Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals
Abstract
1. Introduction
2. Materials and Methods
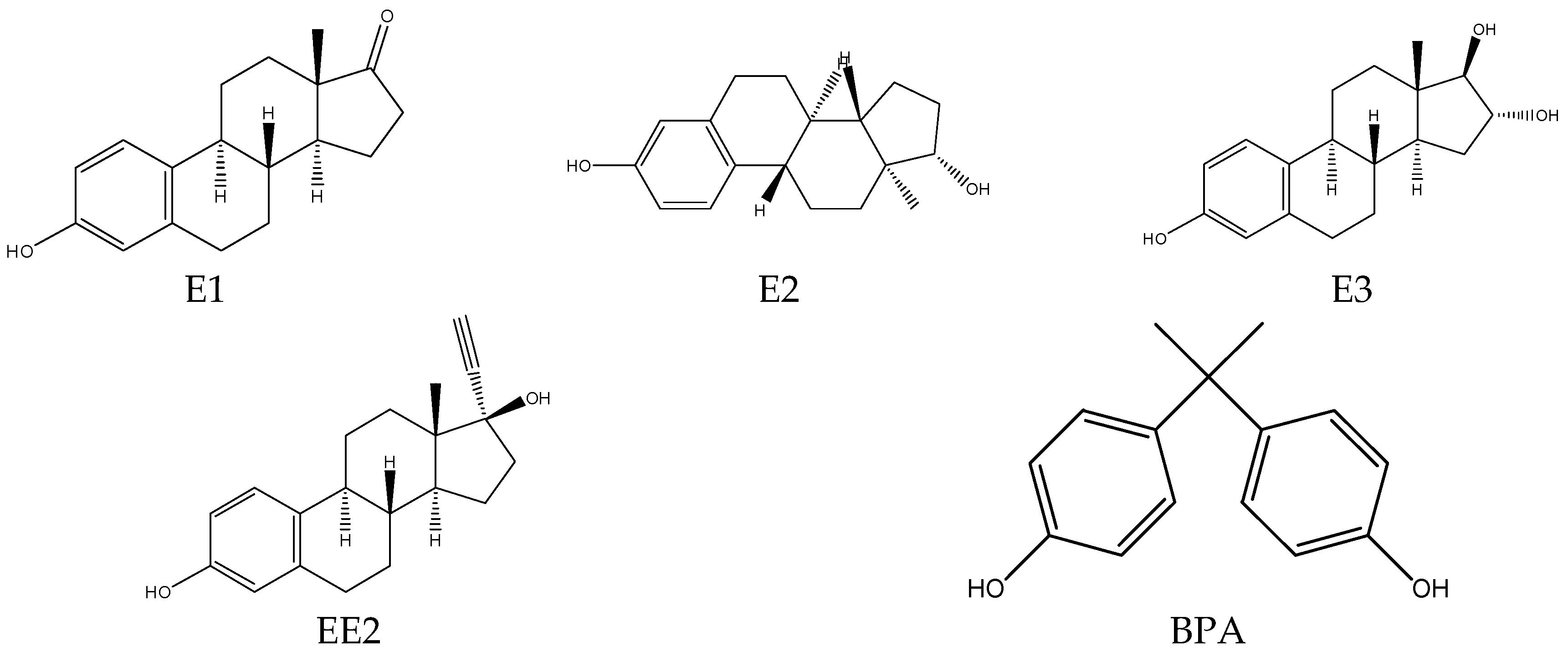
2.1. Chemicals and Reagents
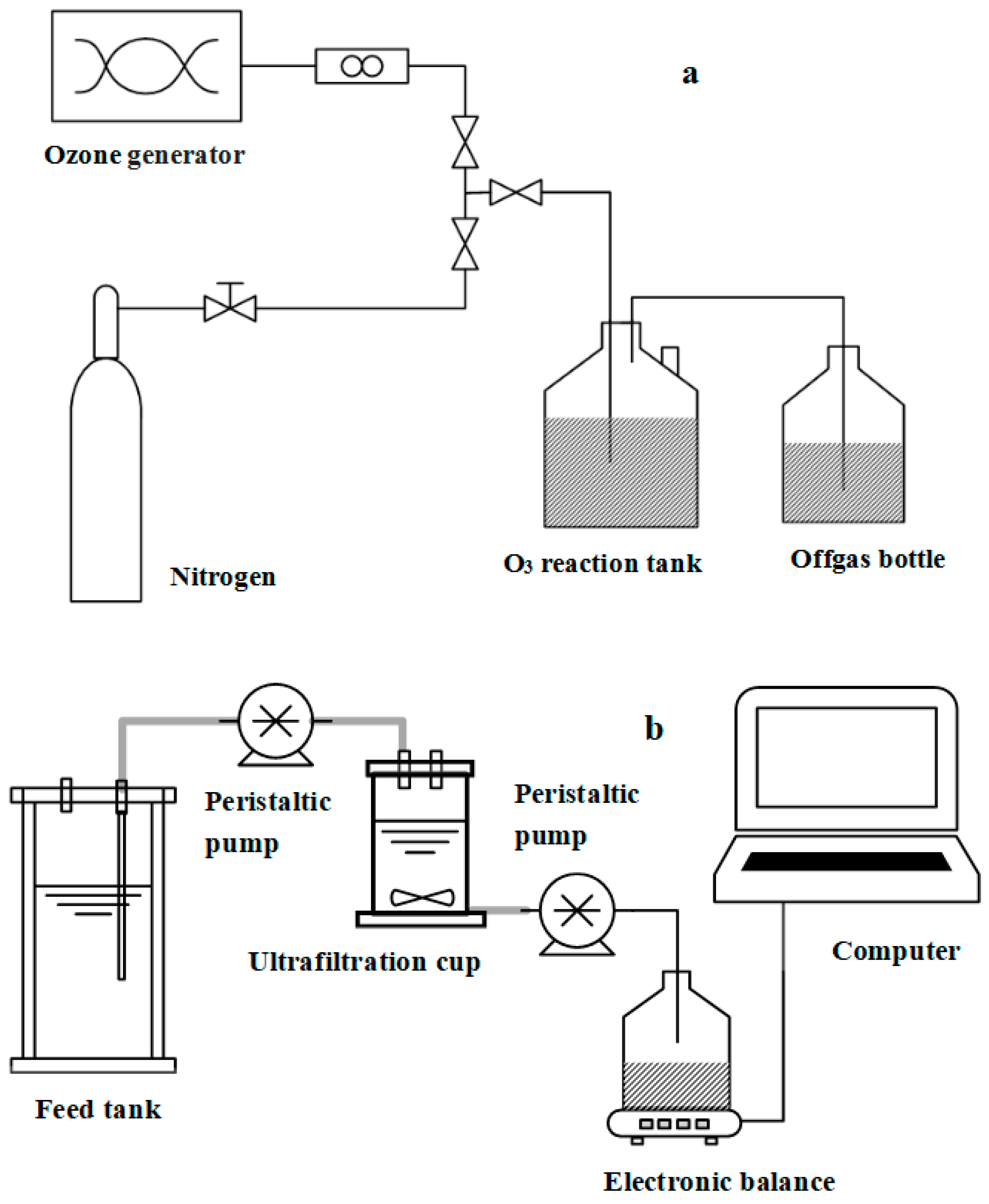
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
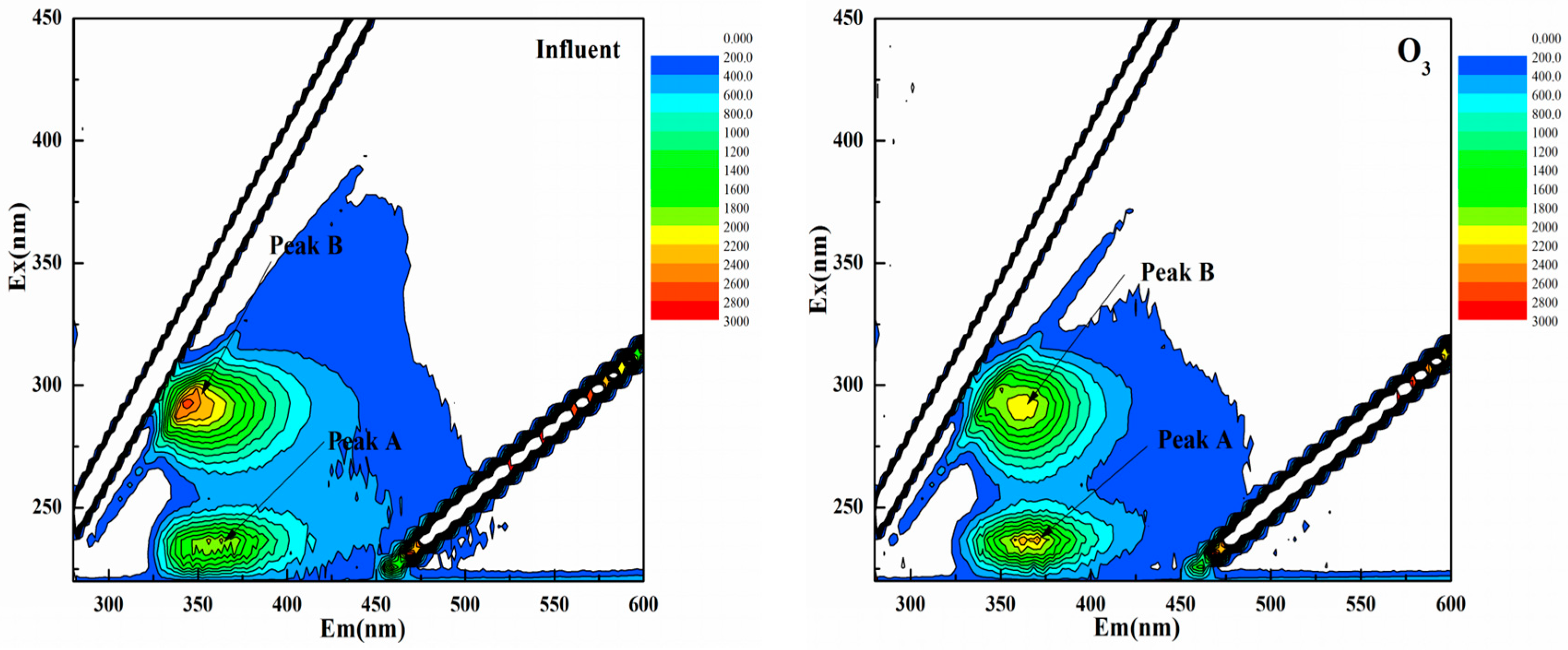
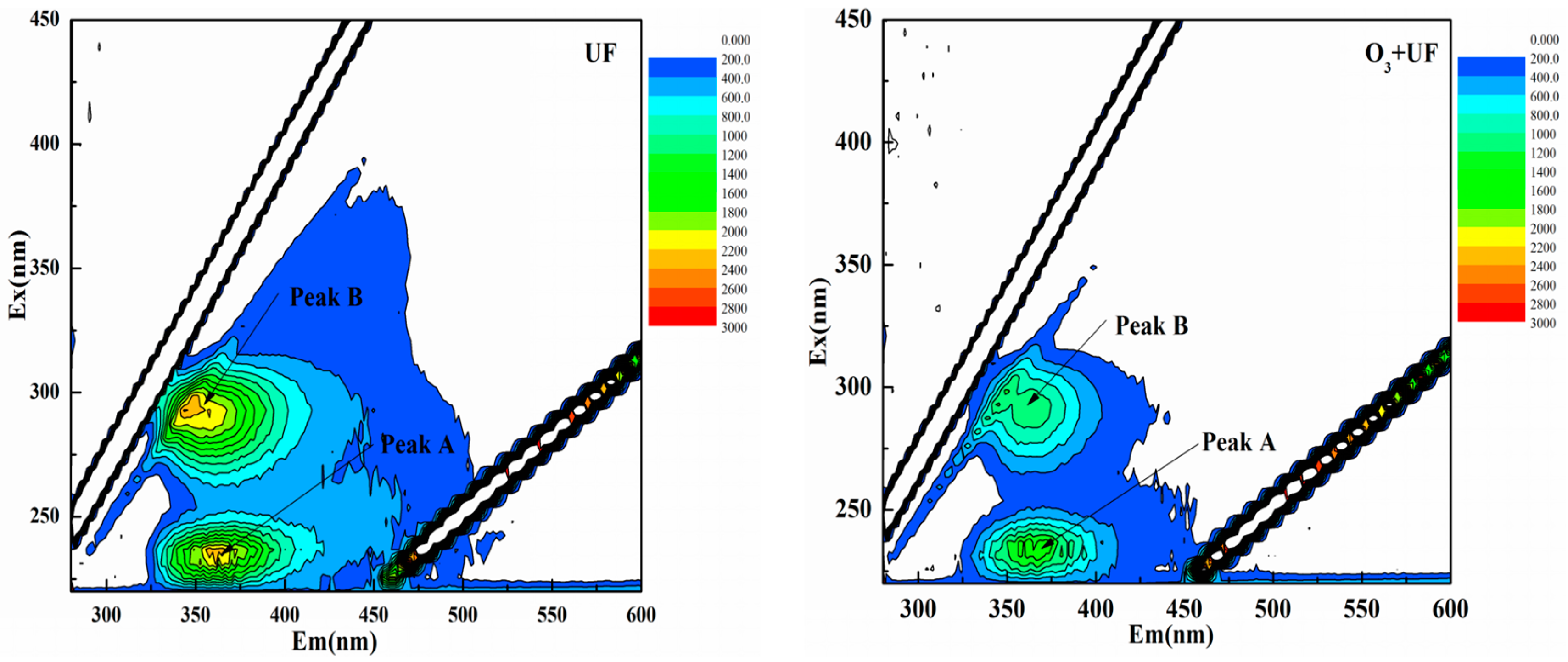
3.1. Effects of UF, O3, and Their Combination on Reduction of Fluorescence Intensity
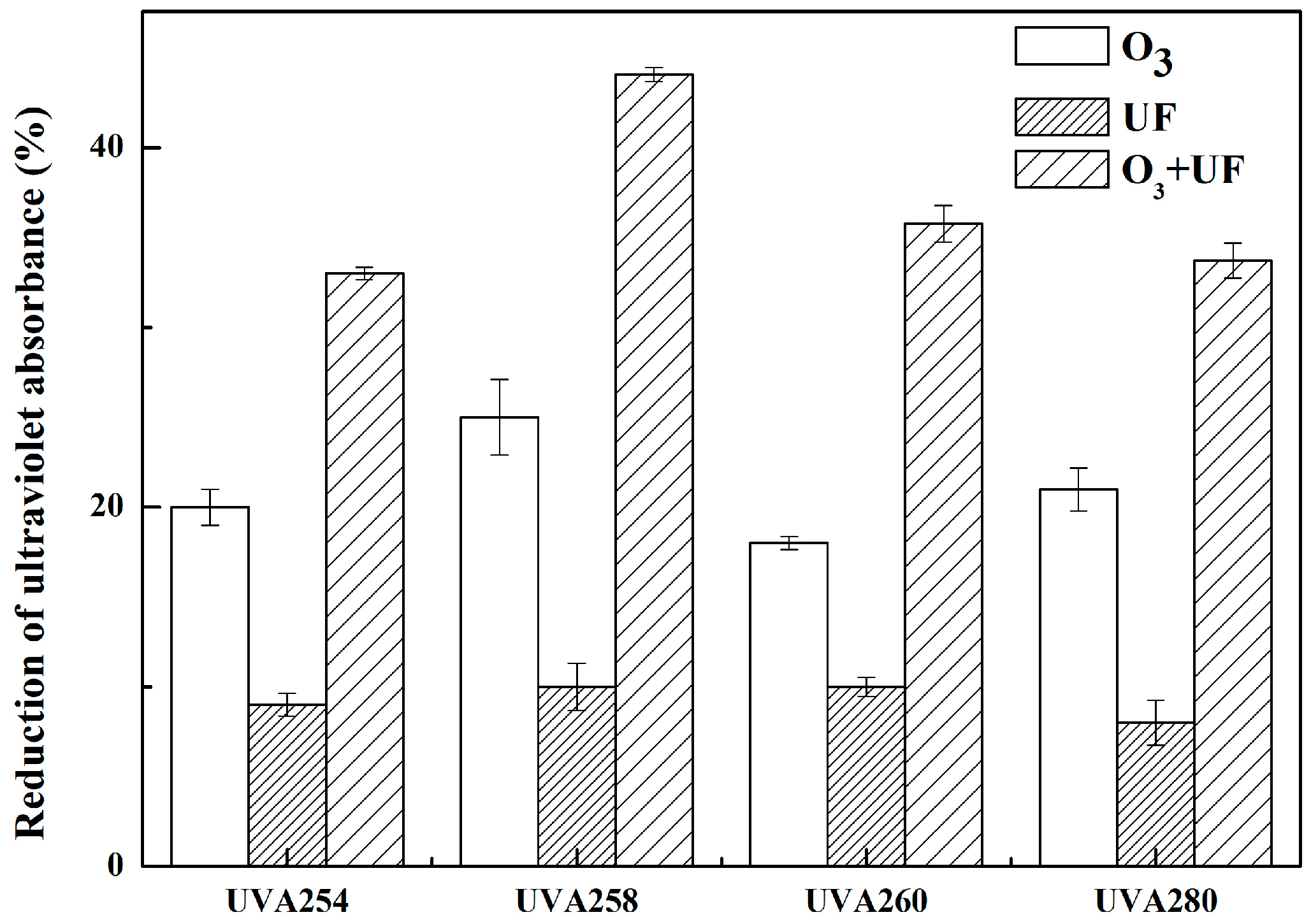
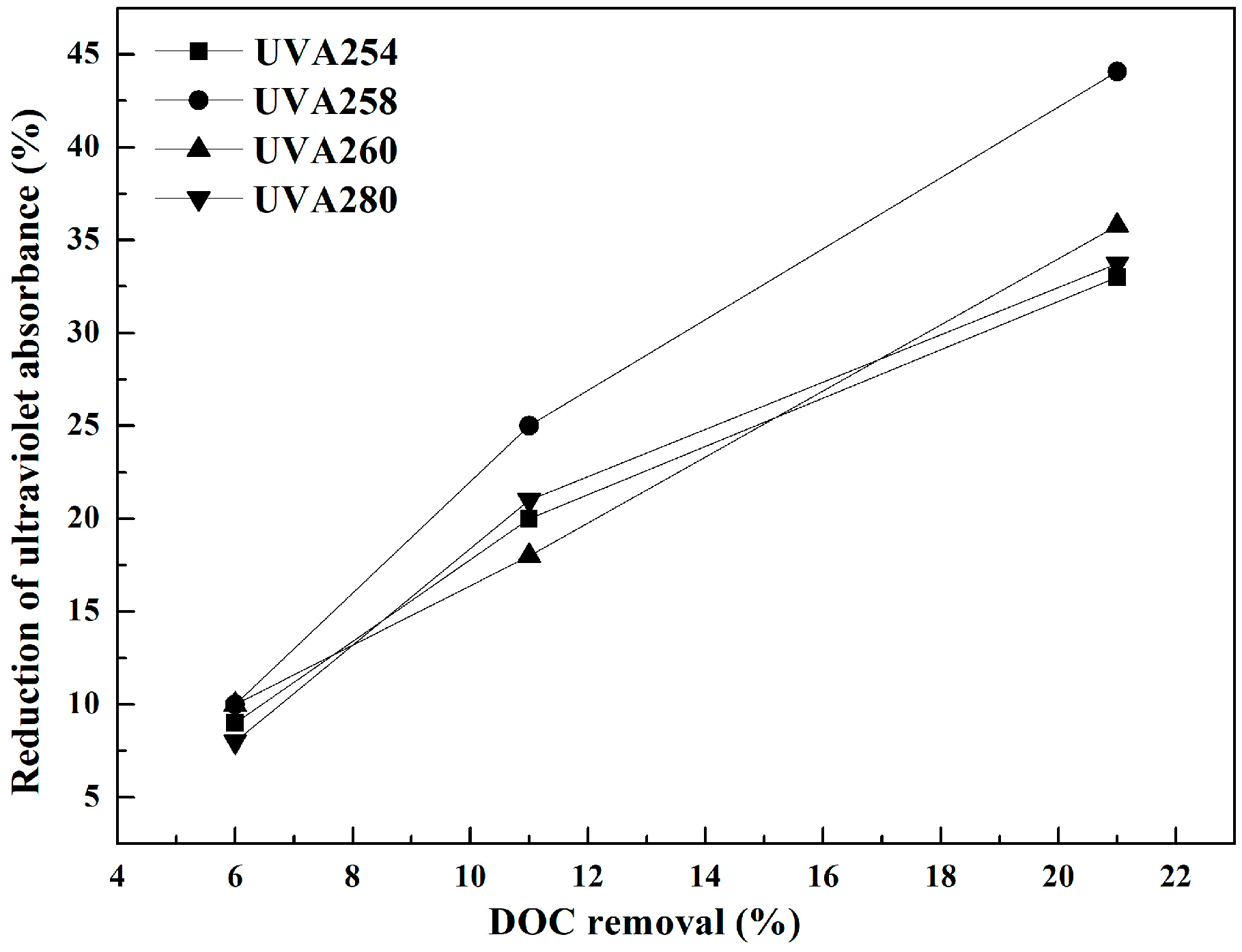
3.2. Effects of UF, O3, and Their Combination on Reduction of Ultraviolet Absorbance (UVA)
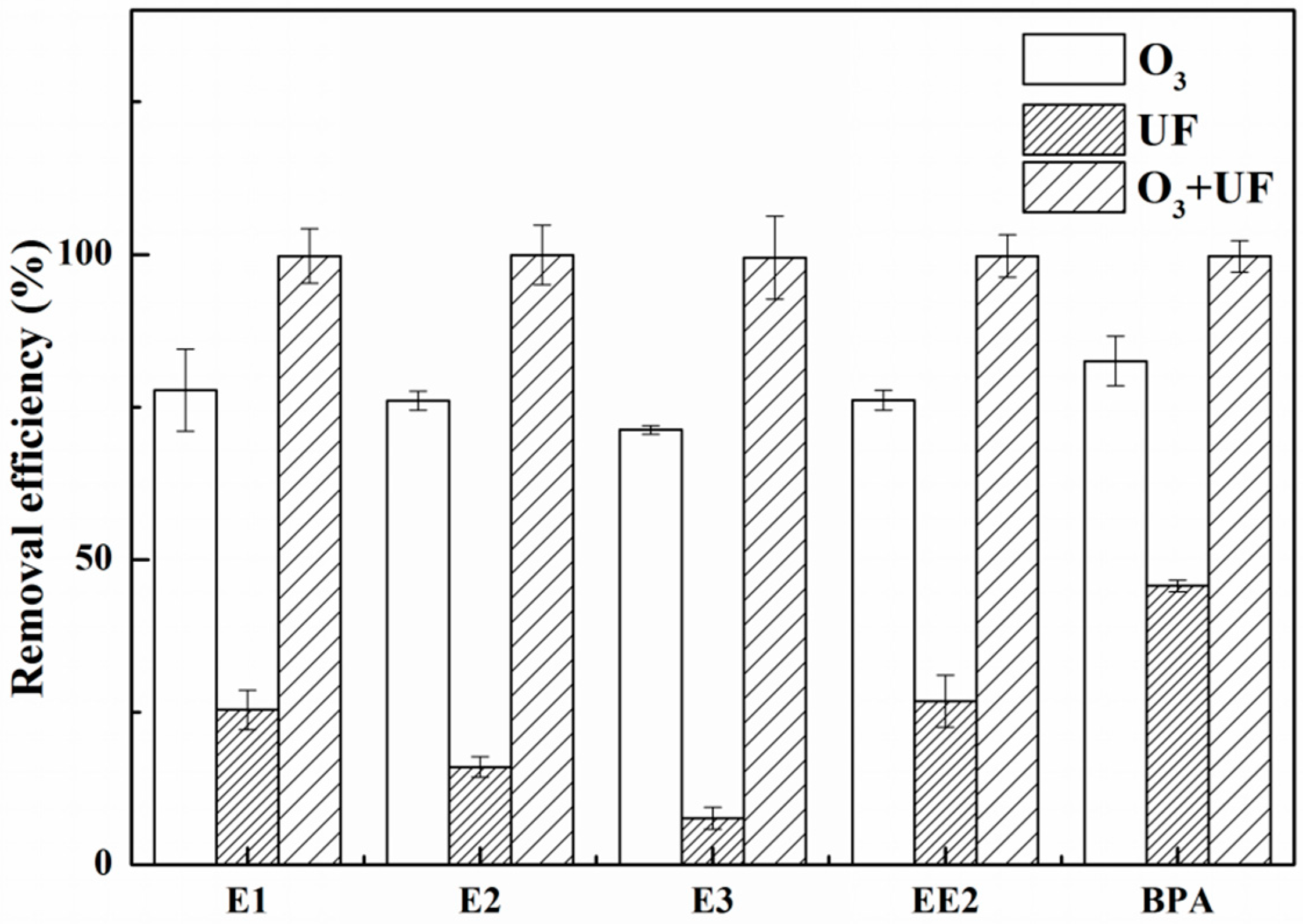
3.3. Effects of UF, O3, and Their Combination on EDC Removal
3.4. Effects of UF, O3, and Their Combination on Estrogenicity Removal
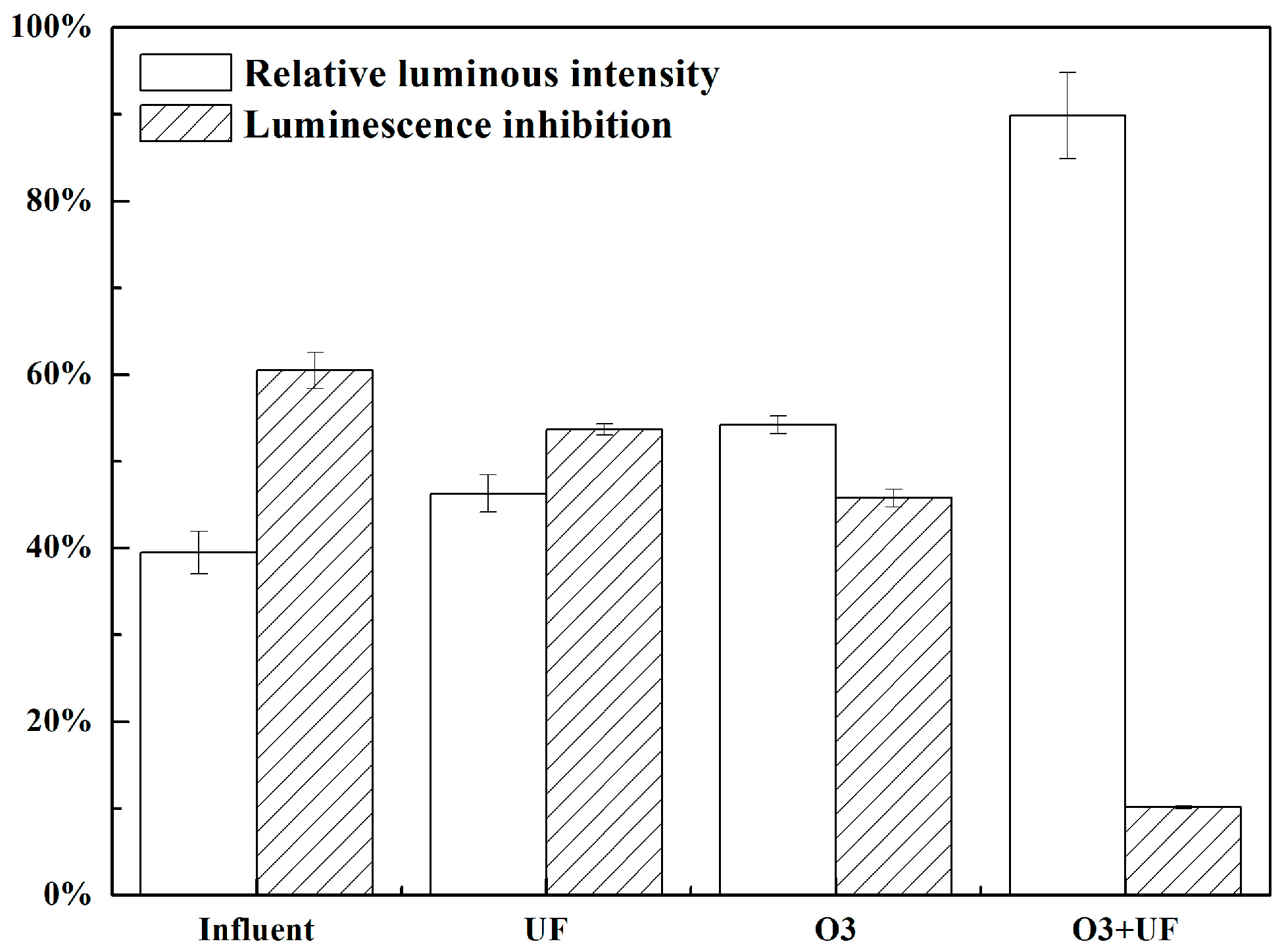
3.5. Effects of UF, O3, and Their Combination on Acute Ecotoxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, V.K.; Ali, I.; Saleh, T.A. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Lakretz, A.; Mamane, H.; Cikurel, H. The role of soil aquifer treatment (SAT) for effective removal of organic matter, trace organic compounds and microorganisms from secondary effluents pre-treated by ozone. Ozone-Sci. Eng. 2017, 39, 385–394. