Distinct Clusters of Testosterone Levels, Symptoms, and Serum Trace Elements in Young Men: A Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Cross-Sectional Study
2.1.2. Inclusion and Exclusion Criteria
2.1.3. Outcomes
2.2. Assessment of Serum Testosterone and Testosterone-Related Hormone Levels
2.3. Analytical Approach to Testosterone Levels
2.4. Symptom Evaluation
2.5. Measurement of Serum Trace Element Concentrations
2.6. Semen Analysis and Other Secondary Outcome Measures
2.7. Statistical Analysis
2.7.1. Preparation of Variables
2.7.2. Dimensionality Reduction and Clustering
2.7.3. Age Adjustment and Comparison of Serum Trace Element Concentrations
2.7.4. Multivariate Regression Analyses
2.7.5. Software and Statistical Significance
3. Results
3.1. Patient Characteristics and Correlations Among Explanatory Variables
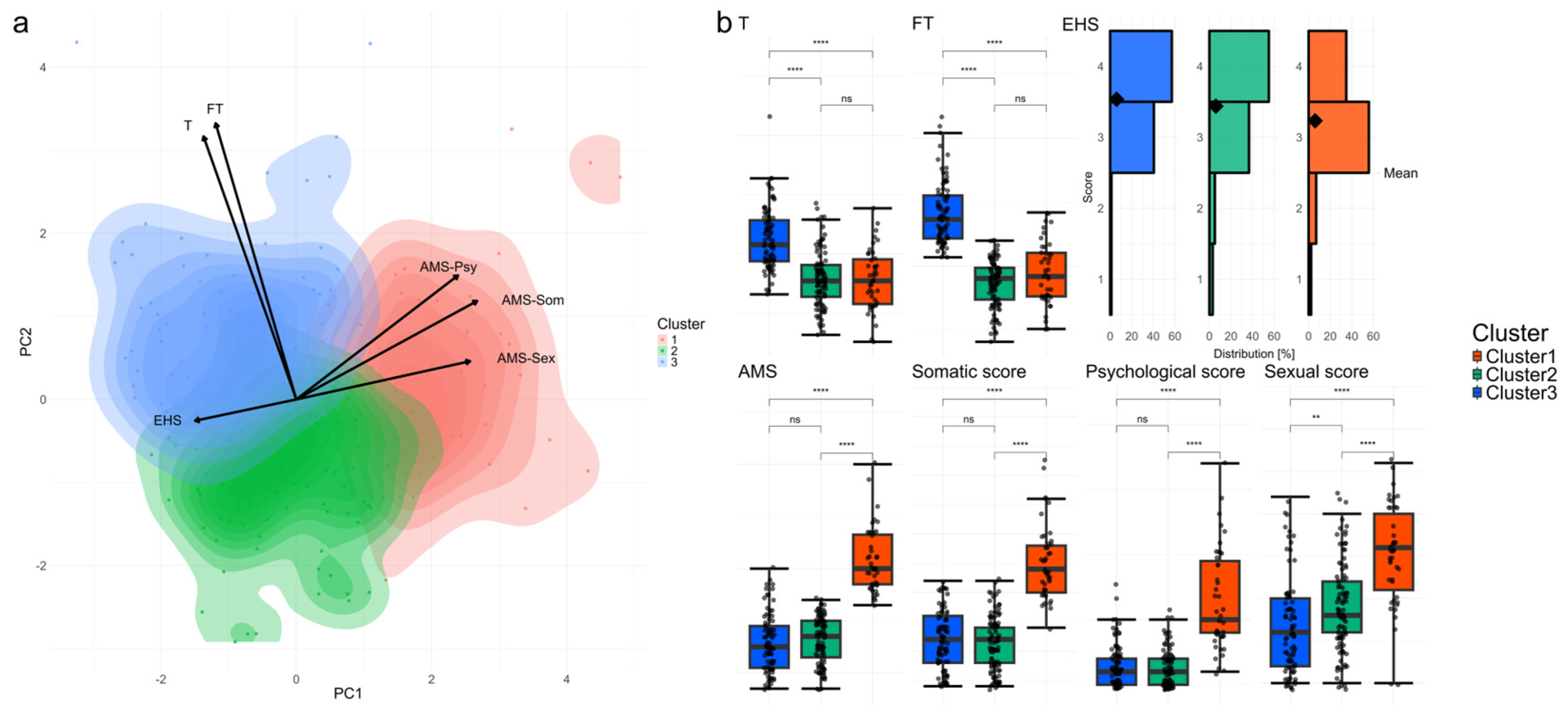
3.2. Dimensionality Reduction, Clustering, and Baseline Characteristics of Explanatory Variables and SDS
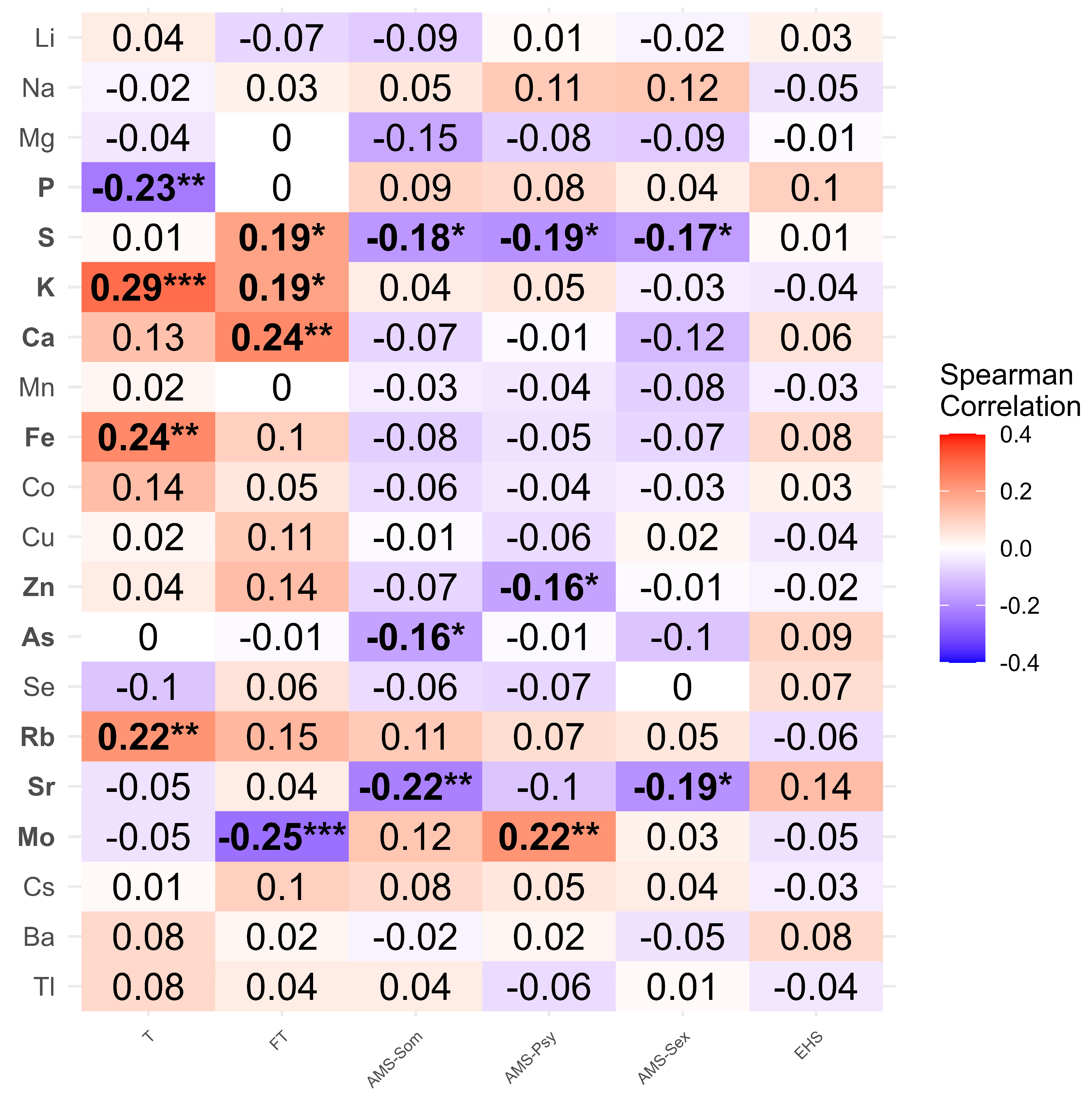
3.3. Identification of Trace Elements Associated with Cluster Classification
4. Discussion
4.1. Overview of Clusters and Key Findings
4.2. Trace Elements Associated with Testosterone Dynamics
4.2.1. Mo, Sr, and Other Negative Contributors
4.2.2. Fe, Zn, and Other Positive or Ambiguous Elements
4.3. Clinical and Sociocultural Factors Affecting AMS, EHS, and Semen Parameters
4.3.1. Semen Parameters and AMS
4.3.2. EHS Prevalence and Sociocultural Influences
4.3.3. Symptom Disclosure and Ejaculatory Dysfunction
4.4. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMS | Aging Males’ Symptoms |
| EHS | Erection Hardness Score |
| PCA | Principal component analysis |
| SDS | Self-Rating Depression Scale |
| WQS | Weighted quantile sum |
| WHO | World Health Organization |
References
- Hutmacher, F. Putting Stress in Historical Context: Why It Is Important That Being Stressed Out Was Not a Way to Be a Person 2,000 Years Ago. Front. Psychol. 2021, 12, 539799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, D.G. Changing Men’s Health: Leading the Future. World J. Mens Health 2018, 36, 1–3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whittaker, J. Dietary trends and the decline in male reproductive health. Hormones 2023, 22, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Vandello, J.A.; Bosson, J.K.; Caswell, T.A.; Cummings, J.R. Healthful eating as a manhood threat. J. Men’s Health 2024, 20, 42–56. [Google Scholar] [CrossRef]
- Hehemann, M.C.; Kashanian, J.A. Can lifestyle modification affect men’s erectile function? Transl. Androl. Urol. 2016, 5, 187–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.; Luke, N. Men’s Economic Dependency, Gender Ideology, and Stress at Midlife. J. Marriage Fam. 2020, 82, 1026–1040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuo, W.C.; Oakley, L.D.; Brown, R.L.; Hagen, E.W.; Barnet, J.H.; Peppard, P.E.; Bratzke, L.C. Gender Differences in the Relationship Between Financial Stress and Metabolic Abnormalities. Nurs. Res. 2021, 70, 123–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weissman, J.D.; Russell, D.; Taylor, J. The Relationship Between Financial Stressors, Chronic Pain, and High-Impact Chronic Pain: Findings From the 2019 National Health Interview Survey. Public Health Rep. 2023, 138, 438–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Courtenay, W.H. Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Soc. Sci. Med. 2000, 50, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Seidler, Z.E.; Dawes, A.J.; Rice, S.M.; Oliffe, J.L.; Dhillon, H.M. The role of masculinity in men’s help-seeking for depression: A systematic review. Clin. Psychol. Rev. 2016, 49, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Frech, A.; Damaske, S. Men’s Income Trajectories and Physical and Mental Health at Midlife. AJS 2019, 124, 1372–1412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viner, R.; Christie, D. Fatigue and somatic symptoms. BMJ 2005, 330, 1012–1015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hidaka, B.H. Depression as a disease of modernity: Explanations for increasing prevalence. J. Affect. Disord. 2012, 140, 205–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, B.Y.; Wilson, G.; Berger, J.; Christman, M.; Reina, B.; Bishop, F.; Klam, W.P.; Doan, A.P. Is Internet Pornography Causing Sexual Dysfunctions? A Review with Clinical Reports. Behav. Sci. 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, H.M.T.; Gabrielson, A.T.; Hellstrom, W.J.G. Erectile Dysfunction in Young Men—A Review of the Prevalence and Risk Factors. Sex. Med. Rev. 2017, 5, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, S.D.; Patel, P.; Fantus, R.J.; Halpern, J.; Chang, C.; Kargi, A.Y.; Ramasamy, R. Decline in Serum Testosterone Levels Among Adolescent and Young Adult Men in the USA. Eur. Urol. Focus 2021, 7, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zueger, R.; Annen, H.; Ehlert, U. Testosterone and cortisol responses to acute and prolonged stress during officer training school. Stress 2023, 26, 2199886. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Sullivan, C.T.; McCoy, S.C.; Yarrow, J.F.; Morrow, M.; Borst, S.E. Review of health risks of low testosterone and testosterone administration. World J. Clin. Cases 2015, 3, 338–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nieschlag, E. Late-onset hypogonadism: A concept comes of age. Andrology 2020, 8, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Eugster, E.A. Etiology and treatment of hypogonadism in adolescents. Pediatr. Clin. N. Am. 2011, 58, 1181–1200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, J.; Nassau, D.E.; Patel, P.; Ramasamy, R. Low Testosterone in Adolescents & Young Adults. Front. Endocrinol. 2019, 10, 916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwamoto, T.; Yanase, T.; Koh, E.; Horie, H.; Baba, K.; Namiki, M.; Nawata, H. Reference ranges of total serum and free testosterone in Japanese male adults. Nihon Hinyokika Gakkai Zasshi 2004, 95, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.D.; Belakovskiy, A.; McGrath, R.; Yarrow, J.F. Testosterone Deficiency, Weakness, and Multimorbidity in Men. Sci. Rep. 2018, 8, 5897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, A.; Andino, J.; Daignault-Newton, S.; Chopra, Z.; Sarma, A.; Dupree, J.M. What Is a Normal Testosterone Level for Young Men? Rethinking the 300 ng/dL Cutoff for Testosterone Deficiency in Men 20–44 Years Old. J. Urol. 2022, 208, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Esche, G.R.; Kupelian, V.; O’Donnell, A.B.; Travison, T.G.; Williams, R.E.; Clark, R.V.; McKinlay, J.B. Prevalence of symptomatic androgen deficiency in men. J. Clin. Endocrinol. Metab. 2007, 92, 4241–4247. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kato-Negishi, M.; Tanaka, K.I. Dietary Trace Elements and the Pathogenesis of Neurodegenerative Diseases. Nutrients 2023, 15, 2067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baj, J.; Bargiel, J.; Cabaj, J.; Skierkowski, B.; Hunek, G.; Portincasa, P.; Flieger, J.; Smolen, A. Trace Elements Levels in Major Depressive Disorder—Evaluation of Potential Threats and Possible Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 15071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rotter, I.; Wiatrak, A.; Ryl, A.; Kotfis, K.; Ciosek, Z.; Laszczynska, M.; Sipak-Szmigiel, O.; Szylinska, A. The Relationship between Selected Bioelements and Depressiveness Associated with Testosterone Deficiency Syndrome in Aging Men. Medicina 2020, 56, 125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, R.; Wunsch, D.C., 2nd. Clustering algorithms in biomedical research: A review. IEEE Rev. Biomed. Eng. 2010, 3, 120–154. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kojo, K.; Nagumo, Y.; Ikeda, A.; Shimizu, T.; Fujimoto, S.; Kakinuma, T.; Uchida, M.; Kimura, T.; Kandori, S.; et al. A new clustering model based on the seminal plasma/serum ratios of multiple trace element concentrations in male patients with subfertility. Reprod. Med. Biol. 2024, 23, e12584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kusakabe, M.; Sato, M.; Nakamura, Y.; Mikami, H.; Lin, J.; Nagase, H. Elemental analysis by Metallobalance provides a complementary support layer over existing blood biochemistry panel-based cancer risk assessment. PeerJ 2021, 9, e12247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, L.; Shen, S.; Zhang, Z.; Song, X.; Jiang, Q. Study on the relationship between age and the concentrations of heavy metal elements in human bone. Ann. Transl. Med. 2018, 6, 320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baudry, J.; Kopp, J.F.; Boeing, H.; Kipp, A.P.; Schwerdtle, T.; Schulze, M.B. Changes of trace element status during aging: Results of the EPIC-Potsdam cohort study. Eur. J. Nutr. 2020, 59, 3045–3058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, Y.; Tanda, H.; Kato, S.; Onishi, S.; Nakajima, H.; Nanbu, A.; Nitta, T.; Koroku, M.; Akagashi, K.; Hanzawa, T.; et al. Serum testosterone levels using the radioimmunoassay method in healthy Japanese male volunteers. Reprod. Med. Biol. 2006, 5, 37–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brambilla, D.J.; Matsumoto, A.M.; Araujo, A.B.; McKinlay, J.B. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J. Clin. Endocrinol. Metab. 2009, 94, 907–913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamaguchi, K.; Ishikawa, T.; Chiba, K.; Fujisawa, M. Assessment of possible effects for testosterone replacement therapy in men with symptomatic late-onset hypogonadism. Andrologia 2011, 43, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.L.; Bhasin, S.; Wu, F.C.W.; Krishna, M.; Matsumoto, A.M.; Jasuja, R. A Reappraisal of Testosterone’s Binding in Circulation: Physiological and Clinical Implications. Endocr. Rev. 2017, 38, 302–324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kacker, R.; Hornstein, A.; Morgentaler, A. Free testosterone by direct and calculated measurement versus equilibrium dialysis in a clinical population. Aging Male 2013, 16, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Ando, F.; Shimokata, H. Serum total and free testosterone level of Japanese men: A population-based study. Int. J. Urol. 2005, 12, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Jockenhovel, F. Late-onset hypogonadism in the aging male (LOH): Definition, diagnostic and clinical aspects. J. Endocrinol. Investig. 2005, 28, 23–27. [Google Scholar] [PubMed]
