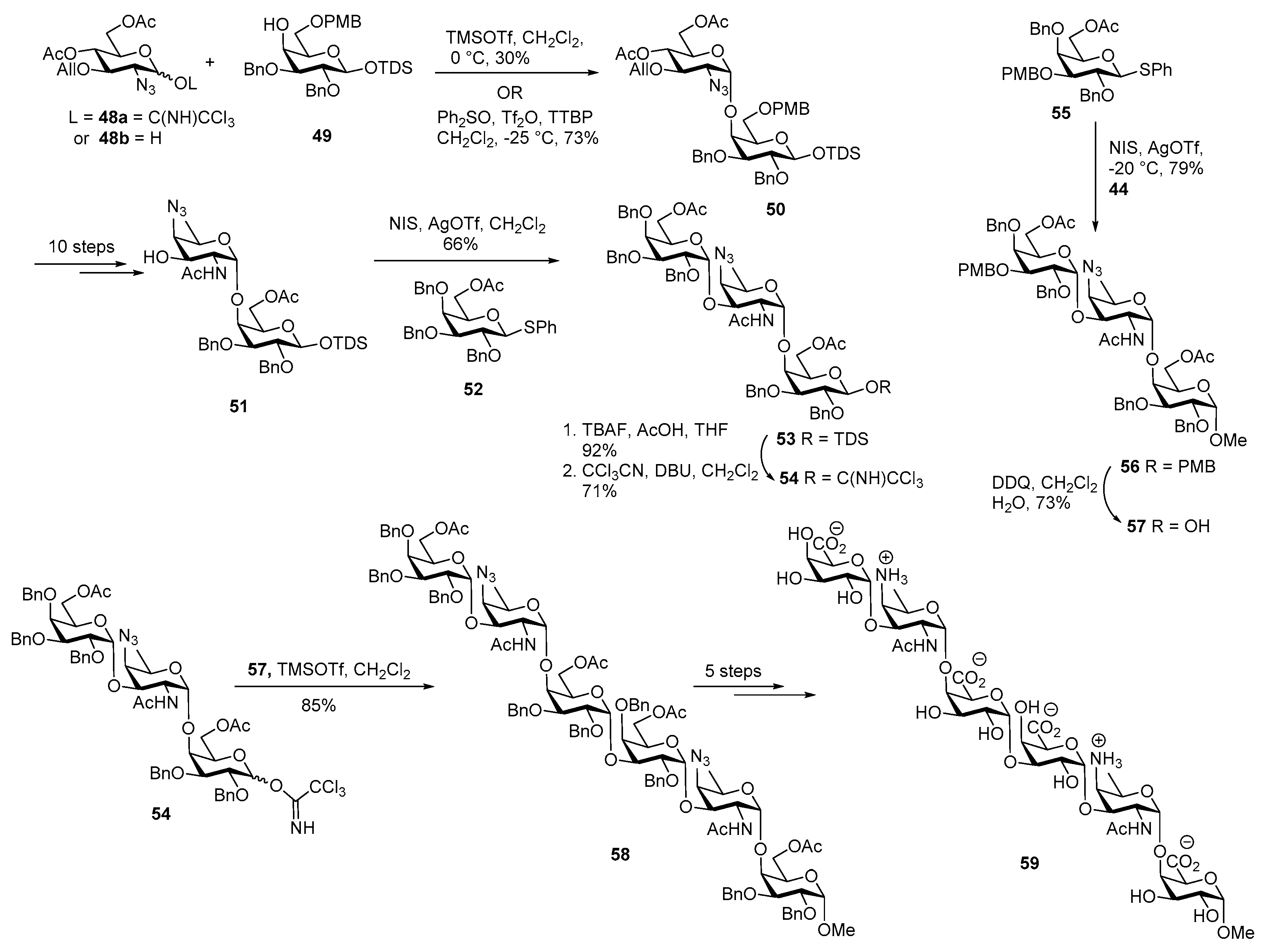
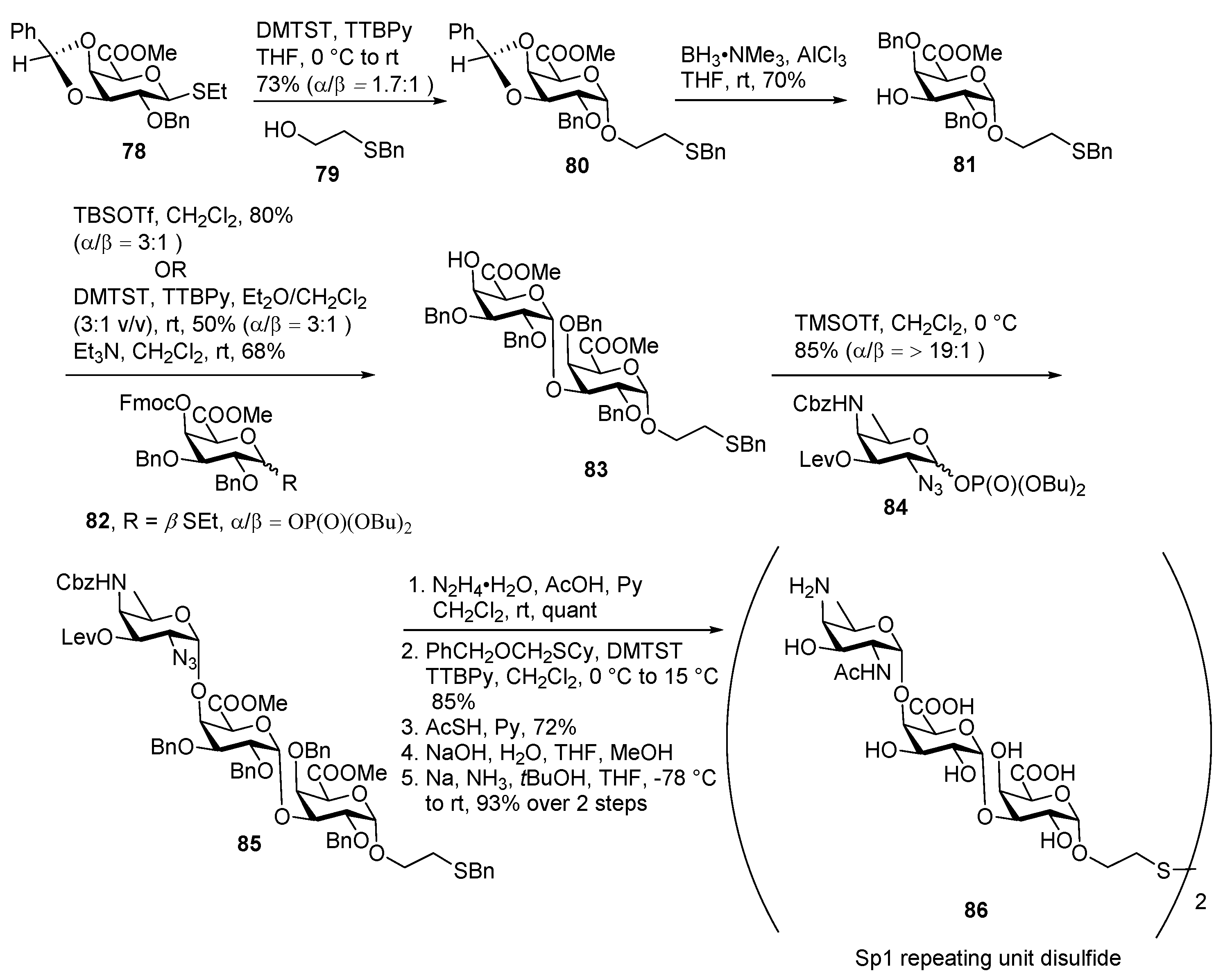
ZPS of Streptococcus pneumoniae
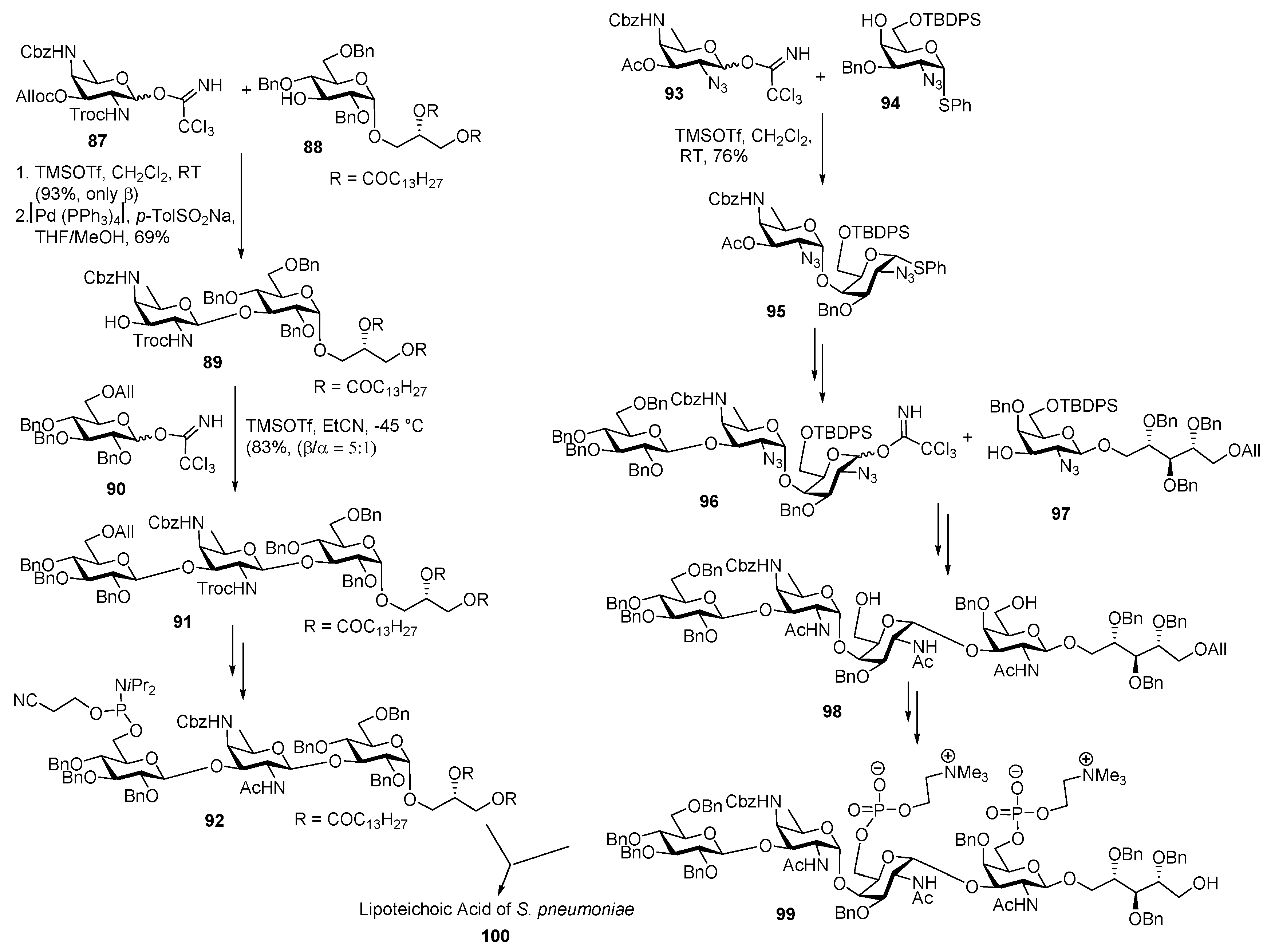
Lipoteichoic Acid of Streptococcus pneumoniae
CPS of Streptococcus pneumoniae Serotype 4
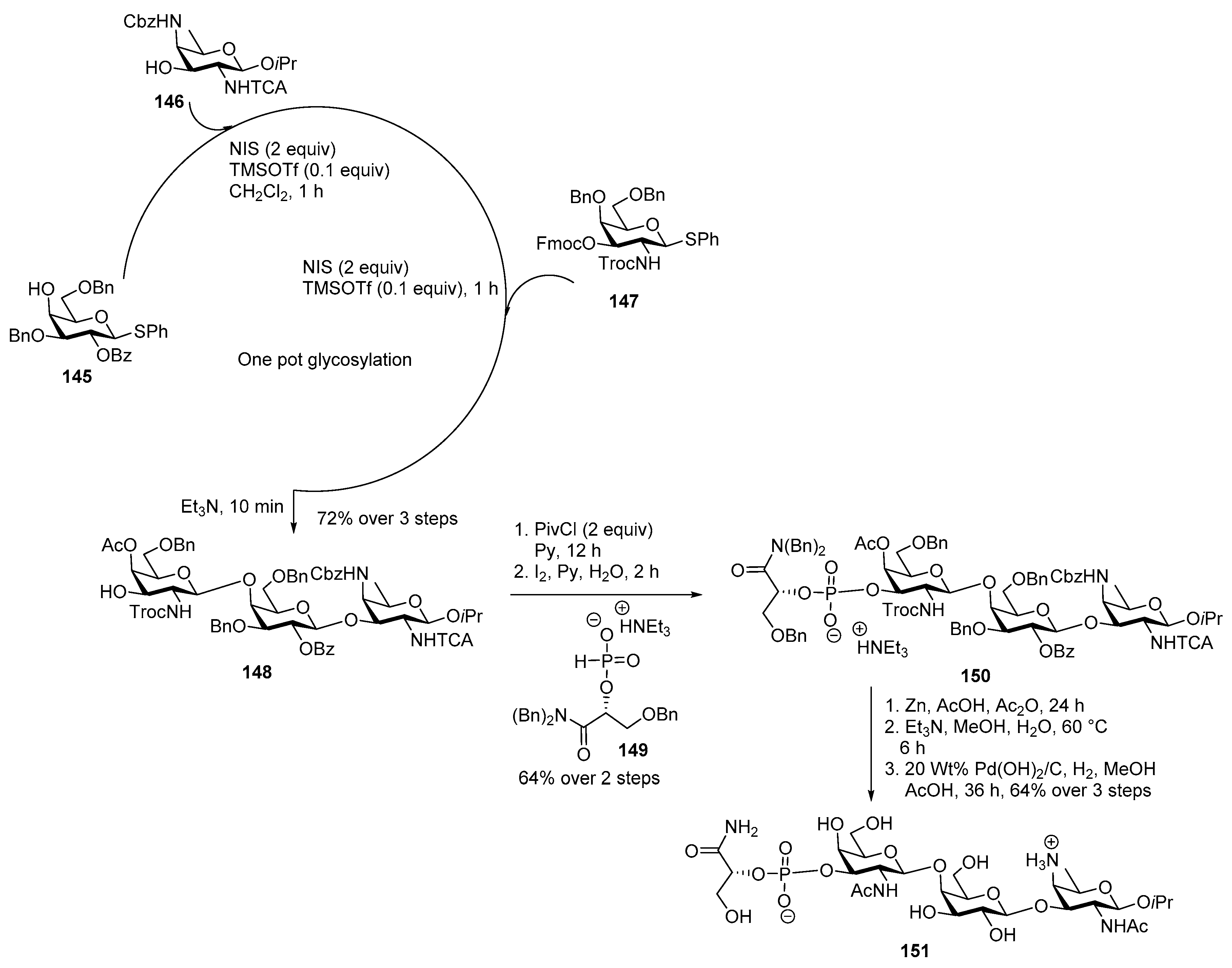
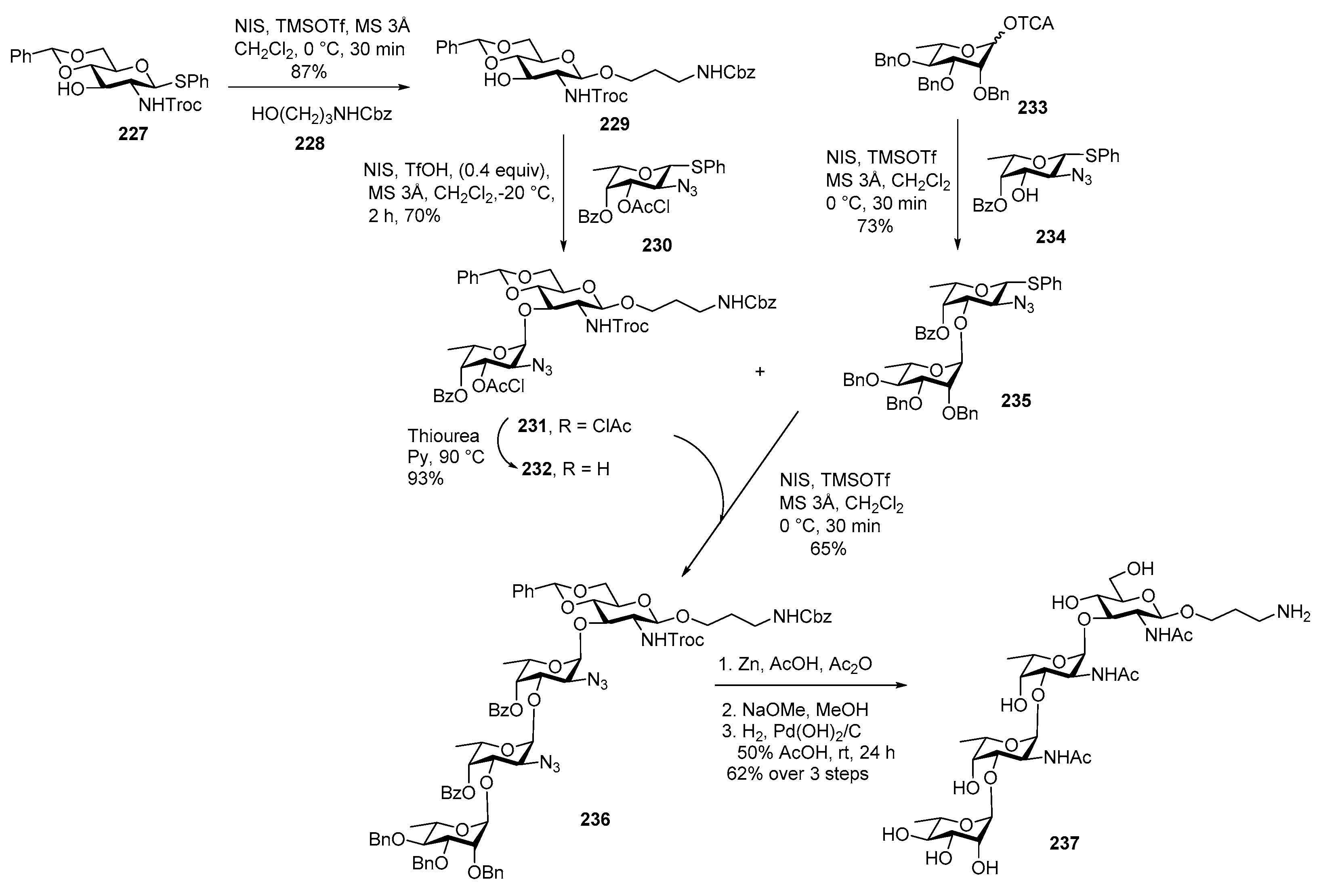
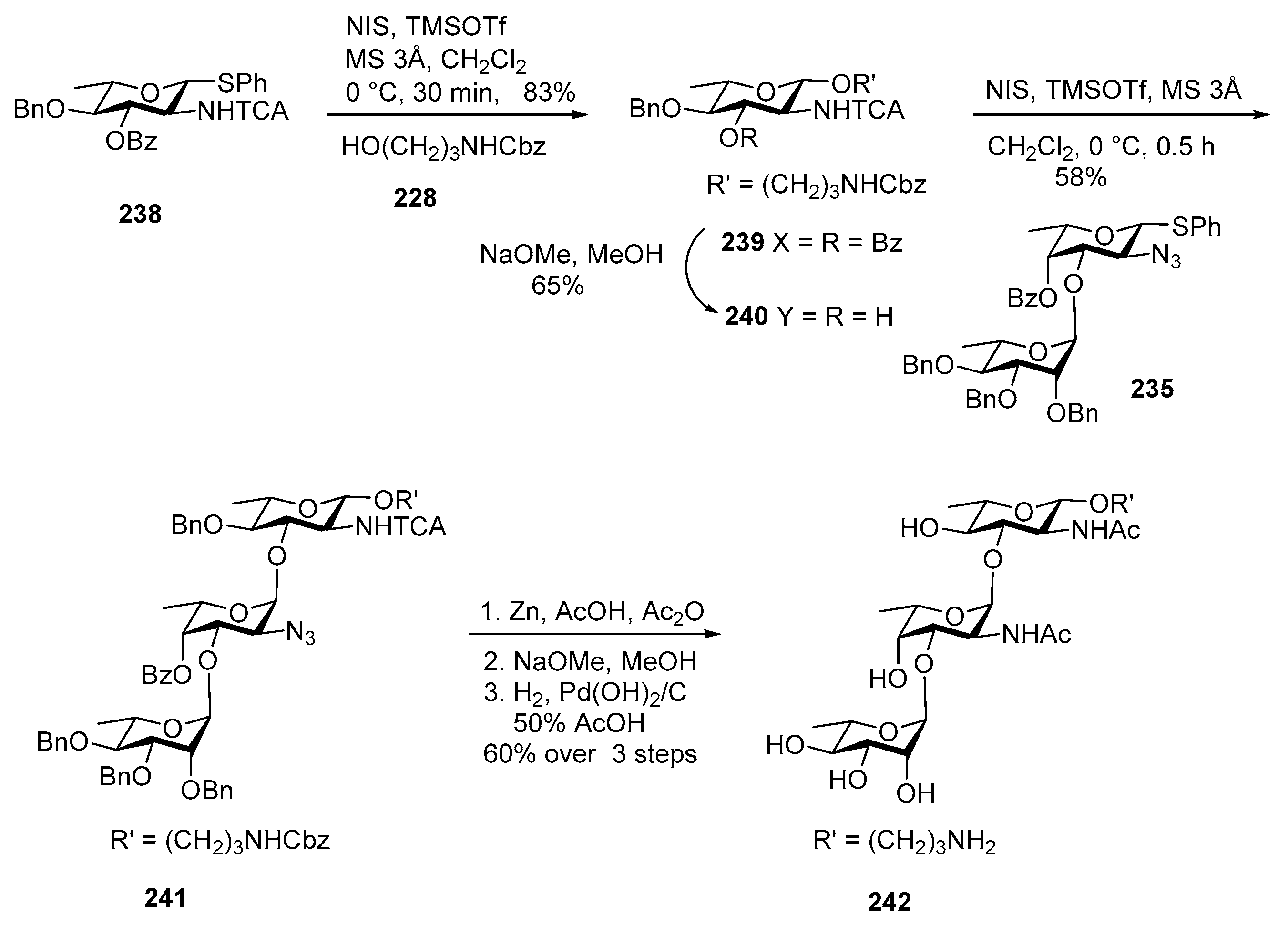
Streptococcus pneumoniae Serotype 12F CPS
CPS of Streptococcus pneumoniae Serotype 5
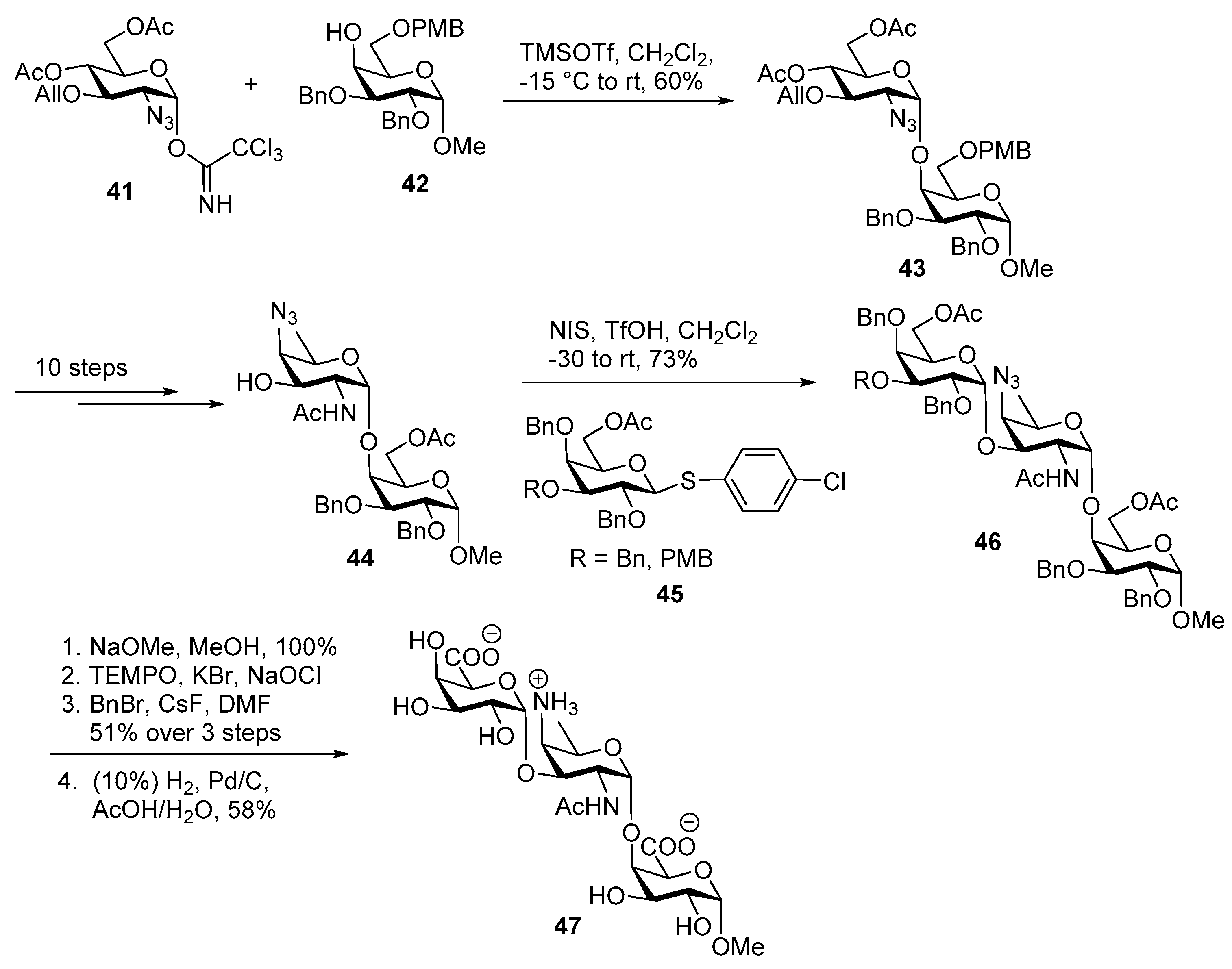
ZPS of Shigella sonnei
Synthesis of Disaccharide AB-Pr (1)
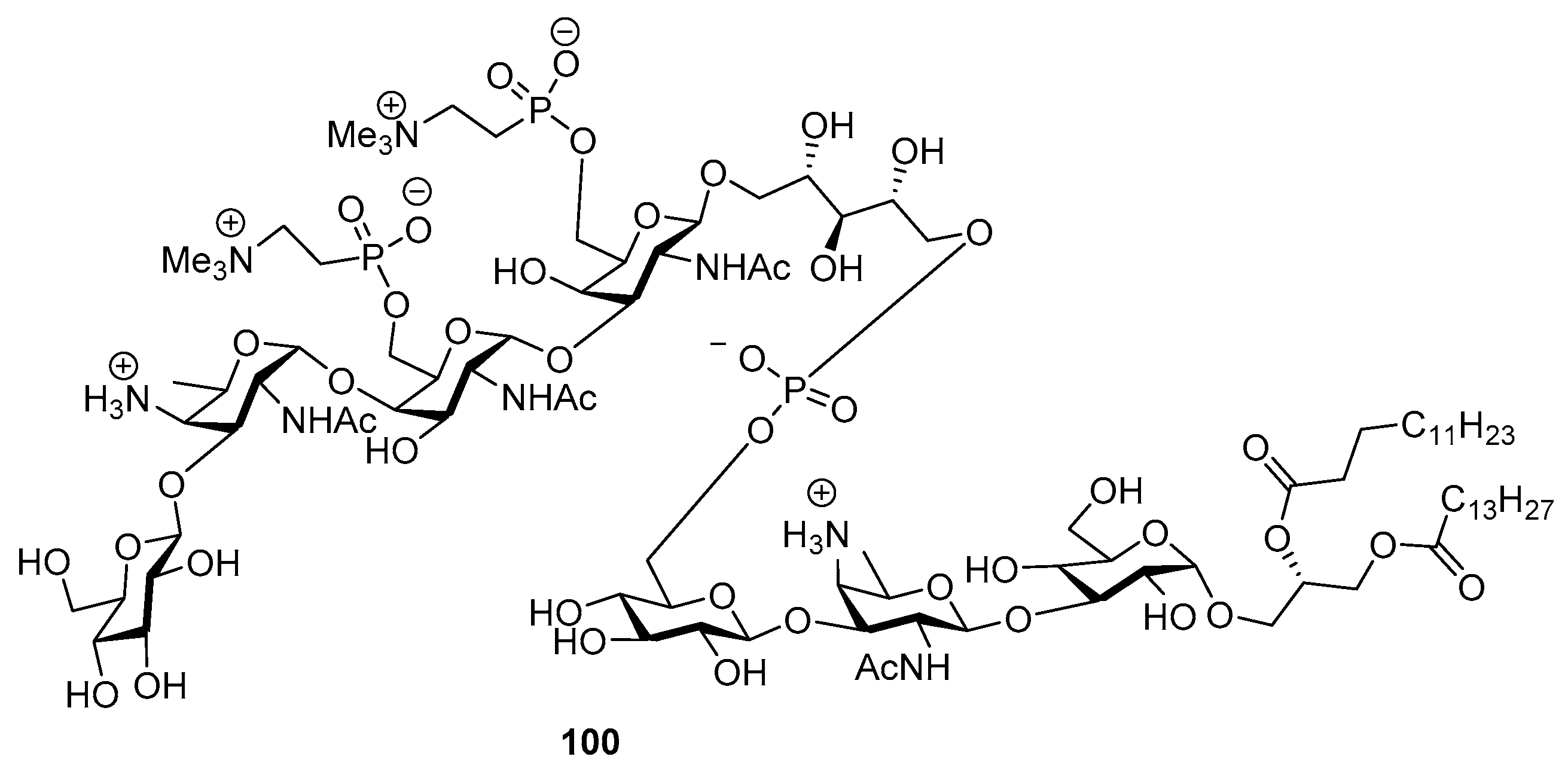
Phosphorylated ZPS of Providencia alcalifaciens O22
Staphylococcus aureus Type 5 Capsular Polysaccharide
Staphylococcus aureus Type 8 Capsular Polysaccharide
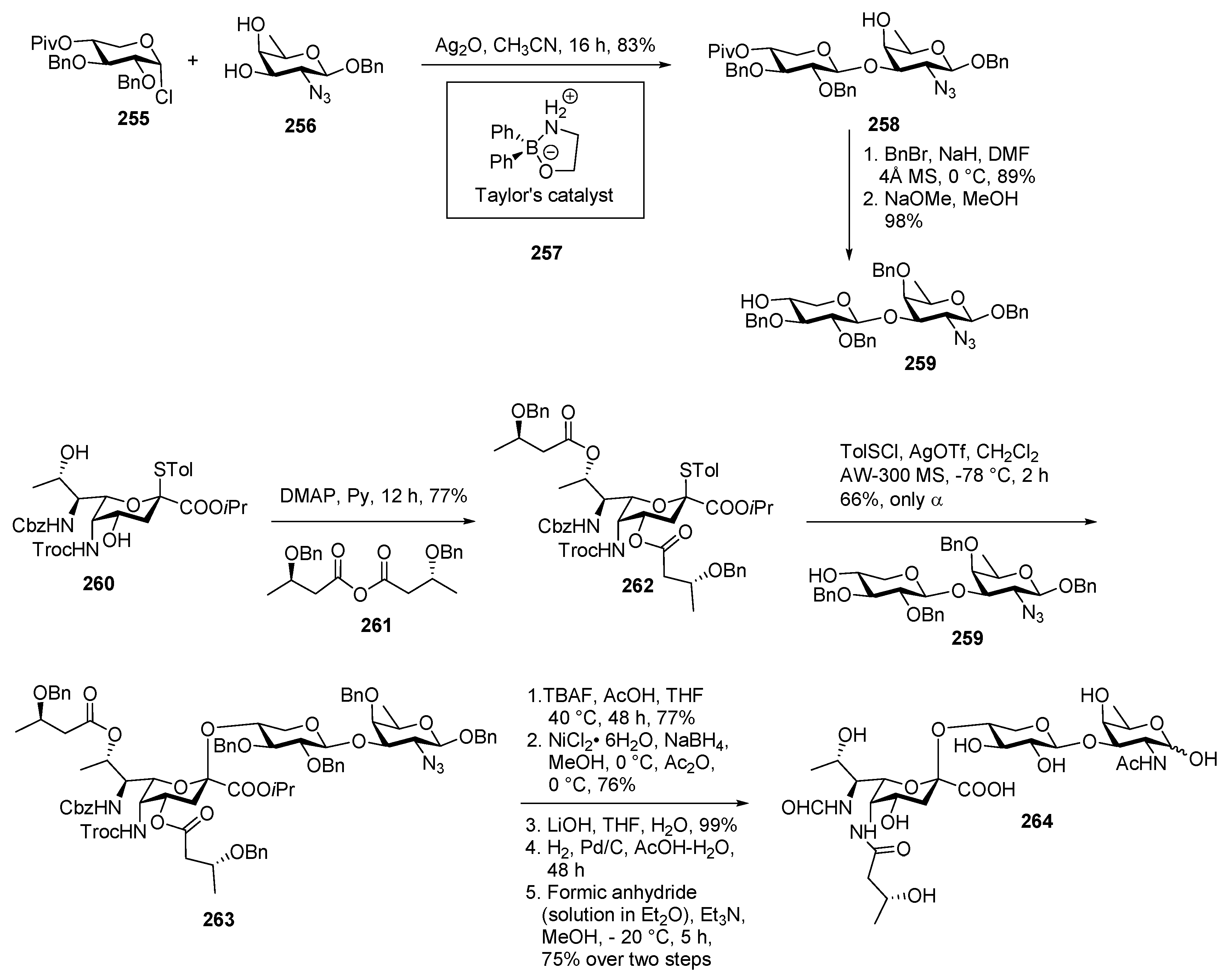
Staphylococcus aureus Strain M Capsular Polysaccharide
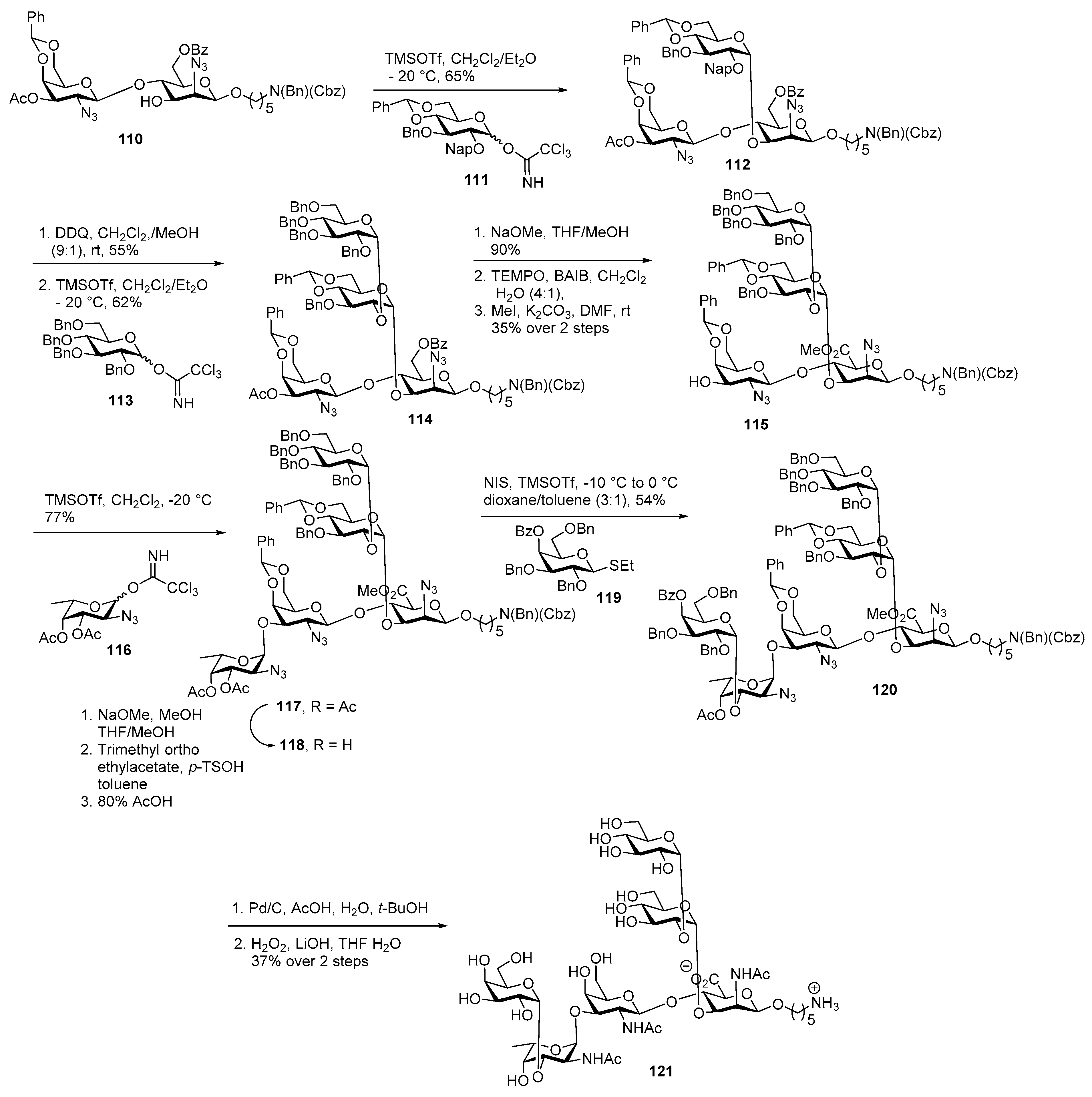
Neisseria meningitidis Pilin Glycans
Bacillus cereus Ch HF-PS
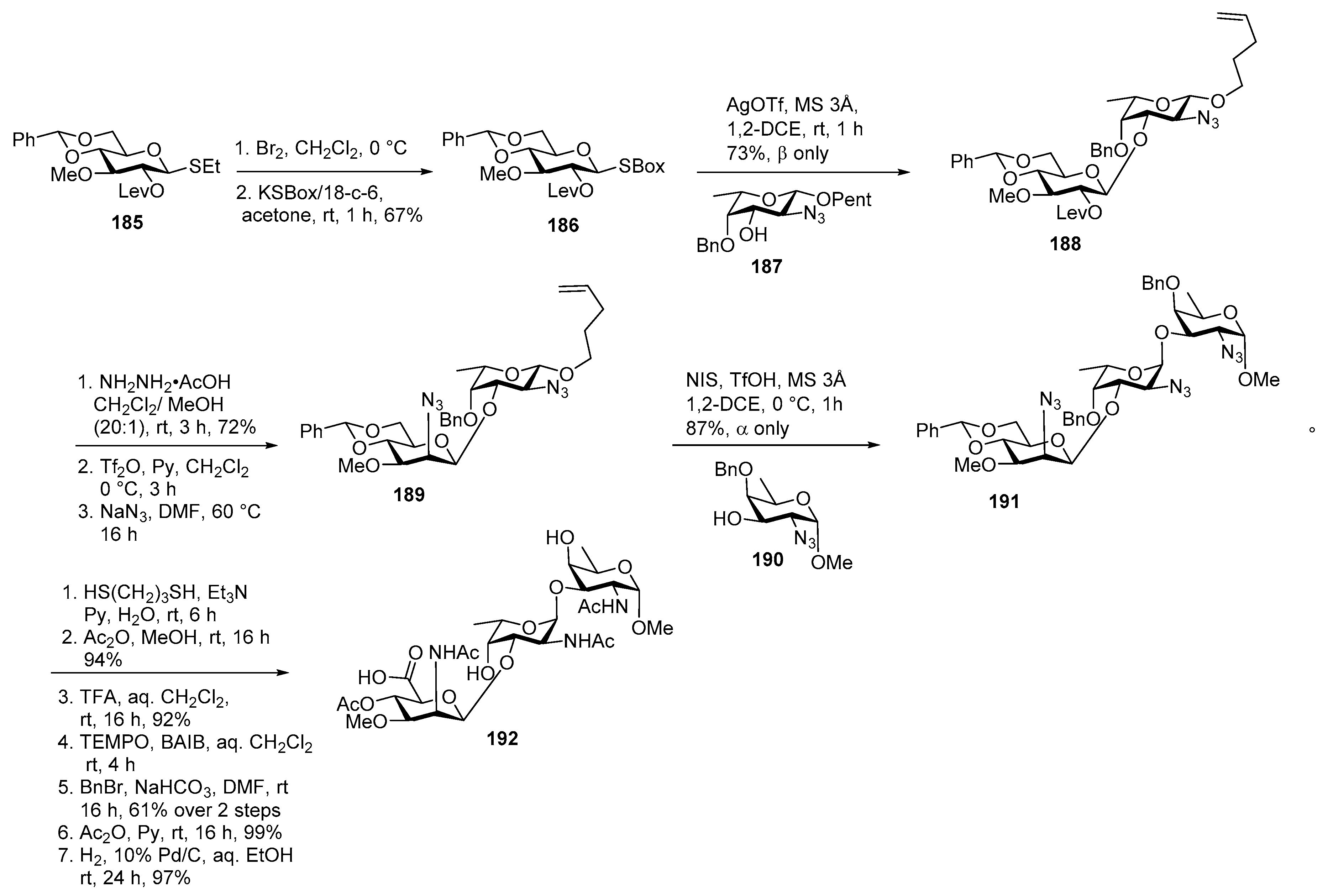
Glycan of Yersinia enterocolitica
P. chlororaphis Subsp. Aureofaciens Strain M71 Glycan
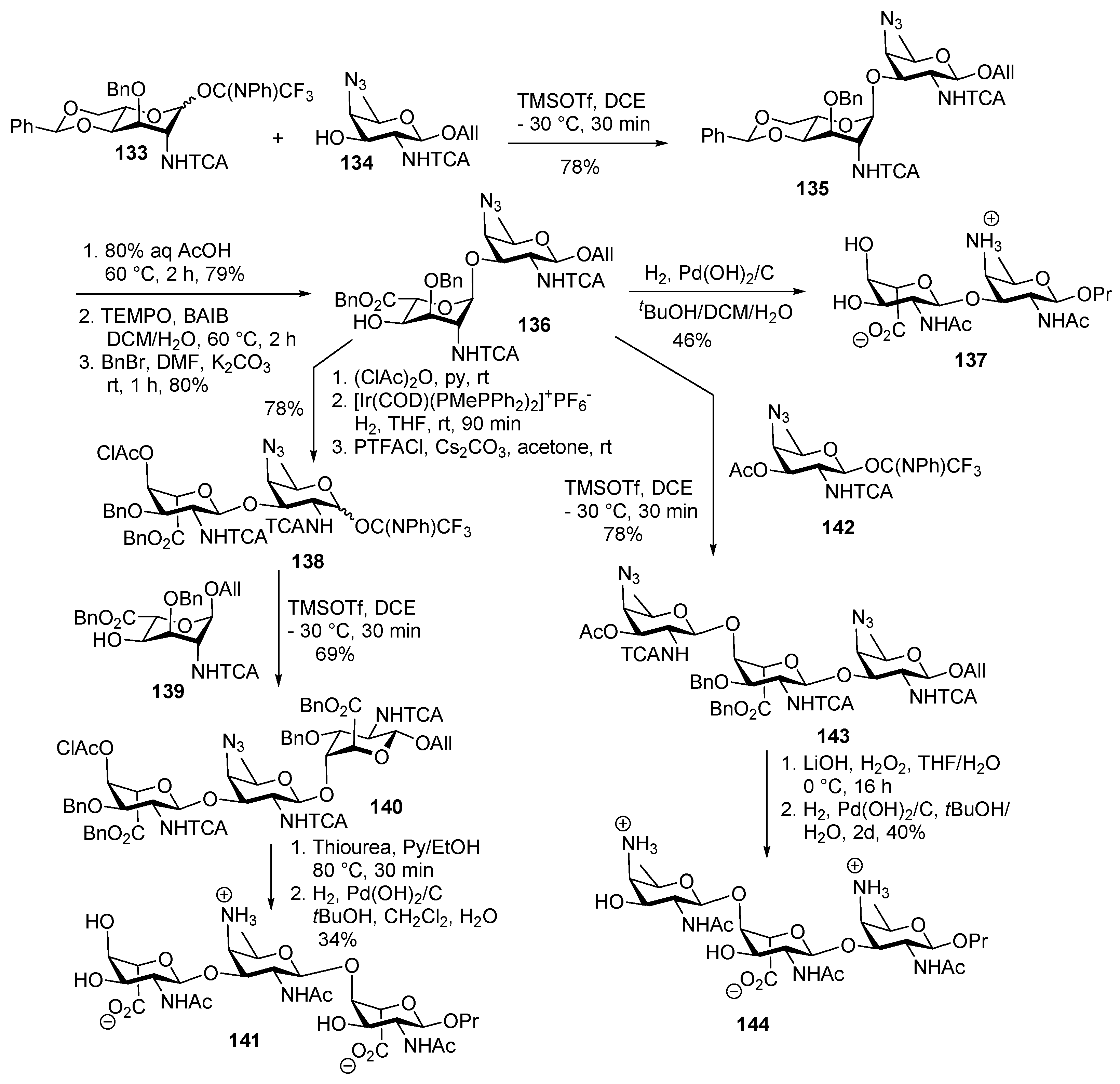
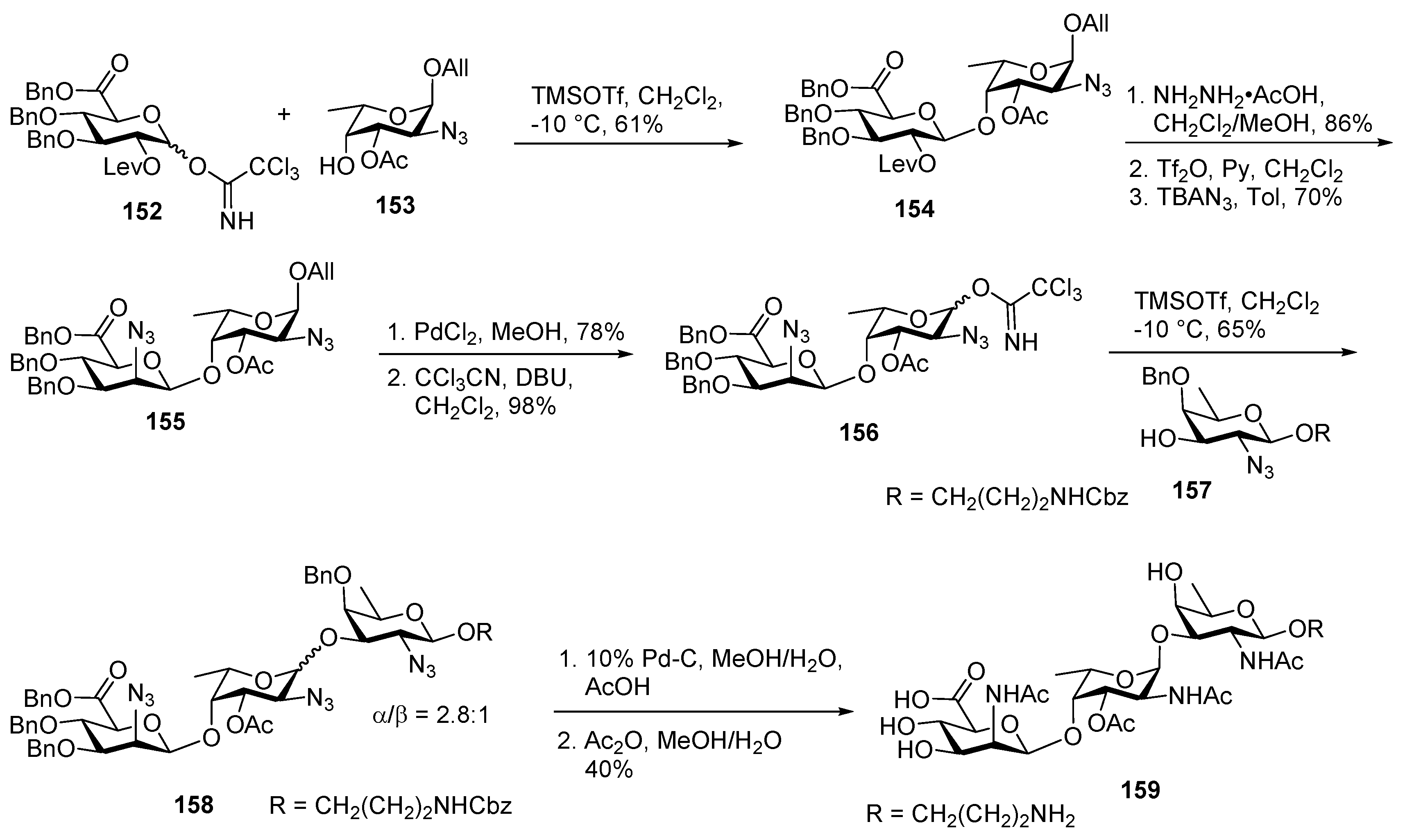
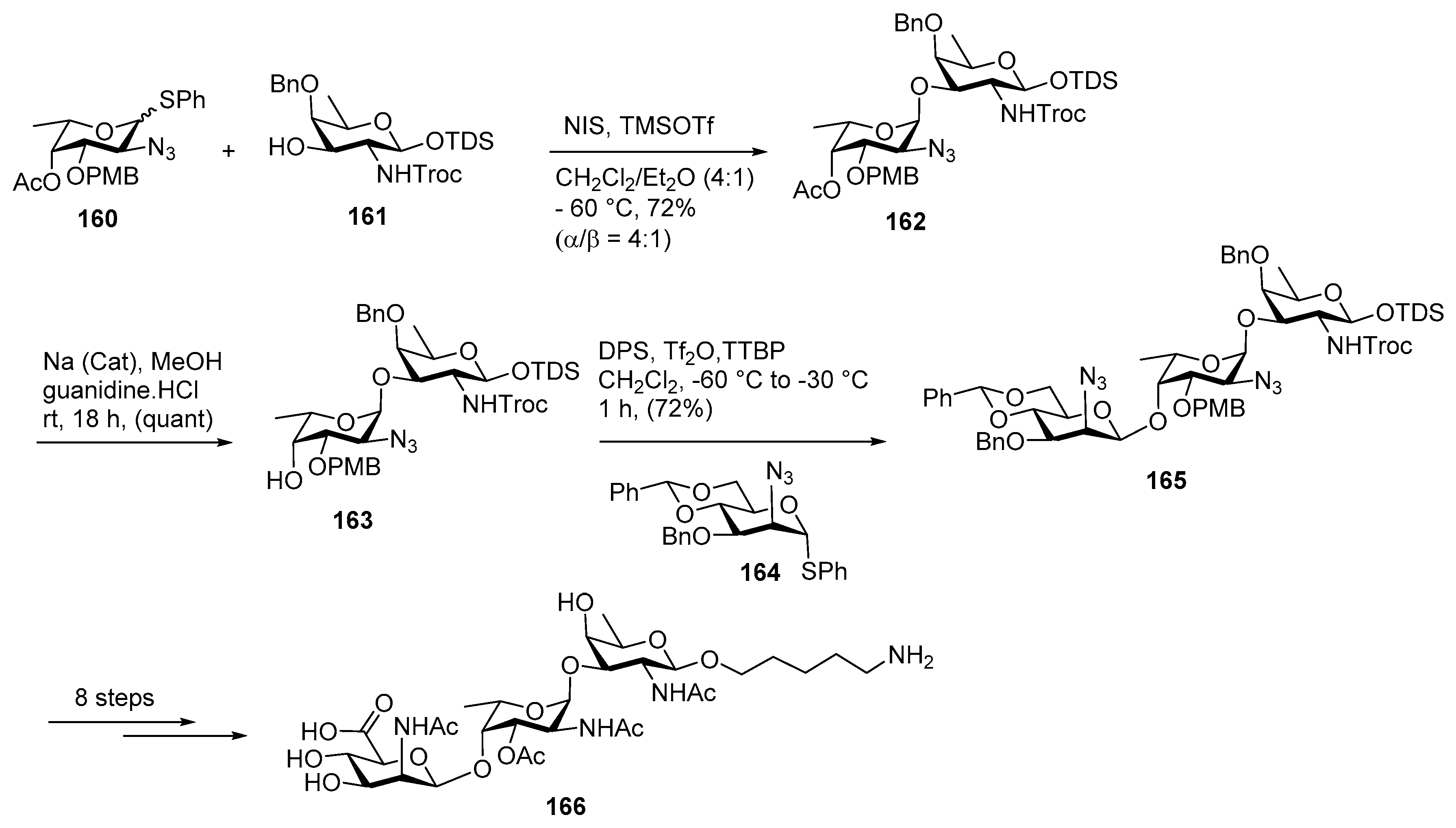
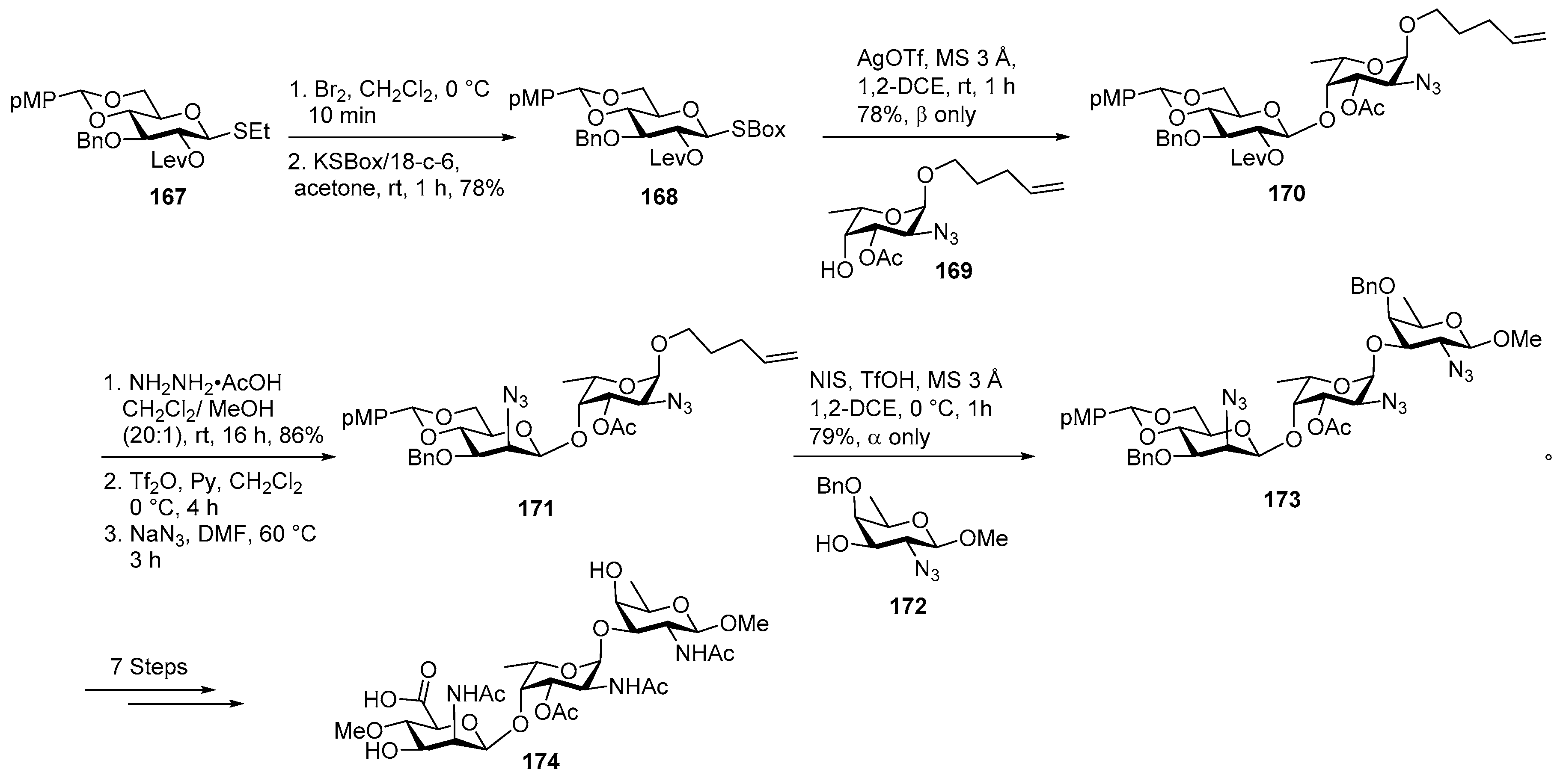
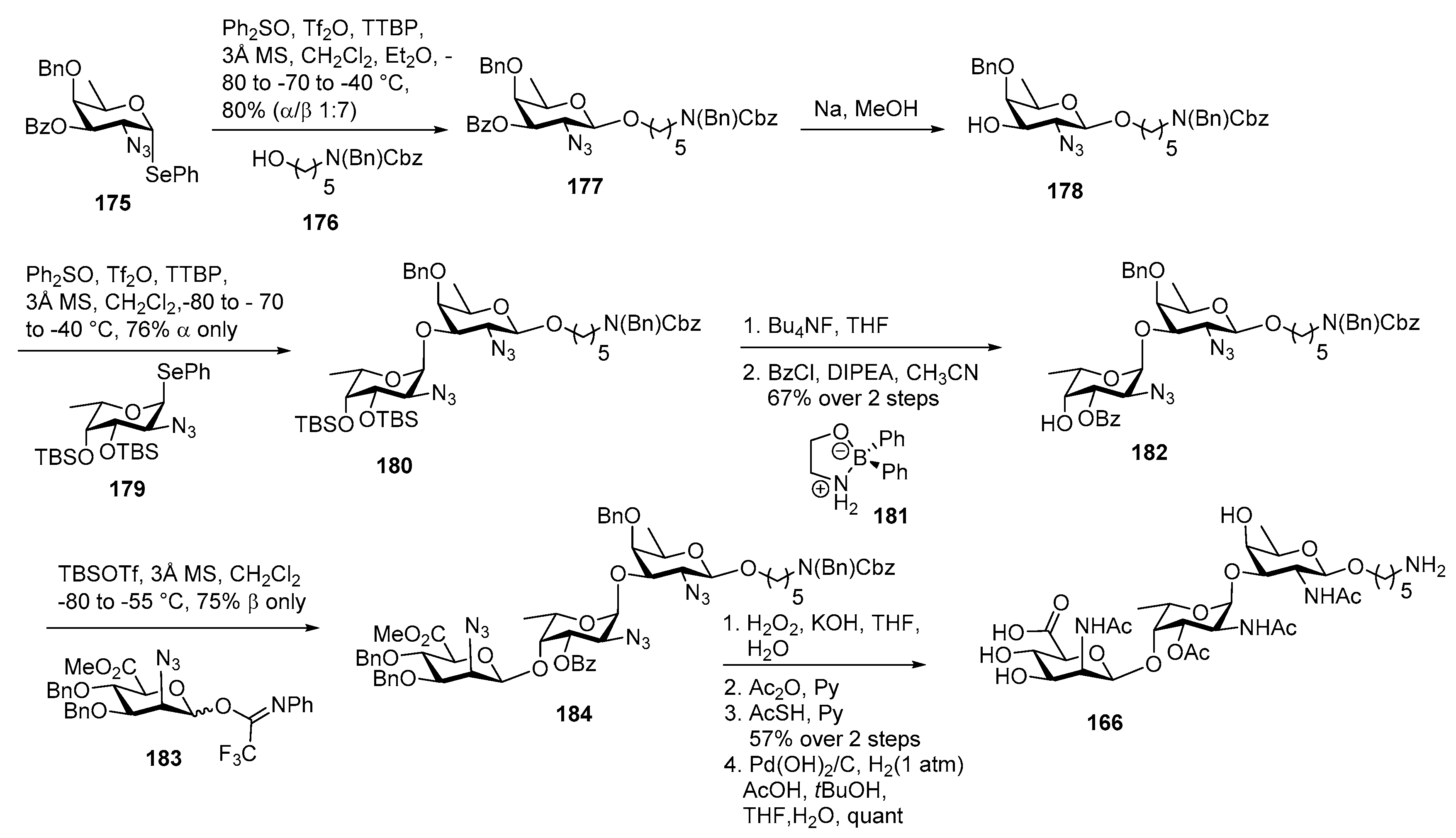
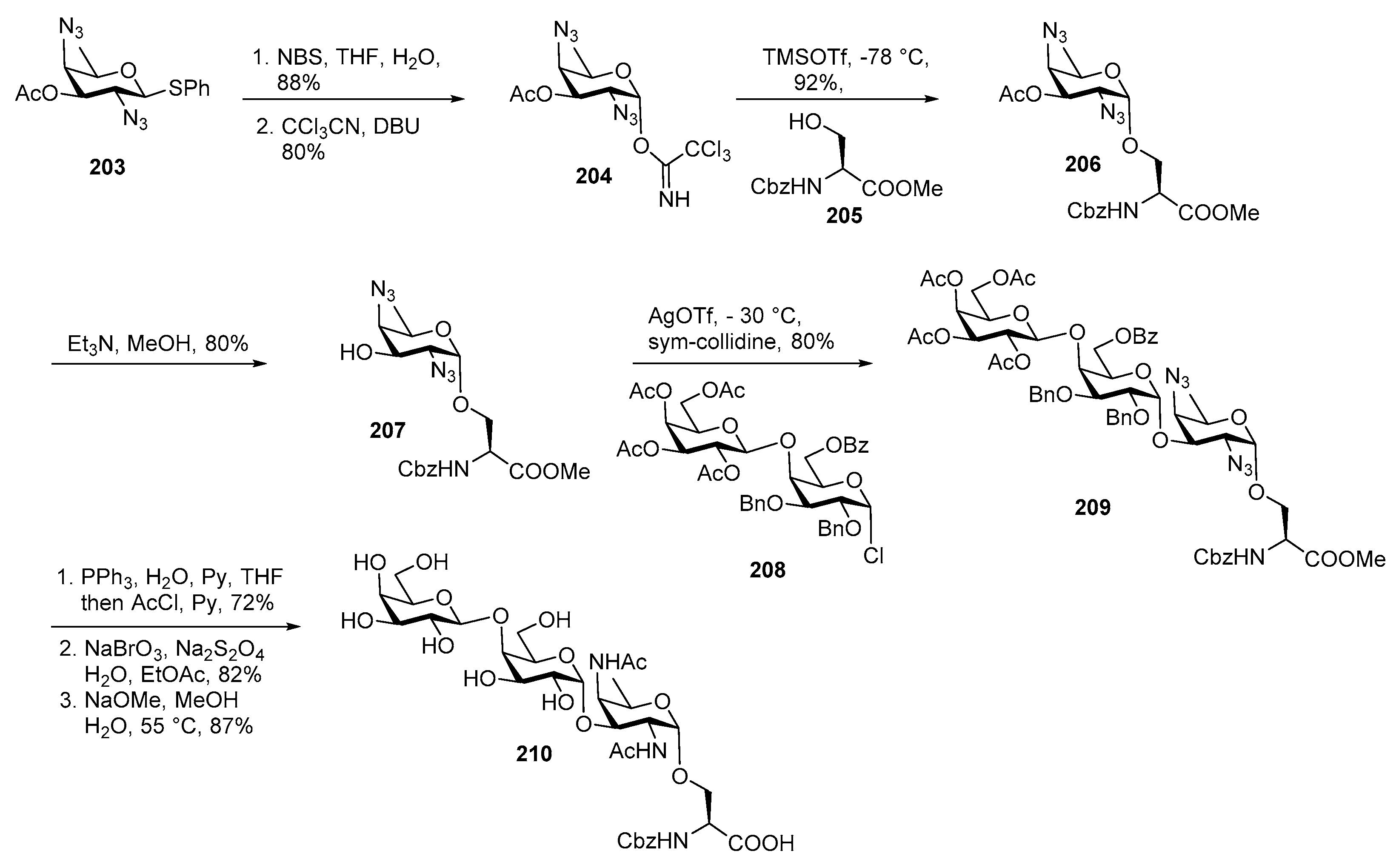
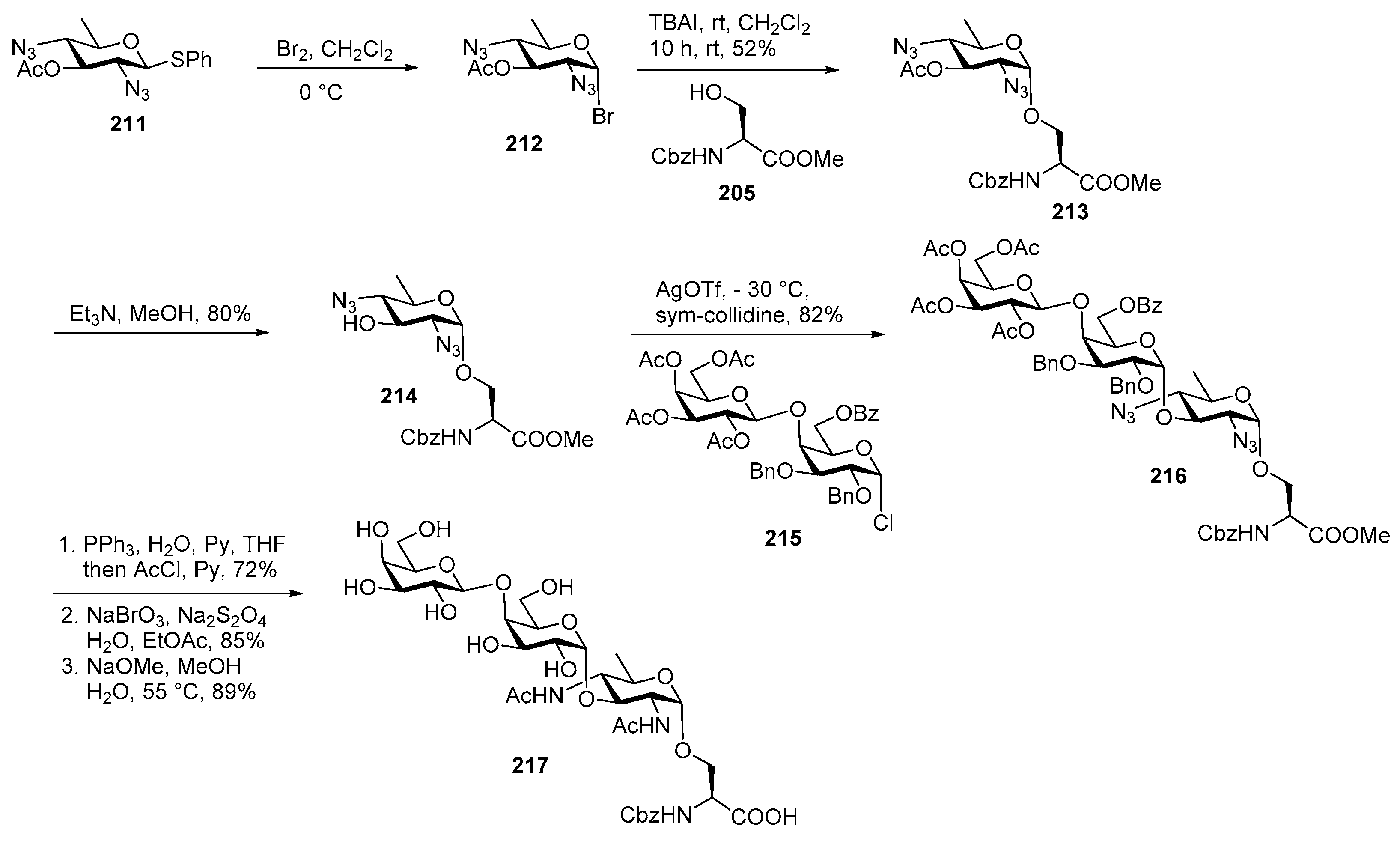
Plesiomonas shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen
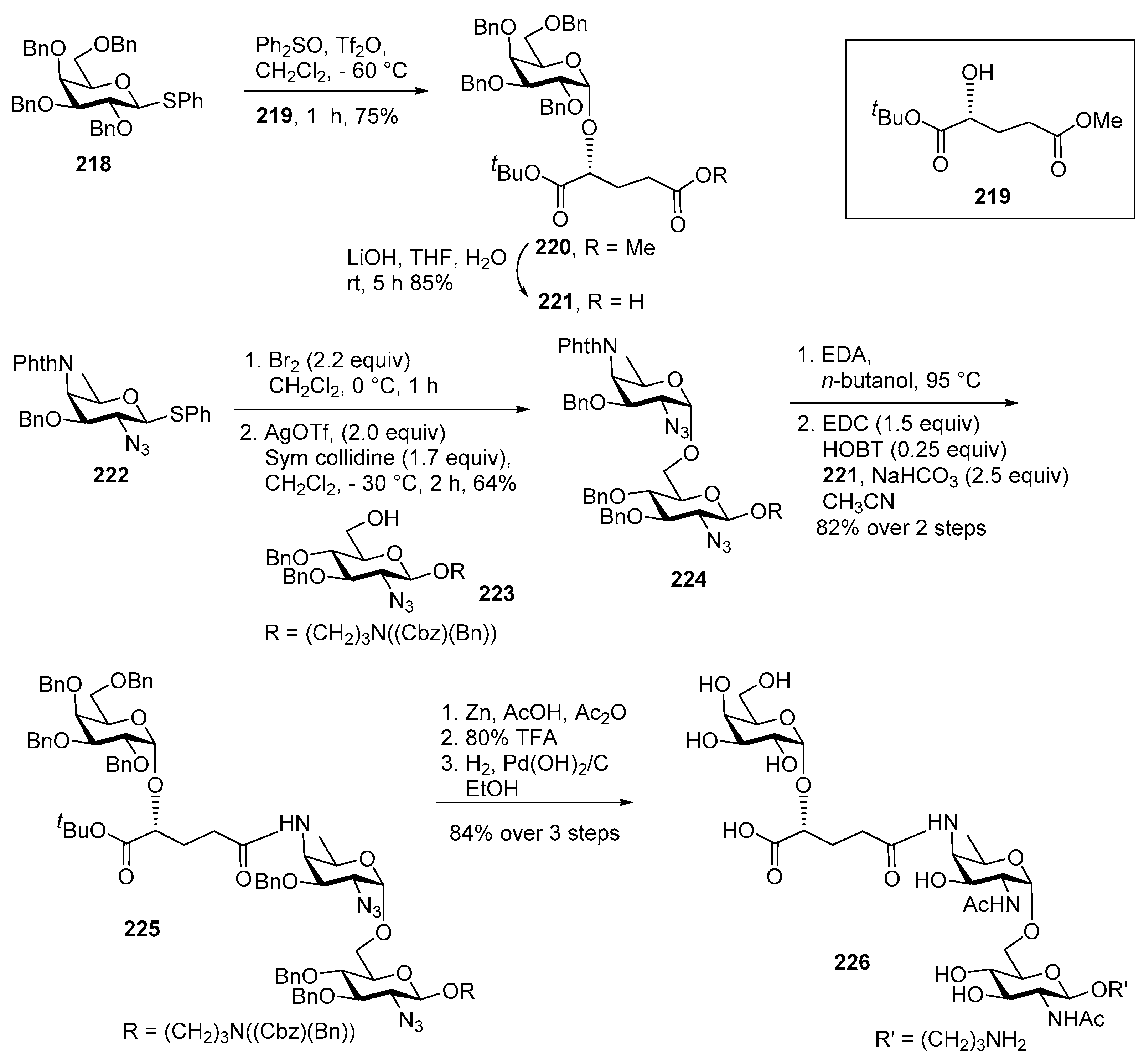
Pseudomonas aeruginosa 1244 Pilin
Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Varki, A. Biological Roles of Oligosaccharides: All of the Theories Are Correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Nishat, S.; Andreana, P. Entirely Carbohydrate-Based Vaccines: An Emerging Field for Specific and Selective Immune Responses. Vaccines 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A Mechanism for Glycoconjugate Vaccine Activation of the Adaptive Immune System and Its Implications for Vaccine Design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micoli, F.; Costantino, P.; Adamo, R. Potential Targets for next Generation Anti-Microbial Glycoconjugate Vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef] [PubMed]
- Dube, D.H.; Champasa, K.; Wang, B. Chemical Tools to Discover and Target Bacterial Glycoproteins. Chem. Commun. 2011, 47, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Longwell, S.A.; Dube, D.H. Deciphering the Bacterial Glycocode: Recent Advances in Bacterial Glycoproteomics. Curr. Opin. Chem. Biol. 2013, 17, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Adibekian, A.; Stallforth, P.; Hecht, M.L.; Werz, D.B.; Gagneux, P.; Seeberger, P.H. Comparative Bioinformatics Analysis of the Mammalian and Bacterial Glycomes. Chem. Sci. 2011, 2, 337–344. [Google Scholar] [CrossRef]
- Morelli, L.; Poletti, L.; Lay, L. Carbohydrates and Immunology: Synthetic Oligosaccharide Antigens for Vaccine Formulation. Eur. J. Org. Chem. 2011, 2011, 5723–5777. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Cañada, F.J.; Jiménez-Barbero, J. Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem. Eur. J. 2015, 21, 10616–10628. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Werz, D.B. Synthesis and Medical Applications of Oligosaccharides. Nature 2007, 446, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.L.; Emmadi, M.; Krupp, K.L.; Podilapu, A.R.; Helble, J.D.; Kulkarni, S.S.; Dube, D.H. Development of Rare Bacterial Monosaccharide Analogs for Metabolic Glycan Labeling in Pathogenic Bacteria. ACS Chem. Biol. 2016, 11, 3365–3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, A.; Malleron, A.; Awwad, M.; Dukan, S.; Vauzeilles, B. Click-Mediated Labeling of Bacterial Membranes through Metabolic Modification of the Lipopolysaccharide Inner Core. Angew. Chem. Int. Ed. 2012, 51, 3143–3146. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Recent Advances in Synthesis of Bacterial Rare Sugar Building Blocks and Their Applications. Nat. Prod. Rep. 2014, 31, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Linton, D.; Hitchen, P.G.; Nita-Lazar, M.; Haslam, S.M.; North, S.J.; Panico, M.; Morris, H.R.; Dell, A.; Wren, B.W. N-Linked Glycosylation in Campylobacter Jejuni and Its Functional Transfer into E. coli. Science 2002, 298, 1790–1793. [Google Scholar] [CrossRef] [PubMed]