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhang, S.H.; Ji, G.X. Occurrence, distribution and risk assessment of suspected endocrine-disrupting chemicals in surface water and suspended particulate matter of Yangtze River (Nanjing section). Ecotoxicol. Environ. Saf. 2017, 135, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Padhye, L.P.; Yao, H.; Kung’u, F.T. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014, 51, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wen, Y.; Wang, Y. Environmental risk assessment of the emerging EDCs contaminants from rural soil and aqueous sources: Analytical and modelling approaches. Chemosphere 2018, 198, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Prusinski, L.; Yang, Q.; Mas, A. Early-life exposure to endocrine-disrupting chemicals (EDCs) leads to the development ofuterine fibroids by impairing DNA repair capacity in myometrial stem cells. Fertil. Steril. 2016, 106, 282. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Boateng, L.K.; Flora, J.R.V. Removal of bisphenol A and 17α-ethinyl estradiol by combined coagulation and adsorption using carbon nanomaterials and powdered activated carbon. Sep. Purif. Technol. 2013, 107, 37–47. [Google Scholar] [CrossRef]
- Ben Fredj, S.; Novakoski, R.T.; Tizaoui, C. Two-phase ozonation for the removal of estrone, 17β-estradiol and 17α-ethinylestradiol in water using ozone-loaded decamethylcyclopentasiloxane. Ozone-Sci. Eng. 2017, 39, 343–356. [Google Scholar] [CrossRef]
- Basile, T.; Petrella, A.; Petrella, M. Review of endocrine-disrupting-compound removal technologies in water and wastewater treatment plants: An EU perspective. Ind. Eng. Chem. Res. 2011, 50, 8389–8401. [Google Scholar] [CrossRef]
- Jin, P.K.; Jin, X.; Wang, X.C.C.; Shi, X.B. An analysis of the chemical safety of secondary effluent for reuse purposes and the requirement for advanced treatment. Chemosphere 2013, 91, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent organic matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Zhang, H.; Yamada, H.; Tsuno, H. Removal of endocrine-disrupting chemicals during ozonation of municipal sewage with brominated byproducts control. Environ. Sci. Technol. 2008, 42, 3375–3380. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Graham, N.J.D.; Fowler, G.D. Coagulation and oxidation for controlling ultrafiltration membrane fouling in drinking water treatment: Application of ozone at low