- Ide, H.; Akehi, Y.; Fukuhara, S.; Ohira, S.; Ogawa, S.; Kataoka, T.; Kumagai, H.; Kobayashi, K.; Komiya, A.; Shigehara, K.; et al. Summary of the clinical practice manual for late-onset hypogonadism. Int. J. Urol. 2023, 30, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.A.; Saad, F.; Zimmermann, T.; Novak, A.; Myon, E.; Badia, X.; Potthoff, P.; T’Sjoen, G.; Pollanen, P.; Goncharow, N.P.; et al. The Aging Males’ Symptoms (AMS) scale: Update and compilation of international versions. Health Qual. Life Outcomes 2003, 1, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, K.; Hashimoto, K.; Kato, R.; Tanaka, T.; Hirose, T.; Masumori, N.; Itoh, N.; Mori, M.; Tsukamoto, T. The aging males’ symptoms scale for Japanese men: Reliability and applicability of the Japanese version. Int. J. Impot. Res. 2008, 20, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, J.P.; Goldstein, I.; Bushmakin, A.G.; Cappelleri, J.C.; Hvidsten, K. Validation of the erection hardness score. J. Sex. Med. 2007, 4, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Shimura, S.; Tai, T.; Kobayashi, H.; Baba, S.; Kano, M.; Nagao, K. A web-based survey of erection hardness score and its relationship to aging, sexual behavior, confidence, and risk factors in Japan. Sex. Med. 2013, 1, 76–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukuda, K.; Kobayashi, S. A study on a self-rating depression scale (author’s transl). Seishin Shinkeigaku Zasshi 1973, 75, 673–679. [Google Scholar] [PubMed]
- Kitamura, T.; Hirano, H.; Chen, Z.; Hirata, M. Factor structure of the Zung Self-rating Depression Scale in first-year university students in Japan. Psychiatry Res. 2004, 128, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Mikami, H.; Nakamura, Y.; Kusakabe, M.; Yamamoto, N.; Takiguchi, N.; Nabeya, Y.; Soda, H.; Fukusawa, S.; Kishida, T.; et al. A Nobel Multivariate Index for Cancer Risk Detection Based On the Serum Trace Elements: Metallo-Balance Method. J. Cancer Epidemol. Prev. 2020, 5, 1–9. [Google Scholar]
- Okamoto, N.; Shimizu, T.; Fujimoto, S.; Kojo, K.; Ikeda, A.; Miyagi, Y.; Mikami, H.; Nakamura, Y.; Nagase, H. Dai 4 setsu: Ketchū biryō genso nōdo no ICP-MS sokutei ni yoru hyōjunchi settei to “ganrisuku” sukurīninghō, Metaro-Baransu kensa, e no ōyō. [Clause 4: Setting of the Reference Values by ICP-MS Measurement of Serum Trace Element Concentrations and Application to “Cancer Risk” Screening Method: Metallo-Balance Test]. In Seimei Kinzoku Dainamikusu: Seitainai ni Okeru Kinzoku no Kyodō to Seigyo [Bio-Metal Dynamics: Behavior and Control of Metals in Living Systems]; Shiro, H., Tsumoto, K., Furukawa, Y., Kambe, T., Eds.; N. T. S. & Co., Ltd.: Tokyo, Japan, 2021; pp. 490–500. ISBN 9784860437060. [Google Scholar]
- Kojo, K.; Oguri, T.; Tanaka, T.; Ikeda, A.; Shimizu, T.; Fujimoto, S.; Nakazono, A.; Nagumo, Y.; Kandori, S.; Negoro, H.; et al. Inductively coupled plasma mass spectrometry performance for the measurement of key serum minerals: A comparative study with standard quantification methods. J. Clin. Lab. Anal. 2025, 39, e25140. [Google Scholar] [CrossRef] [PubMed]
- Takihara, H.; Sakatoku, J.; Fujii, M.; Nasu, T.; Cosentino, M.J.; Cockett, A.T. Significance of testicular size measurement in andrology. I. A new orchiometer and its clinical application. Fertil. Steril. 1983, 39, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mbizvo, M.; Festin, M.P.; Bjorndahl, L.; Toskin, I.; other Editorial Board Members of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Evolution of the WHO “Semen” processing manual from the first (1980) to the sixth edition (2021). Fertil. Steril. 2022, 117, 237–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, Y.; Zhang, L.; Liu, Y.; Shuai, P.; Li, Y.; Li, J.; Zhao, Y.; Wu, Y.; Zhang, D.; Xiang, Q. Serum trace elements show association with thyroperoxidase autoantibodies in Thyroid Imaging Reporting and Data System (TI-RADS) 4 nodules. Sci. Rep. 2024, 14, 19813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ljubicic, M.L.; Madsen, A.; Juul, A.; Almstrup, K.; Johannsen, T.H. The Application of Principal Component Analysis on Clinical and Biochemical Parameters Exemplified in Children with Congenital Adrenal Hyperplasia. Front. Endocrinol. 2021, 12, 652888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, S.; Dutta, S.; Kakar, R.S. Machine learning for lumbar and pelvis kinematics clustering. Comput. Methods Biomech. Biomed. Eng. 2024, 27, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Agodi, A. The Application of Clustering on Principal Components for Nutritional Epidemiology: A Workflow to Derive Dietary Patterns. Nutrients 2022, 15, 195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramezani-Tehrani, F.; Rahmani, K.; Moradi, A.; Montazeri, S.A.; Bidhendi-Yarandi, R.; Darabi, F. Comparison of Two Statistical Methods to Determine Normal Range of Androgen Hormones: K-Means Cluster Analysis and Receiver Operating Characteristic Curve. J. Fam. Reprod. Health 2018, 12, 96–102. [Google Scholar] [PubMed] [PubMed Central]