- Young, N.M.; Brisson, J.R.; Kelly, J.; Watson, D.C.; Tessier, L.; Lanthier, P.H.; Jarrell, H.C.; Cadotte, N.; St. Michael, F.; Aberg, E.; et al. Structure of the N-Linked Glycan Present on Multiple Glycoproteins in the Gram-Negative Bacterium, Campylobacter Jejuni. J. Biol. Chem. 2002, 277, 42530–42539. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, C.M.; Burr, D.H.; Guerry, P. Campylobacter Protein Glycosylation Affects Host Cell Interactions. Infect. Immun. 2002, 70, 2242–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vliet, A.H.M.; Ketley, J.M. Pathogenesis of Enteric Campylobacter Infection. J. Appl. Microbiol. 2001, 90, 45S–56S. [Google Scholar] [CrossRef]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter Species and Guillain-Barre Syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [PubMed]
- Glover, K.J.; Weerapana, E.; Imperiali, B. In Vitro Assembly of the Undecaprenylpyrophosphate-Linked Heptasaccharide for Prokaryotic N-Linked Glycosylation. Proc. Natl. Acad. Sci. USA 2005, 102, 14255–14259. [Google Scholar] [CrossRef] [PubMed]
- Weerapana, E.; Glover, K.J.; Chen, M.M.; Imperiali, B. Investigating Bacterial N-Linked Glycosylation: Synthesis and Glycosyl Acceptor Activity of the Undecaprenyl Pyrophosphate-Linked Bacillosamine. J. Am. Chem. Soc. 2005, 127, 13766–13767. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.J.; Weerapana, E.; Numao, S.; Imperiali, B. Chemoenzymatic Synthesis of Glycopeptides with PglB, a Bacterial Oligosaccharyl Transferase from Campylobacter Jejuni. Chem. Biol. 2005, 12, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, A.; Ohta, S.; Ito, Y. A Stereoselective 1,2-Cis Glycosylation toward the Synthesis of a Novel N-Linked Glycan from the Gram-Negative Bacterium, Campylobacter Jejuni. Carbohydr. Res. 2006, 341, 1557–1573. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Ishiwata, A.; Ito, Y. Synthesis of Asparagine-Linked Bacillosamine. Carbohydr. Res. 2006, 341, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Ishiwata, A.; Ito, Y. Synthesis of N-Linked Glycan Derived from Gram-Negative Bacterium, Campylobacter Jejuni. Tetrahedron 2007, 63, 8181–8198. [Google Scholar] [CrossRef]
- Cobb, B.A.; Kasper, D.L. Zwitterionic Capsular Polysaccharides: The New MHCII-Dependent Antigens. Cell. Microbiol. 2005, 7, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Kasper, D.L. The Love-Hate Relationship between Bacterial Polysaccharides and the Host Immune System. Nat. Rev. Immunol. 2006, 6, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Kasper, D.L. How Bacterial Carbohydrates Influence the Adaptive Immune System. Annu. Rev. Immunol. 2010, 28, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A.; Wang, Q.; Tzianabos, A.O.; Kasper, D.L. Polysaccharide Processing and Presentation by the MHCII Pathway. Cell 2004, 117, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, B.A.; Kasper, D.L. Characteristics of Carbohydrate Antigen Binding to the Presentation Protein HLA-DR. Glycobiology 2008, 18, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Tzianabos, A.O.; Brisson, J.R.; Kasper, D.L.; Jennings, H.J. Structural Elucidation of Two Capsular Polysaccharides from One Strain of Bacteroides Fragilis Using High-Resolution NMR Spectroscopy. Biochemistry 1992, 31, 4081–4089. