Calix[4]arene-Based Amino Extractants Containing n-Alkyl Moieties for Separation of Pd(II) and Pt(IV) from Leach Liquors of Automotive Catalysts
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of 3–6
2.3. Liquid-Liquid Extraction Studies
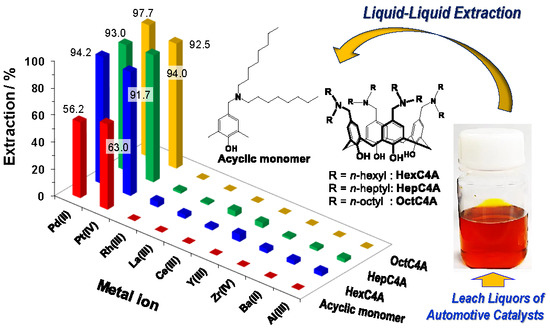
2.3.1. Solvent Extraction of PGM by 3–6
2.3.2. Solvent Extraction of Pd(II) and Pt(IV) Ions from Leach Liquors of Automotive Catalysts
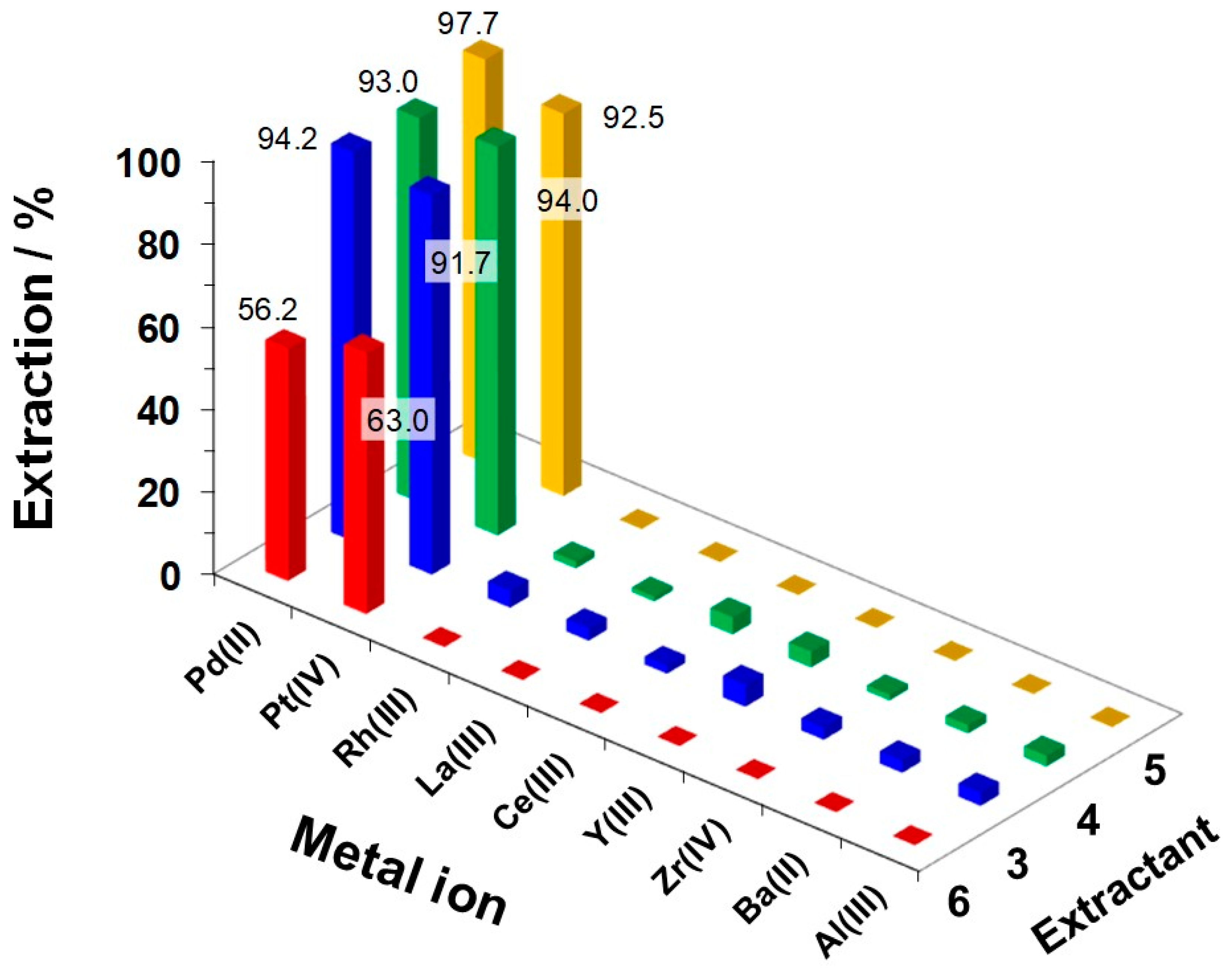
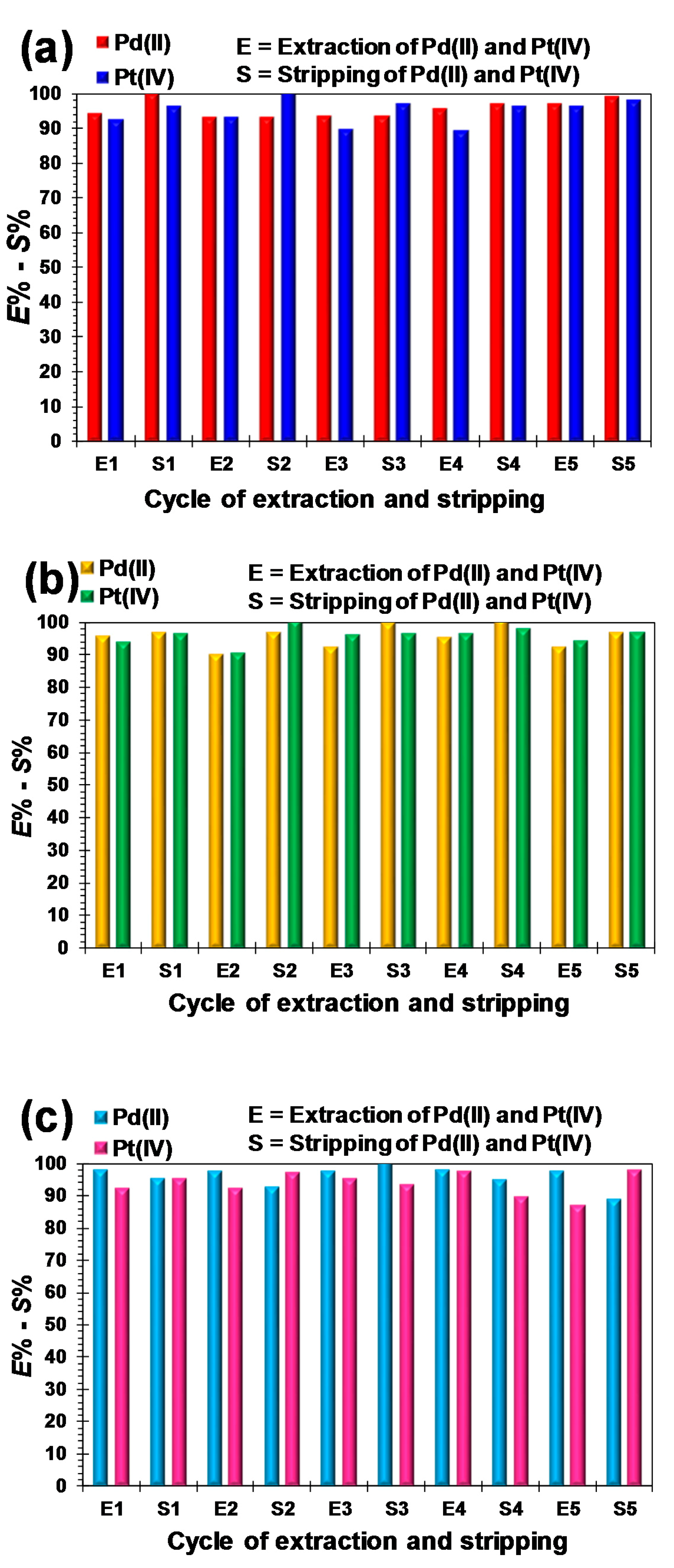
2.3.3. Stripping of Pt(IV) and Pd(II) Ions from Extractant Organic Phases
2.3.4. Reusability of Extractants
2.3.5. 1H NMR and Attenuated Total Refection (ATR)-FTIR Measurements of Native 3–5 and 0.1 M HCl-Treated 3–5
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vicens, J.; Böhmer, V. (Eds.) Calixarenes: A Versatile Class of Macrocyclic Compounds; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; ISBN 0-7923-0714-3. [Google Scholar]
- Gutsche, C.D. Calixarenes Revisited; Royal Society of Chemistry: Cambridge, UK, 1998; ISBN 0-85404-502-3. [Google Scholar]
- Neri, N.; Sessler, J.L.; Wang, M.-X. (Eds.) Calixarene and Beyond; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-31865-3. [Google Scholar]
- Shinkai, S.; Shiramama, Y.; Satoh, H.; Manabe, O. Selective extraction and transport of UO22+ with calixarene-based uranophiles. J. Chem. Soc. Perkin Trans. II 1989, 1167–1171. [Google Scholar] [CrossRef]
- Deligöz, H.; Yilmaz, M. Liquid-liquid extraction of transition metal cations by calixarene-based cyclic ligands. Solvent Extr. Ion Exch. 1995, 13, 19–26. [Google Scholar] [CrossRef]
- Deligöz, H.; Erdem, E. Comparative studies on the solvent extraction of transition metal cations by calixarene, phenol and ester derivatives. J. Hazard. Mater. 2008, 154, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Morisada, S.; Kawakita, H.; Ohto, K.; Kim, Y. Relationship between chemical structure and extraction efficiency toward palladium with ketonic derivatives of p-tert-octylcalix[4]arene in nitric acid media. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 25–32. [Google Scholar] [CrossRef]
- Tanaka, M.; Morisada, S.; Kawakita, H.; Inoue, K.; Ohto, K. Synthesis of a cross phosphonic acid type calix[4]arene with two different spacers and its extractive separation of rare earth metals. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 33–42. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Synergism as a phenomenon in solvent extraction of 4f-elements with calixarenes. RSC Adv. 2016, 6, 11303–11324. [Google Scholar] [CrossRef]