- Lossow, K.; Kopp, J.F.; Schwarz, M.; Finke, H.; Winkelbeiner, N.; Renko, K.; Meci, X.; Ott, C.; Alker, W.; Hackler, J.; et al. Aging affects sex- and organ-specific trace element profiles in mice. Aging 2020, 12, 13762–13790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abreu, M.N.; Siqueira, A.L.; Cardoso, C.S.; Caiaffa, W.T. Ordinal logistic regression models: Application in quality of life studies. Cad. Saude Publica 2008, 24 (Suppl. S4), s581–s591. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, S.; Gennings, C.; Calza, S. A weighted quantile sum regression with penalized weights and two indices. Front. Public Health 2023, 11, 1151821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, T.; Ji, D.; Su, X.; Zhou, X.; Wang, X.; He, S.; Jiang, T.; Yue, X.; Zhang, H.; Zhang, Y.; et al. Using Bayesian and weighted regression to evaluate the association of idiopathic oligoastenoteratozoospermia with seminal plasma metal mixtures. Chemosphere 2024, 351, 141202. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Artino, A.R., Jr. Analyzing and interpreting data from likert-type scales. J. Grad. Med. Educ. 2013, 5, 541–542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Padmanahban, V.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Environmental exposure to metals and male reproductive hormones: Circulating testosterone is inversely associated with blood molybdenum. Fertil. Steril. 2010, 93, 130–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewis, R.C.; Meeker, J.D. Biomarkers of exposure to molybdenum and other metals in relation to testosterone among men from the United States National Health and Nutrition Examination Survey 2011–2012. Fertil. Steril. 2015, 103, 172–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.X.; Sun, Y.; Huang, Z.; Wang, P.; Feng, W.; Li, J.; Yang, P.; Wang, M.; Sun, L.; Chen, Y.J.; et al. Associations of urinary metal levels with serum hormones, spermatozoa apoptosis and sperm DNA damage in a Chinese population. Environ. Int. 2016, 94, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhou, B.; Feng, W.; Wang, Y.X.; Liu, A.L.; Yue, J.; Li, Y.F.; Lu, W.Q. Associations of urinary metal concentrations and circulating testosterone in Chinese men. Reprod. Toxicol. 2013, 41, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Rotter, I.; Kosik-Bogacka, D.I.; Dolegowska, B.; Safranow, K.; Kuczynska, M.; Laszczynska, M. Analysis of the relationship between the blood concentration of several metals, macro- and micronutrients and endocrine disorders associated with male aging. Environ. Geochem. Health 2016, 38, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Dong, W.Y.; Zhao, H.; Shi, X.H.; Zhang, Y.L. Effect of molybdenum on reproductive function of male mice treated with busulfan. Theriogenology 2019, 126, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Barrientos, G.; Toro, V.; Grijota, F.J.; Munoz, D.; Maynar, M. Correlations between Basal Trace Minerals and Hormones in Middle and Long-Distance High-Level Male Runners. Int. J. Environ. Res. Public Health 2020, 17, 9473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Momcilovic, B. A case report of acute human molybdenum toxicity from a dietary molybdenum supplement–A new member of the “Lucor metallicum” family. Arh. Hig. Rada Toksikol. 1999, 50, 289–297. [Google Scholar] [PubMed]
- Mao, Q.; Zhu, X.; Kong, Y. Sleep duration mediates the association between heavy metals and the prevalence of depression: An integrated approach from the NHANES (2005–2020). Front. Psychiatry 2024, 15, 1455896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, G.; Lyndon, B. Depression in hepatolenticular degeneration (Wilson’s disease). Aust. N. Z. J. Psychiatry 1997, 31, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Q.; Wang, N.; Li, S.; Zhang, H.; Zhu, Y.; Guo, H.; Wang, F.; He, L.; Xia, S.; et al. Serum and urinary essential trace elements in association with major depressive disorders: A case-control study. Front. Psychiatry 2023, 14, 1297411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marie, P.J. Strontium as therapy for osteoporosis. Curr. Opin. Pharmacol. 2005, 5, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Q.; Liu, H.; Heroux, P.; Zhang, Q.; Jiang, Z.Y.; Gu, A. Low Serum Testosterone Levels Are Associated with Elevated Urinary Mandelic Acid, and Strontium Levels in Adult Men According to the US 2011–2012 National Health and Nutrition Examination Survey. PLoS ONE 2015, 10, e0127451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, S.A.; Peet, M.; Ward, N.I. Blood levels of vanadium, caesium, and other elements in depressive patients. J. Affect. Disord. 1985, 9, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; Lam, L.Q.; Scarr, E.; Duce, J.A. Cortical biometals: Changed levels in suicide and with mood disorders. J. Affect. Disord. 2019, 243, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, M.D.; Lewis, R.J., Sr.; Lewis, R.A.N. Hawley’s Condensed Chemical Dictionary, 16th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 949–999. ISBN 9781118135150/9781119312468. [Google Scholar] [CrossRef]