dose in submerged membrane tank. Water Res. 2016, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Pan, Y.W.; Chen, S.S. Removal of natural organic matter (NOM) using ozonation and ultrafiltration. Water Sci. 2001, 1, 49–54. [Google Scholar] [CrossRef]
- Chang, H.S.; Choo, K.H.; Lee, B. The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J. Hazard. Mater. 2009, 172, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Rosario-Ortiz, F.L.; Snyder, S.A. Using ultraviolet absorbance and color to assess pharmaceutical oxidation during ozonation of wastewater. Environ. Sci. Technol. 2009, 43, 4858–4863. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Metcalfe, T.L.; Kiparissis, Y. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2001, 20, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, B.V.; Pesonen, M.; Escher, B.I. Comparative analysis of estrogenic activity in sewage treatment plant effluents involving three in vitro assays and chemical analysis of steroids. Environ. Toxicol. Chem. 2004, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wieczerzak, M.; Yotova, G. Environmental risk assessment of Polish wastewater treatment plant activity. Chemosphere 2016, 160, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.; Roccaro, P.; Korshin, G.V. Monitoring the behavior of emerging contaminants in wastewater-impacted rivers based on the use of fluorescence excitation emission matrixes (EEM). Environ. Sci. Technol. 2017, 51, 4306–4316. [Google Scholar] [CrossRef] [PubMed]
- Peleato, N.M.; Legge, R.L.; Andrews, R.C. Neural networks for dimensionality reduction of fluorescence spectra and prediction of drinking water disinfection by-products. Water Res. 2018, 136, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Wang, Z.; He, S. Multifunctional single-drug loaded nanoparticles for enhanced cancer treatment with low toxicity in vivo. RSC Adv. 2016, 6, 20366–20373. [Google Scholar] [CrossRef]
- Meng, F.; Drews, A.; Mehrez, R.; Iversen, V.; Ernst, M.; Yang, F.; Jekel, M.; Kraume, M. Occurrence, source, and fate of dissolved organic matter (DOM) in a pilot-scale membrane bioreactor. Environ. Sci. Technol. 2009, 43, 8821–8826. [Google Scholar] [CrossRef] [PubMed]
- Van Geluwe, S.; Vinckier, C.; Braeken, L. Ozone oxidation of nanofiltration concentrates alleviates membrane fouling in drinking water industry. J. Membrane Sci. 2011, 378, 128–137. [Google Scholar] [CrossRef]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Hu, Z.; Ding, D. Effects