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; McLoughlin, R.M.; Cobb, B.A.; Charrel-Dennis, M.; Zaleski, K.J.; Golenbock, D.; Tzianabos, A.O.; Kasper, D.L. A Bacterial Carbohydrate Links Innate and Adaptive Responses through Toll-Like Receptor 2. J. Exp. Med. 2006, 203, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Cui, H.L.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Perez, B.; Chung, D.R.; Sharpe, A.H.; Yagita, H.; Kalka-Moll, W.M.; Sayegh, M.H.; Kasper, D.L.; Tzianabos, A.O. Modulation of Surgical Fibrosis by Microbial Zwitterionic Polysaccharides. Proc. Natl. Acad. Sci. USA 2005, 102, 16753–16758. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A Polysaccharide from the Human Commensal Bacteroides Fragilis Protects against CNS Demyelinating Disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, L.J.; Boltje, T.J.; Provoost, T.; Mazurek, J.; Overkleeft, H.S.; van der Marel, G.A. A Synthetic Study towards the PSA1 Tetrasaccharide Repeating Unit. Tetrahedron Lett. 2007, 48, 2697–2700. [Google Scholar] [CrossRef]
- Pragani, R.; Seeberger, P.H. Total Synthesis of the Bacteroides Fragilis Zwitterionic Polysaccharide A1 Repeating Unit. J. Am. Chem. Soc. 2011, 133, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Pragani, R.; Seeberger, P.H. De Novo Synthesis of a 2-Acetamido-4-Amino-2,4,6-Trideoxy-d-Galactose (AAT) Building Block for the Preparation of the Zwitterionic Polysaccharide A1 (PS A1) Repeating Subunit of Bacteroides Fragilis. Org. Lett. 2010, 12, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Orthogonally Protected d-Galactosamine Thioglycoside Building Blocks via Highly Regioselective, Double Serial and Double Parallel Inversions of β-d-Thiomannoside. Org. Biomol. Chem. 2013, 11, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.A.; Wang, Q.; Chidley, T.; Appulage, D.K.; Andreana, P.R. Immunological Response from an Entirely Carbohydrate Antigen: Design of Synthetic Vaccines Based on Tn-PS A1 Conjugates. J. Am. Chem. Soc. 2009, 131, 9622–9623. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kleski, K.A.; Trabbic, K.R.; Bourgault, J.-P.; Andreana, P.R. Sialyl-Tn A1 as an Entirely Carbohydrate Immunogen: Synthesis and Immunological Evaluation. J. Am. Chem. Soc. 2016, 138, 14264–14272. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nishat, S.; Andreana, P.R. Synthesis of an Aminooxy Derivative of the Tetrasaccharide Repeating Unit of Streptococcus dysgalactiae 2023 Polysaccharide for a PS A1 Conjugate Vaccine. J. Org. Chem. 2016, 81, 4475–4484. [Google Scholar] [CrossRef] [PubMed]
- Eradi, P.; Ghosh, S.; Andreana, P.R. Total Synthesis of Zwitterionic Tetrasaccharide Repeating Unit from Bacteroides fragilis ATCC 25285/NCTC 9343 Capsular Polysaccharide PS A1 with Alternating Charges on Adjacent Monosaccharides. Org. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Roehrl, M.H.; Kasper, D.L.; Wang, J.Y. A Unique Structural Pattern Shared by T-Cell-Activating and Abscess-Regulating Zwitterionic Polysaccharides. Biochemistry 2002, 41, 15144–15151. [Google Scholar] [CrossRef] [PubMed]
- Zangwill, K.M.; Vadheim, C.M.; Vannier, A.M.; Hemenway, L.S.; Greenberg, D.P.; Ward, J.I. Epidemiology of Invasive Pneumococcal Disease in Southern California: Implications for the Design and Conduct of a Pneumococcal Conjugate Vaccine Efficacy Trial. J. Infect. Dis. 1996, 174, 752–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedrzejas, M.J. Pneumococcal Virulence Factors: Structure and Function. Microbiol. Mol. Biol. Rev. 2001, 65, 187–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowy, F.D. Staphylococcus Aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cui, L.; Lipinski, T.; Bundle, D.R. Synthesis of Monomeric and Dimeric Repeating Units of the Zwitterionic Type 1 Capsular Polysaccharide from Streptococcus Pneumoniae. Chem. Eur. J. 2010, 16, 3476–3488. [Google Scholar] [CrossRef] [PubMed]
- Boebel, T.A.; Gin, D.Y. Sulfoxide Covalent Catalysis: Application to Glycosidic Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5874–5877. [Google Scholar] [CrossRef] [PubMed]