- Crundwell, F.; Moats, M.; Ramachandran, V.; Robinson, T.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt, and Platinum Group Metals; Elsevier: Oxford, UK, 2011; ISBN 978-0-08-096809-4. [Google Scholar]
- Zaera, F. The long and winding road to catalysis. Nature 2017, 541, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson Matthey. Summary of Platinum Supply & Demand in 2017; PGM Market Reports February 2018; Johnson Matthey: Royston, UK, 2018. [Google Scholar]
- Fornalczyk, A. Industrial catalysts as a source of valuable metals. J. Achiev. Mater. Manuf. Eng. 2012, 55, 864–869. [Google Scholar]
- Tollefson, J. Worth its weight in Platinum. Nature 2007, 450, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Kolliopoulos, G.; Balomenos, E.; Giannopoulou, I.; Yakoumis, I.; Panias, D. Behavior of platinum group metals during their pyrometallurgical recovery from spent automotive. Open Access Libr. J. 2014, 1, e735. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Paiva, A.P.; Oliveira, P.C.; Costa, M.C.; da Costa, A.M.R. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters. J. Hazard. Mater. 2014, 278, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinlechner, S.; Antrekowitsch, J. Potential of a hydrometallurgical recycling process for catalysts to cover the demand for critical metals, like PGMs and cerium. JOM 2015, 67, 406–411. [Google Scholar] [CrossRef]
- Okuda, A.; Sawai, H.; Ichiishi, S.; Shibata, J. Extractions of Pd(II) and Pt(IV) from hydrochloric acid solution by dihexyl sulfide and the degradation of dihexyl sulphide. J. MMIJ (Shigen-to-Sozai) 2000, 116, 929–933. [Google Scholar] [CrossRef]
- Cox, M. Solvent extraction in hydrometallurgy. In Principles and Practices, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 455–505. ISBN 0-8247-5063-2. [Google Scholar]
- Rajiv Gandhi, M.; Yamada, M.; Haga, K.; Shibayama, A. Synthesis of pincer-type extractants for selective extraction of palladium from PGMs: An improved liquid-liquid extraction approach to current refining processes. Sci. Rep. 2017, 7, 8709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narita, H.; Suzuki, T.; Motokawa, R. Recent research in solvent extraction of Platinum group metals. J. Jpn. Inst. Met. Mater. 2017, 81, 157–167. [Google Scholar] [CrossRef]