of effluent organic matters on endocrine disrupting chemical removal by ultrafiltration and ozonation in synthetic secondary effluent. J. Environ. Sci. 2018. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Petrella, A.; Mascolo, G.; Murgolo, S.; Petruzzelli, V.; Ranieri, E.; Spasiano, D.; Petruzzelli, D. Photocatalytic oxidation of organic micro-pollutants: Pilot plant investigation and mechanistic aspects of the degradation reaction. Chem. Eng. Commun. 2016, 203, 1298–1307. [Google Scholar] [CrossRef]
- Maniero, M.G.; Bila, D.M.; Dezotti, M. Degradation and estrogenic activity removal of 17β-estradiol and 17α-ethinylestradiol by ozonation and O3/H2O2. Sci. Total Environ. 2008, 407, 105–115. [Google Scholar] [PubMed]
- Akbari, S.; Ghanbari, F.; Moradi, M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: Applying low current density for oxidation mechanism. Chem. Eng. J. 2016, 294, 298–307. [Google Scholar] [CrossRef]
- Carlson, J.C.; Stefan, M.I.; Parnis, J.M. Direct UV photolysis of selected pharmaceuticals, personal care products and endocrine disruptors in aqueous solution. Water Res. 2015, 84, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Bertanza, G.; Papa, M.; Pedrazzani, R. EDCs, estrogenicity and genotoxicity reduction in a mixed (domestic+ textile) secondary effluent by means of ozonation: A full-scale experience. Sci. Total Environ. 