- Wulaningsih, W.; Van Hemelrijck, M.; Michaelsson, K.; Kanarek, N.; Nelson, W.G.; Ix, J.H.; Platz, E.A.; Rohrmann, S. Association of serum inorganic phosphate with sex steroid hormones and vitamin D in a nationally representative sample of men. Andrology 2014, 2, 967–976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, W.; Kim, B.S.; Lee, J.E.; Huh, J.K.; Kim, B.J.; Sung, K.C.; Kang, J.H.; Lee, M.H.; Park, J.R.; Rhee, E.J.; et al. Serum phosphate levels and the risk of cardiovascular disease and metabolic syndrome: A double-edged sword. Diabetes Res. Clin. Pract. 2009, 83, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Choi, K.; Kim, S.K.; Lee, G.I.; Cho, I.C. Phosphorus as predictive factor for erectile dysfunction in middle aged men: A cross sectional study in Korea. Investig. Clin. Urol. 2016, 57, 442–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, R.B. Stress, inflammation, depression, and dementia associated with phosphate toxicity. Mol. Biol. Rep. 2020, 47, 9921–9929. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. Sulfur containing amino acids and human disease. Biomed. Pharmacother. 2004, 58, 47–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vermeulen, A.; Kaufman, J.M.; Giagulli, V.A. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J. Clin. Endocrinol. Metab. 1996, 81, 1821–1826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albalat, E.; Telouk, P.; Albarède, F. Sulfur Isotope Measurement in Human Serum. In Application Note; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2015; p. AN 30300. [Google Scholar]
- Albalat, E.; Telouk, P.; Balter, V.; Fujii, T.; Bondanese, V.P.; Plissonnier, M.-L.; Vlaeminck-Guillem, V.; Baccheta, J.; Thiam, N.; Miossec, P.; et al. Sulfur isotope analysis by MC-ICP-MS and application to small medical samples. J. Anal. At. Spectrom. 2016, 31, 1002–1011. [Google Scholar] [CrossRef]
- McCann, B.S.; Magee, M.S.; Broyles, F.C.; Vaughan, M.; Albers, J.J.; Knopp, R.H. Acute psychological stress and epinephrine infusion in normolipidemic and hyperlipidemic men: Effects on plasma lipid and apoprotein concentrations. Psychosom. Med. 1995, 57, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Al-Marwani, S.; Batieha, A.; Khader, Y.; El-Khateeb, M.; Jaddou, H.; Ajlouni, K. Association between albumin and depression: A population-based study. BMC Psychiatry 2023, 23, 780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barth, J.H.; Fiddy, J.B.; Payne, R.B. Adjustment of serum total calcium for albumin concentration: Effects of non-linearity and of regression differences between laboratories. Ann. Clin. Biochem. 1996, 33 Pt 1, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, J.M. Arsenic Toxicity in Male Reproduction and Development. Dev. Reprod. 2015, 19, 167–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uthus, E.O. Evidence for arsenic essentiality. Environ. Geochem. Health 1992, 14, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Madej, D. Adverse effect after cessation of rats’ unjustified iron or iron and zinc supplementation on hematological parameters but not ferritin concentration. Clin. Nutr. 2015, 34, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Madej, D.; Pietruszka, B.; Kaluza, J. The effect of iron and/or zinc diet supplementation and termination of this practice on the antioxidant status of the reproductive tissues and sperm viability in rats. J. Trace Elem. Med. Biol. 2021, 64, 126689. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Liao, Y.R.; Chang, T.C.; Liew, Y.F.; Liu, C.Y. Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats. Nutrients 2022, 14, 2063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, W.; Abou Ghayda, R.; Schmidt, P.J.; Fleming, M.D.; Bhasin, S. The role of iron in mediating testosterone’s effects on erythropoiesis in mice. FASEB J. 2020, 34, 11672–11684. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Kuhadiya, N.D.; Abuaysheh, S.; Green, K.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Effect of testosterone on hepcidin, ferroportin, ferritin and iron binding capacity in patients with hypogonadotropic hypogonadism and type 2 diabetes. Clin. Endocrinol. 