- Narita, H.; Morisaku, K.; Tamura, K.; Tanaka, M.; Shiwaku, H.; Okamoto, Y.; Suzuki, S.; Yaita, T. Extraction properties of palladium(II) in HCl solution with sulfide-containing monoamide compounds. Ind. Eng. Chem. Res. 2014, 53, 3636–3640. [Google Scholar] [CrossRef]
- Traeger, J.; König, J.; Städtke, A.; Holdt, H.-J. Development of a solvent extraction system with 1,2-Bis(2-Methoxyethylthio)Benzene for the selective separation of Palladium(II) from secondary raw materials. Hydrometallurgy 2012, 127–128, 30–38. [Google Scholar] [CrossRef]
- Yamada, M.; Rajiv Gandhi, M.; Kondo, Y.; Hamada, F. Synthesis and characterisation of p-diethylaminomethylthiacalix[4]arene for selective recovery of platinum from automotive catalyst residue. Supramol. Chem. 2014, 26, 620–630. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Liquid-liquid extraction of palladium(II) from chloride media by N,N-dimethyl-N,N-dicyclohexylthiodiglycolamide. Sep. Purif. Technol. 2015, 156, 363–368. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Development of tertiary thioamide derivatives to recover Palladium(II) from simulated complex chloride solutions. Hydrometallurgy 2015, 151, 33–41. [Google Scholar] [CrossRef]
- Paiva, A.P.; Martins, M.E.; Ortet, O. Palladium(II) recovery from hydrochloric acid solutions by N,N′-dimethyl-N,N′-dibutylthiodiglycolamide. Metals 2015, 5, 2303–2315. [Google Scholar] [CrossRef]
- Yamada, M.; Rajiv Gandhi, M.; Kunda, U.M.R.; Hamada, F. Thiacalixarenes: Emergent supramolecules in crystal engineering and molecular recognition. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 1–18. [Google Scholar] [CrossRef]
- Yamada, M.; Rajiv Gandhi, M.; Sato, D.; Kaneta, Y.; Kimura, N. Comparative study on Palladium(II) extraction using thioamide-modified acyclic and cyclic extractants. Ind. Eng. Chem. Res. 2016, 55, 8914–8921. [Google Scholar] [CrossRef]
- Rajiv Gandhi, M.; Yamada, M.; Kondo, Y.; Shibayama, A.; Hamada, F. Rapid and selective extraction of Pd(II) ions using the SCS type pincer ligand 1,3-Bis(dimethylthiocarbamoyloxy)benzene, and its Pd(II) extraction mechanism. RSC Adv. 2016, 6, 1243–1252. [Google Scholar] [CrossRef]
- Paiva, A.P.; Ortet, O.; Carvalho, G.I.; Nogueira, C.A. Recovery of Palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 2017, 171, 394–401. [Google Scholar] [CrossRef]