2013, 458, 160–168. [Google Scholar] [CrossRef] [PubMed]




| Sample Name | Unit | Amount |
|---|---|---|
| TOC | mg∙L−1 | 6.47 |
| conductivity | μs/cm | 1001 |
| pH | - | 7.8 |
| SS | mg∙L−1 | 10.0 |
| TP | mg∙L−1 | 0.32 |
| TN | mg∙L−1 | 23.05 |
| UVA254 | - | 0.084 |
| specific ultraviolet absorbance | L∙mg−1∙m−1 | 1.3 |
| Ca2+ | mg∙L−1 | 66.94 |
| Mg2+ | mg∙L−1 | 9.35 |
| K+ | mg∙L−1 | 20.00 |
| Na+ | mg∙L−1 | 75.51 |
| E1 | ng∙L−1 | 42.04 |
| E2 | ng∙L−1 | 157.73 |
| E3 | ng∙L−1 | 2.67 |
| EE2 | ng∙L−1 | 138.34 |
| BPA | ng∙L−1 | 34.85 |
| Technique | Component | Conditions |
|---|---|---|
| UPLC analysis conditions | Chromatographic column (100 mm × 2.1 mm I. D. × 1.7 μm) | 90% A 0 min→64% A 0.5 min→maintained to 6 min→10% A 7 min→0% 10 min→returned to the initial state for 10.1 min→maintained to 12 min |
| Temperature of columns | 50 °C | |
| Injection volume | 10 µL | |
| Flow rate of mobile phase | 0.3 mL∙min−1 | |
| MS analysis conditions | Ionization mode | ESI- |
| Temperature of ion source | 120 °C |