2016, 85, 772–780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelhaleim, A.F.; Abdo Soliman, J.S.; Amer, A.Y.; Abdo Soliman, J.S. Association of Zinc Deficiency with Iron Deficiency Anemia and its Symptoms: Results from a Case-control Study. Cureus 2019, 11, e3811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasad, A.S.; Halsted, J.A.; Nadimi, M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am. J. Med. 1961, 31, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Folin, M.; Contiero, E.; Vaselli, G.M. Zinc content of normal human serum and its correlation with some hematic parameters. Biometals 1994, 7, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Te, L.; Liu, J.; Ma, J.; Wang, S. Correlation between serum zinc and testosterone: A systematic review. J. Trace Elem. Med. Biol. 2023, 76, 127124. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y.; Kuriyama, T. Zinc and Reproduction. J. Vitam. Soc. Jpn. 2008, 82, 539–542. [Google Scholar] [CrossRef]
- Okumus, M.; Ceceli, E.; Tuncay, F.; Kocaoglu, S.; Palulu, N.; Yorgancioglu, Z.R. The relationship between serum trace elements, vitamin B12, folic acid and clinical parameters in patients with myofascial pain syndrome. J. Back Musculoskelet. Rehabil. 2010, 23, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zheng, Y.; Shen, Z.; Li, Y.; Tian, X.; Dou, X.; Qian, J.; Shen, H. Psychological stress-induced lower serum zinc and zinc redistribution in rats. Biol. Trace Elem. Res. 2013, 155, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Swardfager, W.; Herrmann, N.; Mazereeuw, G.; Goldberger, K.; Harimoto, T.; Lanctot, K.L. Zinc in depression: A meta-analysis. Biol. Psychiatry 2013, 74, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Khedun, S.M.; Naicker, T.; Maharaj, B. Zinc, hydrochlorothiazide and sexual dysfunction. Cent. Afr. J. Med. 1995, 41, 312–315. [Google Scholar] [PubMed]
- Besong, E.E.; Akhigbe, T.M.; Ashonibare, P.J.; Oladipo, A.A.; Obimma, J.N.; Hamed, M.A.; Adeyemi, D.H.; Akhigbe, R.E. Zinc improves sexual performance and erectile function by preventing penile oxidative injury and upregulating circulating testosterone in lead-exposed rats. Redox Rep. 2023, 28, 2225675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cinar, V.; Polat, Y.; Baltaci, A.K.; Mogulkoc, R. Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol. Trace Elem. Res. 2011, 140, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.C.; Kingston, W.; Jozefowicz, R.F.; Herr, B.E.; Forbes, G.; Halliday, D. Effect of testosterone on muscle mass and muscle protein synthesis. J. Appl. Physiol. 1989, 66, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.; Corcoran, C.; Lee, K.; Burrows, B.; Hubbard, J.; Katznelson, L.; Walsh, M.; Guccione, A.; Cannan, J.; Heller, H.; et al. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J. Clin. Endocrinol. Metab. 1996, 81, 4051–4058. [Google Scholar] [CrossRef] [PubMed]
- Pedigo, N.G.; George, W.J.; Anderson, M.B. Effects of acute and chronic exposure to cobalt on male reproduction in mice. Reprod. Toxicol. 1988, 2, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yang, C.; Gu, W.; Liu, R.; Chen, D. Associations between serum copper, zinc, selenium level and sex hormones among 6–19 years old children and adolescents in NHANES 2013–2016. Front. Endocrinol. 2022, 13, 924338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banerji, T.K.; Parkening, T.A.; Collins, T.J. Lithium: Short-term and chronic effects on plasma testosterone and luteinizing hormone concentrations in mice. Life Sci. 1982, 30, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.C.; Thakur, S.S.; Chaube, S.K.; Singh, S.P. Subchronic supplementation of lithium carbonate induces reproductive system toxicity in male rat. Reprod. Toxicol. 2003, 17, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Dees, W.L.; Hiney, J.K.; Srivastava, V.K. Influences of manganese on pubertal development. J. Endocrinol. 2017, 235, R33–R42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Zhang, G.H.; Zhang, G.; Yang, H.; Ling, X.; Xi, J.; Wu, W.; Liu, W.; Zhou, Z.; Ren, J.; et al. Trajectory model to analyze the effect of multi-metal exposures on sperm parameters and sex hormones of the MARHCS cohort in China. Environ. Res. 2024, 262, 119789. [Google Scholar] [CrossRef] [PubMed]
- Vinichuk, M.; Rosen, K.; Johanson, K.J.; Dahlberg, A. Correlations between potassium, rubidium and cesium ((133)Cs and (137)Cs) in sporocarps of Suillus variegatus in a Swedish boreal forest. J. Environ. Radioact. 2011, 102, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Trussell, J.C.; Coward, R.M.; Santoro, N.; Stetter, C.; Kunselman, A.; Diamond, M.P.; Hansen, K.R.; Krawetz, S.A.; Legro, R.S.; Heisenleder, D.; et al. Association between testosterone, semen parameters, and live birth in men with unexplained infertility in an intrauterine insemination population. Fertil. Steril. 2019, 111, 1129–1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ichioka, K.; Okubo, K.; Terai, A.; Itoh, N.; Nakayama, T.; Hatayama, H.; Nishiyama, H. The Aging Male’s Symptoms (AMS) scale in Japanese men attending an infertility clinic. Hinyokika Kiyo 2006, 52, 615–618. [Google Scholar] [PubMed]