- Paiva, A.P. Recycling of Palladium from spent catalysts using solvent extraction- some critical points. Metals 2018, 7, 505. [Google Scholar] [CrossRef]
- Yamada, M.; Rajiv Gandhi, M.; Kaneta, Y.; Kimura, N.; Katagiri, H. Thiodiphenol-based n-dialkylamino extractants for selective Platinum group metal separation from automotive catalysts. Ind. Eng. Chem. Res. 2018, 57, 8914–8921. [Google Scholar] [CrossRef]
- Yamada, M.; Rajiv Gandhi, M.; Kaneta, Y.; Hu, Y.; Shibayama, A. Calix[4]arene-based n-dialkylamino extractants for selective Platinum group metal separation from automotive catalysts. ChemistySelect 2017, 2, 1052–1057. [Google Scholar] [CrossRef]
- Stepniak-Biniakiewicz, D.; Szymanowski, J. Influence of the structure of alkyl derivatives of salicylaldehyde oxime upon the extraction rate of copper from diluted acidic solutions. Polyhedron 1987, 6, 197–203. [Google Scholar] [CrossRef]
- Ainscow, T.A.; Aldalur, I.; Beezer, A.E.; Connor, J.A.; Garbett, N.C.; Mitchell, J.C.; Page, A.L.; Tindale, N.; Turner, K.A.; Willson, R.J. Influence of alkyl chain length and structure on the extraction of Copper(II) from aqueous acid by 5-alkyl-2-hydroxybenzaldoximes in hydrocarbon solvents: Diffusion coefficients of extractants and their complexes. J. Colloid Interface Sci. 1999, 213, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from automotive catalyst residue in various chloride based solutions. Mater. Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the Platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Katagiri, H.; Iki, N.; Matsunaga, Y.; Kabuto, C.; Miyano, S. ‘Thiacalix[4]aniline’ as a highly specific extractant for Au(III) and Pd(II) ions. Chem. Commun. 2002, 2080–2081. [Google Scholar] [CrossRef]
- Fontàs, C.; Anticó, E.; Vocanson, F.; Lamartine, R.; Seta, P. Efficient thiacalix[4]arenes for the extraction and separation of Au(III), Pd(II) and Pt(IV) metal ions from acidic media incorporated in membranes and solid phases. Sep. Purif. Technol. 2007, 54, 322–328. [Google Scholar] [CrossRef]
- Chamberlain, N.F. The Practice of NMR Spectroscopy with Spectra-Structure Correlations for Hydrogen-1; Springer: New York, NY, USA, 1974; Chapter 2; pp. 15–43. [Google Scholar]