| Peak | Ex/Em | Influent FL (A.U.) | O3 FL (A.U.) | UF FL (A.U.) | O3 + UF FL (A.U.) |
|---|---|---|---|---|---|
| Peak A | 230/350 | 1854 | 1535 | 1883 | 1183 |
| 230/400 | 2406 | 1921 | 1880 | 1450 | |
| 235/390 | 1098 | 995 | 992 | 680 | |
| 240/350 | 776 | 716 | 724 | 467 | |
| 225–250/325/400 | 1533 | 1291 | 1369 | 945 | |
| Peak B | 280/350 | 1642 | 1489 | 1578 | 892 |
| 275/400 | 2074 | 2094 | 2056 | 1000 | |
| 280/400 | 2060 | 1971 | 2049 | 1095 | |
| 285/376 | 1469 | 1320 | 1410 | 801 | |
| 275–300/325–400 | 1811 | 1718 | 1773 | 947 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, X.; Hu, Z.; Huang, S. Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals. Appl. Sci. 2018, 8, 1240. https://doi.org/10.3390/app8081240
Si X, Hu Z, Huang S. Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals. Applied Sciences. 2018; 8(8):1240. https://doi.org/10.3390/app8081240
Chicago/Turabian StyleSi, Xiurong, Zunfang Hu, and Shiyuan Huang. 2018. "Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals" Applied Sciences 8, no. 8: 1240. https://doi.org/10.3390/app8081240
APA StyleSi, X., Hu, Z., & Huang, S. (2018). Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals. Applied Sciences, 8(8), 1240. https://doi.org/10.3390/app8081240