- Christina, A.E.; Van Den Bos, L.J.; Overkleeft, H.S.; Van Der Marel, G.A.; Codée, J.D.C. Galacturonic Acid Lactones in the Synthesis of All Trisaccharide Repeating Units of the Zwitterionic Polysaccharide Sp1. J. Org. Chem. 2011, 76, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Schumann, B.; Pragani, R.; Anish, C.; Pereira, C.L.; Seeberger, P.H. Synthesis of Conjugation-Ready Zwitterionic Oligosaccharides by Chemoselective Thioglycoside Activation. Chem. Sci. 2014, 5, 1992–2002. [Google Scholar] [CrossRef]
- Schumann, B.; Reppe, K.; Kaplonek, P.; Wahlbrink, A.; Anish, C.; Witzenrath, M.; Pereira, C.L.; Seeberger, P.H. Development of an Efficacious, Semisynthetic Glycoconjugate Vaccine Candidate against Streptococcus pneumoniae Serotype 1. ACS Cent. Sci. 2018, 4, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Behr, T.; Hartmann, R.; Peter-Katalinic, J.; Egge, H. Teichoic Acid and Lipoteichoic Acid of Streptococcus Pneumoniae Possess Identical Chain Structures: A Reinvestigation of Teichoid Acid (C Polysaccharide). Eur. J. Biochem. 1993, 215, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.W.; Fischer, W.; Joiner, K.A. Influence of Lipoteichoic Acid Structure on Recognition by the Macrophage Scavenger Receptor. Infect. Immun. 1996, 64, 3318–3325. [Google Scholar] [PubMed]
- Fischer, W. Pneumococcal Lipoteichoic and Teichoic Acid. Microb. Drug Resist. 1997, 3, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.M.; Figueroa-Perez, I.; Lindner, B.; Ulmer, A.J.; Zähringer, U.; Schmidt, R.R. Total Synthesis of Lipoteichoic Acid of Streptococcus Pneumoniae. Angew. Chem. Int. Ed. 2010, 49, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.M.; Figueroa-Perez, I.; Boruwa, J.; Lindner, B.; Ulmer, A.J.; Zähringer, U.; Schmidt, R.R. Synthesis of the Core Structure of the Lipoteichoic Acid of Streptococcus Pneumoniae. Chem. Eur. J. 2010, 16, 12627–12641. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.E.; Lindberg, B.; Lindquist, U. Structural Studies of the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1981, 95, 73–80. [Google Scholar] [CrossRef]
- Jones, C.; Currie, F. The Pneumococcal Polysaccharide S4: A Structural Re-Assessment. Carbohydr. Res. 1988, 184, 279–284. [Google Scholar] [CrossRef]
- Higginbotham, J.D.; Heidelberger, M. The Specific Capsular Polysaccharide of Pneumococcus Type IV. Carbohydr. Res. 1972, 23, 165–173. [Google Scholar] [CrossRef]
- Jow, Y.L.; Heidelberger, M. Note Linkage of Pyruvyl Groups in the Specific Capsular Poiysaccharide of Pneumococcus Type IV. Carbohydr. Res. 1976, 52, 255–258. [Google Scholar]
- Jones, C. A Novel Method for the Determination of the Stereochemistry of Pyruvate Acetal Substituents Applied to the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1990, 198, 353–357. [Google Scholar] [CrossRef]
- Jones, C.; Currie, F.; Forster, M.J. Nmr and Conformational Analysis of the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1991, 221, 95–121. [Google Scholar] [CrossRef]
- Horito, S.; Lorentzen, J.P.; Paulsen, H. Bausteine von Oligosacchariden, LXXVII. Synthese Einer Trisaccharideinheit Des Kapselpolysaccharides VonStreptococcus Pneumoniae Typ 4. Liebigs Ann. Chem. 1986, 1986, 1880–1890. [Google Scholar] [CrossRef]
- Pereira, C.L.; Geissner, A.; Anish, C.; Seeberger, P.H. Chemical Synthesis Elucidates the Immunological Importance of a Pyruvate Modification in the Capsular Polysaccharide of Streptococcus Pneumoniae Serotype 4. Angew. Chem. Int. Ed. 2015, 54, 10016–10019. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.; Henrichsen, J. Laboratory Diagnosis, Serology and Epidemiology of Streptococcus Pneumoniae. Methods Microbiol. 1978, 12, 241–262. [Google Scholar]