| No. | Extractants | Structural Formulas | [Extractant] (M) | [Pd(II)] or [Pt(IV)] (M) | pH or [HCl] in Aqueous Solution | Pd(II) E (%) | Pt(IV) E (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | p-diethylamino-thiacalix[4]arene |  | 1 × 10−3 | Pt(IV): 1 × 10−3 | pH 2.0 | - | 80.0 | [24] |
| 2 | p-tert-butylthia-calix[4]aniline | 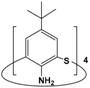 | 5 × 10−4 | Pd(II): 1 × 10−4 Pt(IV): 1 × 10−4 | pH 3.1 | 100 | 0 | [39] |
| 3 | p-tert-butyl-tetra-kis[(diethyl-amide)methoxy]-thiacalix[4]arene | 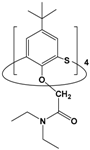 | 1 × 10−3 | Pd(II): 1 × 10−4 Pt(IV): 1 × 10−4 | pH 1.7 | 57.6 | 26.5 | [40] |
| 4 | 1 |  | 1 × 10−3 | Pd(II): 1 × 10−4 Pt(IV): 1 × 10−4 | 0.1 M HCl | 98.3 | 94.7 | [34] |
| 5 | 2 |  | 1 × 10−3 | Pd(II): 1 × 10−4 Pt(IV): 1 × 10−4 | 0.1 M HCl | 99.2 | 91.8 | [34] |
| 6 | 3 | 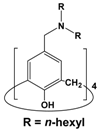 | 1 × 10−3 | Pd(II): 1 × 10−4 Pt(IV): 1 × 10−4 | 0.1 M HCl | 99.5 | 98.8 | Present study |
| 7 | 4 | 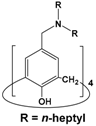 | 1 × 10−3 | Pd(II): 1 × 10−4 Pt(IV): 1 × 10−4 | 0.1 M HCl | 99.3 | 99.5 | Present study |
| 8 | 5 | 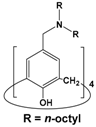 | 1 × 10−3 | Pd(II): 1 × 10−4 Pt(IV): 1 × 10−4 | 0.1 M HCl | 99.0 | 90.8 | Present study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, M.; Kaneta, Y.; Rajiv Gandhi, M.; Kunda, U.M.R.; Shibayama, A. Calix[4]arene-Based Amino Extractants Containing n-Alkyl Moieties for Separation of Pd(II) and Pt(IV) from Leach Liquors of Automotive Catalysts. Metals 2018, 8, 517. https://doi.org/10.3390/met8070517
Yamada M, Kaneta Y, Rajiv Gandhi M, Kunda UMR, Shibayama A. Calix[4]arene-Based Amino Extractants Containing n-Alkyl Moieties for Separation of Pd(II) and Pt(IV) from Leach Liquors of Automotive Catalysts. Metals. 2018; 8(7):517. https://doi.org/10.3390/met8070517
Chicago/Turabian StyleYamada, Manabu, Yu Kaneta, Muniyappan Rajiv Gandhi, Uma Maheswara Rao Kunda, and Atsushi Shibayama. 2018. "Calix[4]arene-Based Amino Extractants Containing n-Alkyl Moieties for Separation of Pd(II) and Pt(IV) from Leach Liquors of Automotive Catalysts" Metals 8, no. 7: 517. https://doi.org/10.3390/met8070517
APA StyleYamada, M., Kaneta, Y., Rajiv Gandhi, M., Kunda, U. M. R., & Shibayama, A. (2018). Calix[4]arene-Based Amino Extractants Containing n-Alkyl Moieties for Separation of Pd(II) and Pt(IV) from Leach Liquors of Automotive Catalysts. Metals, 8(7), 517. https://doi.org/10.3390/met8070517