- Iwatsuki, S.; Umemoto, Y.; Takeda, T.; Nozaki, S.; Yasui, T. 208 Age, Testosterone Levels, and Hypogonadism-Related Symptoms Among Men with Azoospermia. J. Sex. Med. 2022, 19, S205–S206. [Google Scholar] [CrossRef]
- Tsujimura, A.; Fukuhara, S.; Chiba, K.; Yoshizawa, T.; Tomoe, H.; Shirai, M.; Kimura, K.; Kikuchi, E.; Maeda, E.; Sato, Y.; et al. Erectile Function and Sexual Activity Are Declining in the Younger Generation: Results from a National Survey in Japan. World J. Mens Health 2024, 43, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Yumura, Y.; Tsujimura, A.; Imamoto, T.; Umemoto, Y.; Kobayashi, H.; Shiraishi, K.; Shin, T.; Taniguchi, H.; Chiba, K.; Miyagawa, Y.; et al. Nationwide survey of urological specialists regarding male infertility: Results from a 2015 questionnaire in Japan. Reprod. Med. Biol. 2018, 17, 44–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chin, B.; Murphy, M.L.M.; Janicki-Deverts, D.; Cohen, S. Marital status as a predictor of diurnal salivary cortisol levels and slopes in a community sample of healthy adults. Psychoneuroendocrinology 2017, 78, 68–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenthal, L.; Carroll-Scott, A.; Earnshaw, V.A.; Santilli, A.; Ickovics, J.R. The importance of full-time work for urban adults’ mental and physical health. Soc. Sci. Med. 2012, 75, 1692–1696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jannini, E.A.; Sternbach, N.; Limoncin, E.; Ciocca, G.; Gravina, G.L.; Tripodi, F.; Petruccelli, I.; Keijzer, S.; Isherwood, G.; Wiedemann, B.; et al. Health-related characteristics and unmet needs of men with erectile dysfunction: A survey in five European countries. J. Sex. Med. 2014, 11, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Otani, T. Clinical review of ejaculatory dysfunction. Reprod. Med. Biol. 2019, 18, 331–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukuhara, S.; Shirai, M.; Ueda, N.; Takezawa, K.; Ishikawa, K.; Hiramatsu, I.; Uesaka, Y.; Nozaki, T.; Iwasa, A.; Kobayashi, K.; et al. Therapeutic efficacy and safety of a free-standing motorized ejaculation aid for patients with intravaginal ejaculatory dysfunction. Reprod. Med. Biol. 2023, 22, e12530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015, 18, 5–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thaler, M.A.; Seifert-Klauss, V.; Luppa, P.B. The biomarker sex hormone-binding globulin—From established applications to emerging trends in clinical medicine. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Chambers, A.; Stern, B.R.; Aggett, P.J.; Plunkett, L.; Rudenko, L. Development of a copper database for exposure-response analysis. J. Toxicol. Environ. Health A 2010, 73, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Douron, M. U-shaped dose-response curves: Implications for risk characterization of essential elements and other chemicals. J. Toxicol. Environ. Health A 2010, 73, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Omu, A.E.; Al-Bader, A.A.; Dashti, H.; Oriowo, M.A. Magnesium in human semen: Possible role in premature ejaculation. Arch. Androl. 2001, 46, 59–66. [Google Scholar] [CrossRef] [PubMed]


| Median (IQR) | Range | |
|---|---|---|
| Age (years) | 37 (33–42) | 22–75 |
| Height (cm) | 172.0 (168.0–176.0) | 155.0–186.0 |
| Weight (kg) | 68 (62–77) | 46–130 |
| BMI (kg/m2) | 23.0 (20.9–25.5) | 17.6–44.5 |
| Before Matching by Age | After Matching by Age | |||||||
|---|---|---|---|---|---|---|---|---|
| Cluster 1 (n = 43) | Cluster 2 (n = 98) | Cluster 3 (n = 84) | p-Value | Cluster 1 (n = 27) | Cluster 2 (n = 57) | Cluster 3 (n = 84) | p-Value | |
| T (ng/mL) | 3.87 (2.90–4.78) | 3.87 (3.20–4.54) | 5.40 (4.70–6.42) | <0.001 | 3.70 (3.01–4.48) | 3.85 (3.22–4.43) | 5.40 (4.70–6.42) | <0.001 |
| FT (pg/mL) | 9.2 (7.7–11.1) | 9.1 (7.4–9.9) | 13.7 (12.2–15.5) | <0.001 | 9.7 (7.7–11.0) | 9.1 (7.6–9.6) | 13.7 (12.2–15.5) | <0.001 |
| AMS (points) | 40 (37–47) | 27 (23–30) | 25 (21–29) | <0.001 | 40 (36–45) | 25 (23–30) | 25 (21–29) | <0.001 |
| Somatic (points) | 17 (15–19) | 11 (9–12) | 11 (9–13) | <0.001 | 17 (16–19) | 11 (9–12) | 11 (9–13) | <0.001 |
| Psychological (points) | 10 (9–15) | 6 (5–7) | 6 (5–7) | <0.001 | 10 (9–14) | 6 (5–7) | 6 (5–7) | <0.001 |
| Sexual (points) | 13 (11–15) | 9 (8–11) | 8 (6–10) | <0.001 | 12 (10–14) | 9 (7–10) | 8 (6–10) | <0.001 |
| EHS (points) | 3.23 (0.10) | 3.44 (0.07) | 3.54 (0.06) | 0.038 | 3.41 (0.10) | 3.47 (0.10) | 3.54 (0.06) | 0.4 |
| Grade 1 | 2.3% | 3.1% | 1.2% | 0% | 3.5% | 1.2% | ||
| Grade 2 | 7.0% | 5.1% | 1.2% | 0% | 3.5% | 1.2% | ||
| Grade 3 | 56% | 37% | 40% | 59% | 35% | 40% | ||
| Grade 4 | 35% | 55% | 57% | 41% | 58% | 57% | ||
| SDS (points) | 44 (40–48) | 37 (31–40) | 38 (33–41) | <0.001 | 44 (41–48) | 38 (32–41) | 38 (33–41) | <0.001 |
| Affective (points) | 16 (15–18) | 12 (10–14) | 13 (11–14) | <0.001 | 16 (15–18) | 12 (11–14) | 13 (11–14) | <0.001 |
| Cognitive Score (points) | 18 (15–19) | 15 (13–17) | 16 (13–18) | <0.001 | 18 (15–19) | 16 (13–17) | 16 (13–18) | 0.017 |