- Robbins, J.B.; Austrian, R.; Lee, C.-J.; Rastogi, S.C.; Schiffman, G.; Henrichsen, J.; Makela, P.H.; Broome, C.V.; Facklam, R.R.; Tiesjema, R.H.; et al. Considerations for Formulating the Second-Generation Pneumococcal Capsular Polysaccharide Vaccine with Emphasis on the Cross-Reactive Types within Groups. J. Infect. Dis. 1983, 148, 1136–1159. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, M.; Avery, O.T. The Soluble Specific Subtance of Pneumococcus. J. Exp. Med. 1923, 38, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Pereira, C.L.; Govindan, S. Total Synthesis of a Streptococcus Pneumoniae Serotype 12F CPS Repeating Unit Hexasaccharide. Beilstein J. Org. Chem. 2017, 13, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, M.P.; Khan, N.; Martin, C.; Xu, F.-F.; Reppe, K.; Geissner, A.; Govindan, S.; Witzenrath, M.; Pereira, C.L.; Seeberger, P.H. Semisynthetic Glycoconjugate Vaccine Candidate against Streptococcus Pneumoniae Serotype 5. Proc. Natl. Acad. Sci. USA 2017, 114, 11063–11068. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.L.; Deloria-Knoll, M.; Levine, O.S.; Stoszek, S.K.; Hance, L.F.; Reithinger, R.; Muenz, L.R.; O’Brien, K.L. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children under Five: The Pneumococcal Global Serotype Project. PLoS Med. 2010, 7, e1000348. [Google Scholar] [CrossRef] [PubMed]
- How, M.J.; Brimacombe, J.S.; Stacey, M. The Pneumococcal Polysaccharides. Adv. Carbohydr. Chem. 1964, 19, 303–358. [Google Scholar] [PubMed]
- Niyogi, S.K. Shigellosis. J. Microbiol. 2005, 43, 133–143. [Google Scholar] [PubMed]
- Barry, E.M.; Pasetti, M.F.; Sztein, M.B.; Fasano, A.; Kotloff, K.L.; Levine, M.M. Progress and Pitfalls in Shigella Vaccine Research. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kenne, L.; Lindberg, B.; Petersson, K.; Katzenellenbogen, E.; Romanowska, E. Structural Studies of the O-Specific Side-Chains of the Shigella Sonnei Phase I Lipopolysaccharide. Carbohydr. Res. 1980, 78, 119–126. [Google Scholar] [CrossRef]
- Pfister, H.B.; Mulard, L.A. Synthesis of the Zwitterionic Repeating Unit of the O-Antigen from Shigella Sonnei and Chain Elongation at Both Ends. Org. Lett. 2014, 16, 4892–4895. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, O.G.; Kocharova, N.A.; Bialczak-Kokot, M.; Shashkov, A.S.; Rozalski, A.; Knirel, Y.A. Structure of the O-Polysaccharide of Providencia Alcalifaciens O22 Containing d-Glyceramide 2-Phosphate. Eur. J. Org. Chem. 2012, 2012, 3500–3506. [Google Scholar] [CrossRef]
- Yoh, M.; Matsuyama, J.; Ohnishi, M.; Takagi, K.; Miyagi, H.; Mori, K.; Park, K.S.; Ono, T.; Honda, T. Importance of Providencia Species as a Major Cause of Travellers’ Diarrhoea. J. Med. Microbiol. 2005, 54, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Odoyo, E.; Larson, P.S.; Apondi, E.; Kathiiko, C.; Miringu, G.; Nakashima, M.; Ichinose, Y. First Report of a Foodborne Providencia Alcalifaciens Outbreak in Kenya. Am. J. Trop. Med. Hyg. 2015, 93, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Dieli, M.; Brucato, A.; Pedrotti, P.; Brambilla, P.; Curri, S.F.; Senni, M.; Pericotti, S.; Suter, F.; Ferrazzi, P. Images in Cardiovascular Medicine. Bacterial Pericarditis Due to Providencia Stuartii: An Atypical Case of Relapsing Pericarditis. Circulation 2010, 122, e401–e403. [Google Scholar] [CrossRef] [PubMed]
- Krake, P.R.; Tandon, N. Infective Endocarditis Due to Providenca Stuartii. South. Med. J. 2004, 97, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, O.R.; Bardak-Ozcem, S.; Ozgiray, E.; Aydemir, S.; Yurtseven, T.; Yamazhan, T.; Tasbakan, M.; Ulusoy, S. Meningitis Due to Providencia Stuartii. J. Clin. Microbiol. 2010, 48, 4667–4668. [Google Scholar] [CrossRef] [PubMed]
- Koreishi, A.F.; Schechter, B.A.; Karp, C.L. Ocular Infections Caused by Providencia Rettgeri. Ophthalmology 2006, 113, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.M.; Brenner, F.W.; Miller, J.M. Classification, Identification, and Clinical Significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000, 13, 534–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podilapu, A.R.; Kulkarni, S.S. Total Synthesis of Repeating Unit of O-Polysaccharide of Providencia Alcalifaciens O22 via One-Pot Glycosylation. Org. Lett. 2017, 19, 5466–5469. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.; Lee, J.C. Staphylococcus Aureus Capsular Polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Daum, R.S.; Spellberg, B. Progress toward a Staphylococcus Aureus Vaccine. Clin. Infect. Dis. 2012, 54, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, A.; Cervera, C.; Moreno, A.; Moreillon, P.; Miró, J.M. Patients at Risk of Complications of Staphylococcus Aureus Bloodstream Infection. Clin. Infect. Dis. 2009, 48, S246–S253. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Revised Structures for the Capsular Polysaccharides from Staphylococcus Aureus Types 5 and 8, Components of Novel Glycoconjugate Vaccines. Carbohydr. Res. 2005, 340, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A.I.; Schneerson, R.; Szu, S.C.; Vann, W.F.; Shiloach, J.; Karakawa, W.W.; Robbins, J.B. Synthesis and Immunologic Properties in Mice of Vaccines Composed of Staphylococcus aureus Type 5 and Type 8 Capsular Polysaccharides Conjugated to Pseudomonas aeruginosa Exotoxin A. Infect. Immun. 1990, 58, 2367–2374. [Google Scholar] [PubMed]
- Fattom, A.I.; Sarwar, J.; Ortiz, A.; Naso, R. A Staphylococcus Aureus Capsular Polysaccharide (CP) Vaccine and CP-Specific Antibodies Protect Mice against Bacterial Challenge. Infect. Immun. 1996, 64, 1659–1665. [Google Scholar] [PubMed]
- Danieli, E.; Proietti, D.; Brogioni, G.; Romano, M.R.; Cappelletti, E.; Tontini, M.; Berti, F.; Lay, L.; Costantino, P.; Adamo, R. Synthesis of Staphylococcus Aureus Type 5 Capsular Polysaccharide Repeating Unit Using Novel L-FucNAc and D-FucNAc Synthons and Immunochemical Evaluation. Bioorg. Med. Chem. 2012, 20, 6403–6415. [Google Scholar] [CrossRef] [PubMed]
- Gagarinov, I.A.; Fang, T.; Liu, L.; Srivastava, A.D.; Boons, G.J. Synthesis of Staphylococcus Aureus Type 5 Trisaccharide Repeating Unit: Solving the Problem of Lactamization. Org. Lett. 2015, 17, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Yasomanee, J.P.; Visansirikul, S.; Pornsuriyasak, P.; Thompson, M.; Kolodziej, S.A.; Demchenko, A.V. Synthesis of the Repeating Unit of Capsular Polysaccharide Staphylococcus Aureus Type 5 to Study Chemical Activation and Conjugation of Native CP5. J. Org. Chem. 2016, 81, 5981–5987. [Google Scholar] [CrossRef] [PubMed]
- Hagen, B.; Ali, S.; Overkleeft, H.S.; Van der Marel, G.A.; Codée, J.D.C. Mapping the Reactivity and Selectivity of 2-Azidofucosyl Donors for the Assembly of N-Acetylfucosamine-Containing Bacterial Oligosaccharides. J. Org. Chem. 2017, 82, 848–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Taylor, M.S. Borinic Acid-Catalyzed Regioselective Acylation of Carbohydrate Derivatives. J. Am. Chem. Soc. 2011, 133, 3724–3727. [Google Scholar] [CrossRef] [PubMed]
- Visansirikul, S.; Yasomanee, J.P.; Pornsuriyasak, P.; Kamat, M.N.; Podvalnyy, N.M.; Gobble, C.P.; Thompson, M.; Kolodziej, S.A.; Demchenko, A.V. A Concise Synthesis of the Repeating Unit of Capsular Polysaccharide Staphylococcus Aureus Type 8. Org. Lett. 2015, 17, 2382–2384. [Google Scholar] [CrossRef] [PubMed]
- Ala’Aldeen, D.A.A.; Hiramatsu, K. Staphylococcus Aureus: Molecular and Clinical Aspects; Elsevier: New York, NY, USA, 2004. [Google Scholar]
- Hagen, B.; Van Dijk, J.H.M.; Zhang, Q.; Overkleeft, H.S.; Van Der Marel, G.A.; Codée, J.D.C. Synthesis of the Staphylococcus Aureus Strain M Capsular Polysaccharide Repeating Unit. Org. Lett. 2017, 19, 2514–2517. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Melly, M.A.; Harris, T.M.; Hellerqvist, C.G.; Hash, J.H. The Repeating Sequence of the Capsular Polysaccharide of Staphylococcus Aureus M. Carbohydr. Res. 1983, 117, 113–123. [Google Scholar] [CrossRef]