| Somatic Score (points) | 11 (9–12) | 9 (7–11) | 9 (8–10) | <0.001 | 11 (9–12) | 9 (7–11) | 9 (8–10) | <0.001 |
| Before Matching by Age | After Matching by Age | |||||||
|---|---|---|---|---|---|---|---|---|
| Cluster 1 (n = 43) | Cluster 2 (n = 98) | Cluster 3 (n = 84) | p-Value | Cluster 1 (n = 27) | Cluster 2 (n = 57) | Cluster 3 (n = 84) | p-Value | |
| Age (years) | 35 (33–41) | 39 (34–44) | 36 (32–39) | 0.007 | 34 (32–39) | 37 (32–40) | 36 (32–39) | 0.5 |
| Height (cm) | 171.0 (168.0–174.0) | 170.8 (168.0–174.0) | 174.0 (170.0–177.0) | 0.010 | 170.0 (168.0–173.0) | 171.0 (168.0–174.0) | 174.0 (170.0–177.0) | 0.013 |
| Weight (kg) | 70 (62–80) | 67 (60–76) | 67 (63–75) | 0.7 | 68 (59–81) | 70 (60–79) | 67 (63–75) | >0.9 |
| BMI (kg/m2) | 23.5 (21.3–27.2) | 22.9 (20.7–25.9) | 23.0 (21.1–24.6) | 0.4 | 23.3 (20.9–26.7) | 23.0 (20.8–26.6) | 23.0 (21.1–24.6) | 0.8 |
| SV (mL) | 3.10 (2.05–3.95) | 3.50 (2.60–4.30) | 3.60 (2.90–4.93) | 0.085 | 3.20 (2.70–4.10) | 3.70 (2.60–4.70) | 3.60 (2.90–4.93) | 0.4 |
| SC (×106 mL) | 36 (7–82) | 40 (14–102) | 41 (8–89) | 0.5 | 25 (4–58) | 42 (14–105) | 41 (8–89) | 0.2 |
| SM (%) | 52 (35–67) | 51 (31–68) | 55 (38–66) | 0.8 | 53 (35–67) | 54 (30–69) | 55 (38–66) | 0.9 |
| Left TV (mL) | 22 (16–24) | 20 (16–22) | 20 (18–22) | 0.5 | 21 (16–23) | 20 (16–22) | 20 (18–22) | >0.9 |
| Right TV (mL) | 22 (18–24) | 20 (18–22) | 20 (18–24) | 0.7 | 21 (18–23) | 20 (18–24) | 20 (18–24) | 0.9 |
| LH (mIU/mL) | 2.66 (1.84–3.53) | 2.58 (1.79–3.69) | 2.70 (2.15–3.60) | 0.4 | 2.71 (1.84–3.47) | 2.47 (1.67–3.50) | 2.70 (2.15–3.60) | 0.3 |
| FSH (mIU/mL) | 4.5 (3.0–6.1) | 4.3 (2.8–6.7) | 4.3 (3.2–6.0) | >0.9 | 4.9 (2.9–6.1) | 4.0 (2.7–5.8) | 4.3 (3.2–6.0) | 0.4 |
| PRL (ng/mL) | 7.6 (5.5–10.0) | 8.0 (6.0–10.8) | 8.3 (6.3–10.1) | 0.5 | 7.9 (5.8–11.2) | 8.2 (6.5–10.5) | 8.3 (6.3–10.1) | >0.9 |
| E2 (pg/mL) | 18 (13–26) | 17 (12–22) | 20 (14–24) | 0.3 | 20 (16–27) | 18 (12–22) | 20 (14–24) | 0.6 |
| Cluster 1 (n = 27) | Cluster 2 (n = 57) | Cluster 3 (n = 84) | p-Value | |
|---|---|---|---|---|
| Li (μg/L) | 0.46 (0.38–0.57) | 0.48 (0.42–0.62) | 0.50 (0.35–0.67) | 0.6 |
| Na (mg/L) | 3180 (3140–3210) | 3160 (3110–3200) | 3160 (3085–3200) | 0.12 |
| Mg (mg/L) | 20.00 (18.80–20.55) | 19.90 (19.00–20.60) | 19.95 (18.90–20.78) | 0.9 |
| P (mg/L) | 119 (114–131) | 116 (112–127) | 118 (108–129) | 0.6 |
| S (mg/L) | 1130 (1105–1150) | 1130 (1090–1170) | 1150 (1118–1190) | 0.029 |
| K (mg/L) | 163 (161–168) | 159 (156–168) | 167 (158–174) | 0.014 |
| Ca (mg/L) | 93.6 (91.5–95.7) | 92.1 (89.9–94.6) | 94.6 (92.0–96.6) | 0.009 |
| Mn (μg/L) | 0.47 (0.39–0.55) | 0.47 (0.41–0.54) | 0.49 (0.41–0.59) | 0.4 |
| Fe (μg/L) | 912 (777–1245) | 1030 (811–1220) | 1075 (895–1368) | 0.10 |
| Co (μg/L) | 0.086 (0.075–0.099) | 0.082 (0.074–0.098) | 0.089 (0.079–0.104) | 0.3 |
| Cu (μg/L) | 718 (657–790) | 741 (682–794) | 757 (676–828) | 0.4 |
| Zn (μg/L) | 749 (702–844) | 784 (694–864) | 829 (731–895) | 0.080 |
| As (μg/L) | 0.85 (0.67–1.33) | 1.35 (0.94–1.97) | 1.09 (0.75–2.41) | 0.019 |
| Se (μg/L) | 146 (138–156) | 141 (135–148) | 143 (135–154) | 0.2 |
| Rb (μg/L) | 175 (152–185) | 161 (151–183) | 176 (159–197) | 0.049 |
| Sr (μg/L) | 28 (21–33) | 26 (23–32) | 27 (22–33) | 0.8 |
| Mo (μg/L) | 1.25 (0.82–1.80) | 1.22 (0.92–1.76) | 1.01 (0.86–1.42) | 0.12 |
| Cs (μg/L) | 0.64 (0.58–0.74) | 0.56 (0.50–0.72) | 0.66 (0.56–0.76) | 0.035 |
| Ba (μg/L) | 0.45 (0.35–0.68) | 0.36 (0.29–0.50) | 0.42 (0.31–0.60) | 0.060 |
| Tl (μg/L) | 0.036 (0.030–0.044) | 0.035 (0.027–0.044) | 0.037 (0.032–0.044) | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Kojo, K.; Suetomi, T.; Nagumo, Y.; Midorikawa, H.; Matsuda, T.; Nakazono, A.; Shimizu, T.; Fujimoto, S.; Ikeda, A.; et al. Distinct Clusters of Testosterone Levels, Symptoms, and Serum Trace Elements in Young Men: A Cross-Sectional Analysis. Nutrients 2025, 17, 867. https://doi.org/10.3390/nu17050867
Tanaka T, Kojo K, Suetomi T, Nagumo Y, Midorikawa H, Matsuda T, Nakazono A, Shimizu T, Fujimoto S, Ikeda A, et al. Distinct Clusters of Testosterone Levels, Symptoms, and Serum Trace Elements in Young Men: A Cross-Sectional Analysis. Nutrients. 2025; 17(5):867. https://doi.org/10.3390/nu17050867
Chicago/Turabian StyleTanaka, Takazo, Kosuke Kojo, Takahiro Suetomi, Yoshiyuki Nagumo, Haruhiko Midorikawa, Takaaki Matsuda, Ayumi Nakazono, Takuya Shimizu, Shunsuke Fujimoto, Atsushi Ikeda, and et al. 2025. "Distinct Clusters of Testosterone Levels, Symptoms, and Serum Trace Elements in Young Men: A Cross-Sectional Analysis" Nutrients 17, no. 5: 867. https://doi.org/10.3390/nu17050867
APA StyleTanaka, T., Kojo, K., Suetomi, T., Nagumo, Y., Midorikawa, H., Matsuda, T., Nakazono, A., Shimizu, T., Fujimoto, S., Ikeda, A., Kandori, S., Negoro, H., Takayama, T., & Nishiyama, H. (2025). Distinct Clusters of Testosterone Levels, Symptoms, and Serum Trace Elements in Young Men: A Cross-Sectional Analysis. Nutrients, 17(5), 867. https://doi.org/10.3390/nu17050867