- WHO Fact Sheets: Meningococcal Meningitis. Available online: http://www.who.int/mediacentre/factsheets/fs141/en/index.html (accessed on 9 August 2018).
- Stimson, E.; Virji, M.; Makepeace, K.; Dell, A.; Morris, H.R.; Payne, G.; Saunders, J.R.; Jennings, M.P.; Barker, S.; Panico, M.; et al. Meningococcal pilin: A glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 1995, 17, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Expeditious Synthesis of Bacterial, Rare Sugar Building Blocks to Access the Prokaryotic Glycome. Org. Biomol. Chem. 2013, 11, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Synthesis of Orthogonally Protected Bacterial, Rare-Sugar and d-Glycosamine Building Blocks. Nat. Protoc. 2013, 8, 1870–1889. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Total Synthesis of the Bacillosamine Containing α-l-Serine Linked Trisaccharide of Neisseria Meningitidis. Carbohydr. Res. 2014, 399, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Candela, T.; Maes, E.; Garénaux, E.; Rombouts, Y.; Krzewinski, F.; Gohar, M.; Guérardel, Y. Environmental and Biofilm-Dependent Changes in a Bacillus Cereus Secondary Cell Wall Polysaccharide. J. Biol. Chem. 2011, 286, 31250–31262. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Available online: http://www.phac-aspc.gc.ca/labbio/res/psds-ftss/bacillus-cereus-eng.php (accessed on 9 August 2018).
- Logan, N.A.; Rodrigez-Diaz, M. Bacillus Spp. and Related Genera. In Principles and Practice of Clinical Bacteriology, 2nd ed.; Gillespie, S.H., Hawkey, P.M., Eds.; John Wiley and Sons Ltd.: West Sussex, UK, 2006; pp. 139–158. [Google Scholar]
- Rosovitz, M.J.; Voskuil, M.I.; Chambliss, G.H. Bacillus. In Topley & Wilson’s Microbiology and Microbial Infection: Systematic Bacteriology, 9th ed.; Collier, L., Balows, A., Sussman, M., Balows, A., Duerden, B.I., Eds.; Hodder Education Publishers: London, UK, 1998; pp. 709–729. [Google Scholar]
- Drobniewski, F.A. Bacillus Cereus and Related Species. Am. Soc. Microbiol. 1993, 6, 324–338. [Google Scholar] [CrossRef]
- Pinna, A.; Sechi, L.A.; Zanetti, S.; Usai, D.; Delogu, G.; Cappuccinelli, P.; Carta, F. Bacillus Cereus Keratitis Associated with Contact Lens Wear. Ophthalmology 2001, 108, 1830–1834. [Google Scholar] [CrossRef]
- Cowan, C.L.; Madden, W.M.; Hatem, G.F.; Merritt, J.C. Endogenous Bacillus Cereus Panophthalmitis. Ann. Ophthalmol. 1987, 19, 65–68. [Google Scholar] [PubMed]
- O’Day, D.M.; Smith, R.S.; Gregg, C.R.; Turnbull, P.C.B.; Head, W.S.; Ives, J.A.; Ho, P.C. The Problem of Bacillus Species Infection with Special Emphasis on the Virulence of Bacillus Cereus. Ophthalmology 1981, 88, 833–838. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Salazar, L.G.; True, L.D.; Disis, M.L. Fatal Bacillus Cereus Sepsis Following Resolving Neutropenic Enterocolitis during the Treatment of Acute Leukemia. Am. J. Hematol. 2003, 72, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Podilapu, A.R.; Kulkarni, S.S. First Synthesis of Bacillus Cereus Ch HF-PS Cell Wall Trisaccharide Repeating Unit. Org. Lett. 2014, 16, 4336–4339. [Google Scholar] [CrossRef] [PubMed]
- Skurnik, M.; Toivonen, S. Identification of Distinct Lipopolysaccharide Patterns among Yersinia Enterocolitica and Y. Enterocolitica-Like Bacteria. Biochem. 2011, 76, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Vila, J. Yersinia Enterocolitica: Pathogenesis, Virulence and Antimicrobial Resistance. Enferm. Infecc. Microbiol. Clin. 2012, 30, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Beczała, A.; Duda, K.A.; Skurnik, M.; Holst, O. The Structure of the O-Specific Polysaccharide of the Lipopolysaccharide from Yersinia Enterocolitica Serotype O:50 Strain 3229. Carbohydr. Res. 2012, 359, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sanapala, S.R.; Kulkarni, S.S. Expedient Route to Access Rare Deoxy Amino L-Sugar Building Blocks for the Assembly of Bacterial Glycoconjugates. J. Am. Chem. Soc. 2016, 138, 4938–4947. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, G.; Puopolo, G.; Carillo, S.; Zoina, A.; Lanzetta, R.; Parrilli, M.; Evidente, A.; Corsaro, M.M. Structural Characterization of the O-Chain Polysaccharide from an Environmentally Beneficial Bacterium Pseudomonas Chlororaphis Subsp. Aureofaciens Strain M71. Carbohydr. Res. 2011, 346, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Michael Janda, J.; Abbott, S.L.; McIver, C.J. Plesiomonas Shigelloides Revisited. Clin. Microbiol. Rev. 2016, 29, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Stock, I. Plesiomonas Shigelloides: An Emerging Pathogen with Unusual Properties. Rev. Med. Microbiol. 2004, 15, 129–139. [Google Scholar] [CrossRef]
- Maciejewska, A.; Lukasiewicz, J.; Niedziela, T.; Szewczuk, Z.; Lugowski, C. Structural Analysis of the O-Specific Polysaccharide Isolated from Plesiomonas Shigelloides O51 Lipopolysaccharide. Carbohydr. Res. 2009, 344, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Schumann, B.; Zou, X.; Pereira, C.L.; Tian, G.; Hu, J.; Seeberger, P.H.; Yin, J. Total Synthesis of a Densely Functionalized Plesiomonas Shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen. J. Am. Chem. Soc. 2018, 140, 3120–3127. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.J.; Imperiali, B. The Renaissance of Bacillosamine and Its Derivatives: Pathway Characterization and Implications in Pathogenicity. Biochemistry 2014, 53, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Desa, N.; Hansen, E.E.; Knirel, Y.A.; Gordon, J.I.; Gagneux, P.; Nizet, V.; Varki, A. Innovations in Host and Microbial Sialic Acid Biosynthesis Revealed by Phylogenomic Prediction of Nonulosonic Acid Structure. Proc. Natl. Acad. Sci. USA 2009, 106, 13552–13557. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramphal, R.; Sadoff, J.C.; Pyle, M.; Silipigni, J.D. Role of Pili in the Adherence of Pseudomonas Aeruginosa to Injured Tracheal Epithelium. Infect. Immun. 1984, 44, 38–40. [Google Scholar] [PubMed]
- Castric, P.; Cassels, F.J.; Carlson, R.W. Structural Characterization of the Pseudomonas Aeruginosa 1244 Pilin Glycan. J. Biol. Chem. 2001, 276, 26479–26485. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.E.; Marshall, M.A.; Blanch, V.J.; Deal, C.D.; Castric, P. Identification of the Pseudomonas Aeruginosa 1244 Pilin Glycosylation Site. Infect. Immun. 2002, 70, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Wei, R.; Andolina, G.; Li, X. Total Synthesis of Pseudomonas Aeruginosa 1244 Pilin Glycan via de Novo Synthesis of Pseudaminic Acid. J. Am. Chem. Soc. 2017, 139, 13420–13428. [Google Scholar] [CrossRef] [PubMed]
- Gouliaras, C.; Lee, D.; Chan, L.; Taylor, M.S. Regioselective Activation of Glycosyl Acceptors by a Diarylborinic Acid-Derived Catalyst. J. Am. Chem. Soc. 2011, 133, 13926–13929. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Wang, C.-C.; Sabbavarapu, N.M.; Podilapu, A.R.; Liao, P.-H.; Hung, S.-C. “One-Pot” Protection, Glycosylation, and Protection−Glycosylation Strategies of Carbohydrates. Chem. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, A.; Kulkarni, S.S. Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates. Molecules 2018, 23, 1997. https://doi.org/10.3390/molecules23081997
Behera A, Kulkarni SS. Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates. Molecules. 2018; 23(8):1997. https://doi.org/10.3390/molecules23081997
Chicago/Turabian StyleBehera, Archanamayee, and Suvarn S. Kulkarni. 2018. "Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates" Molecules 23, no. 8: 1997. https://doi.org/10.3390/molecules23081997
APA StyleBehera, A., & Kulkarni, S. S. (2018). Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates. Molecules, 23(8), 1997. https://doi.org/10.3390/molecules23081997





