Metabolomic Insights into the Allelopathic Effects of Ailanthus altissima (Mill.) Swingle Volatile Organic Compounds on the Germination Process of Bidens pilosa (L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Germination Indexes
2.3. VOC Characterisation: HS-SPME-GC–MS Analysis
2.4. Untargeted Metabolomic Analysis
2.5. GC/MS Data Analysis Using MS-DIAL
2.6. Statistical Analysis
3. Results
3.1. Germination Index
3.2. VOC Characterisation
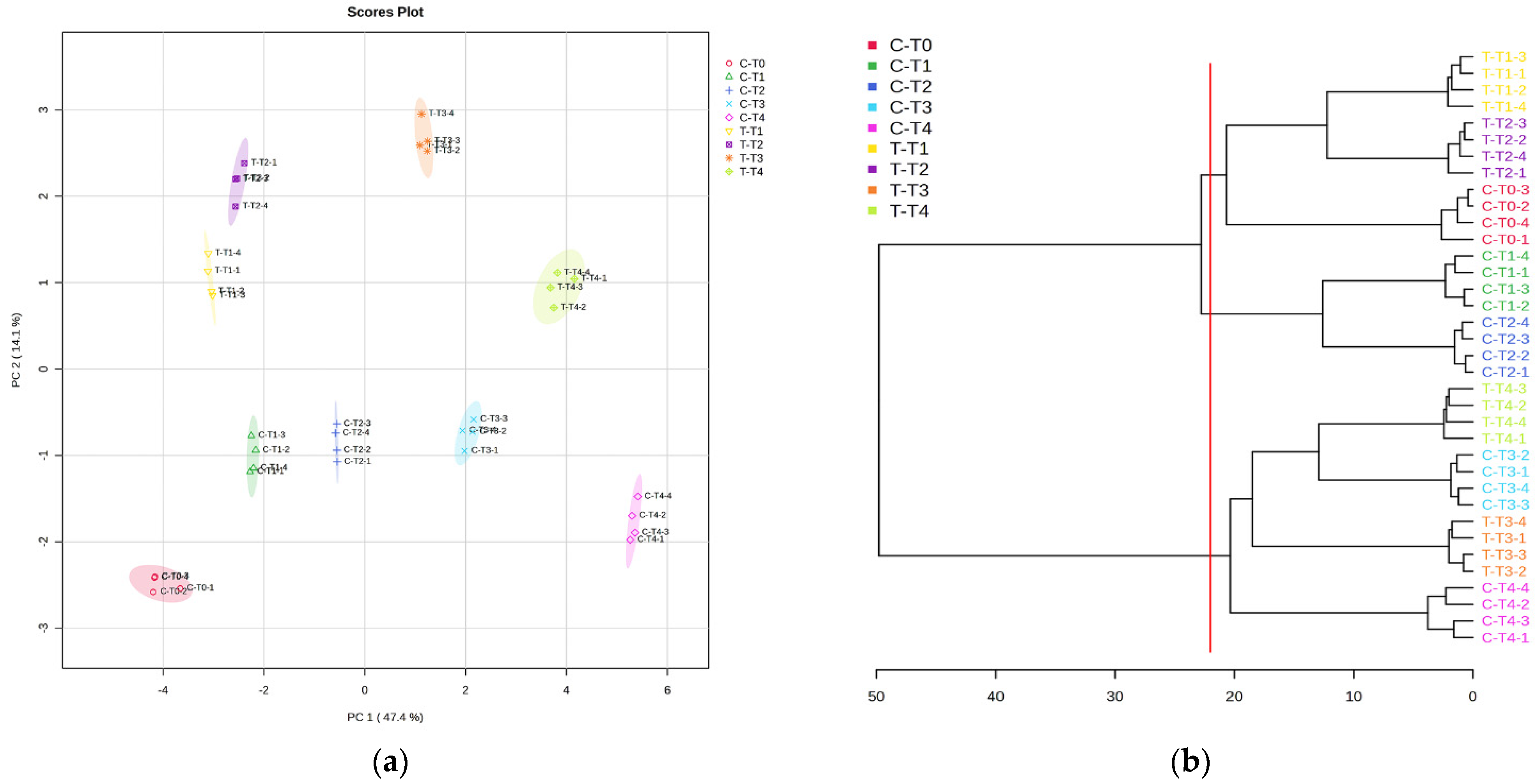
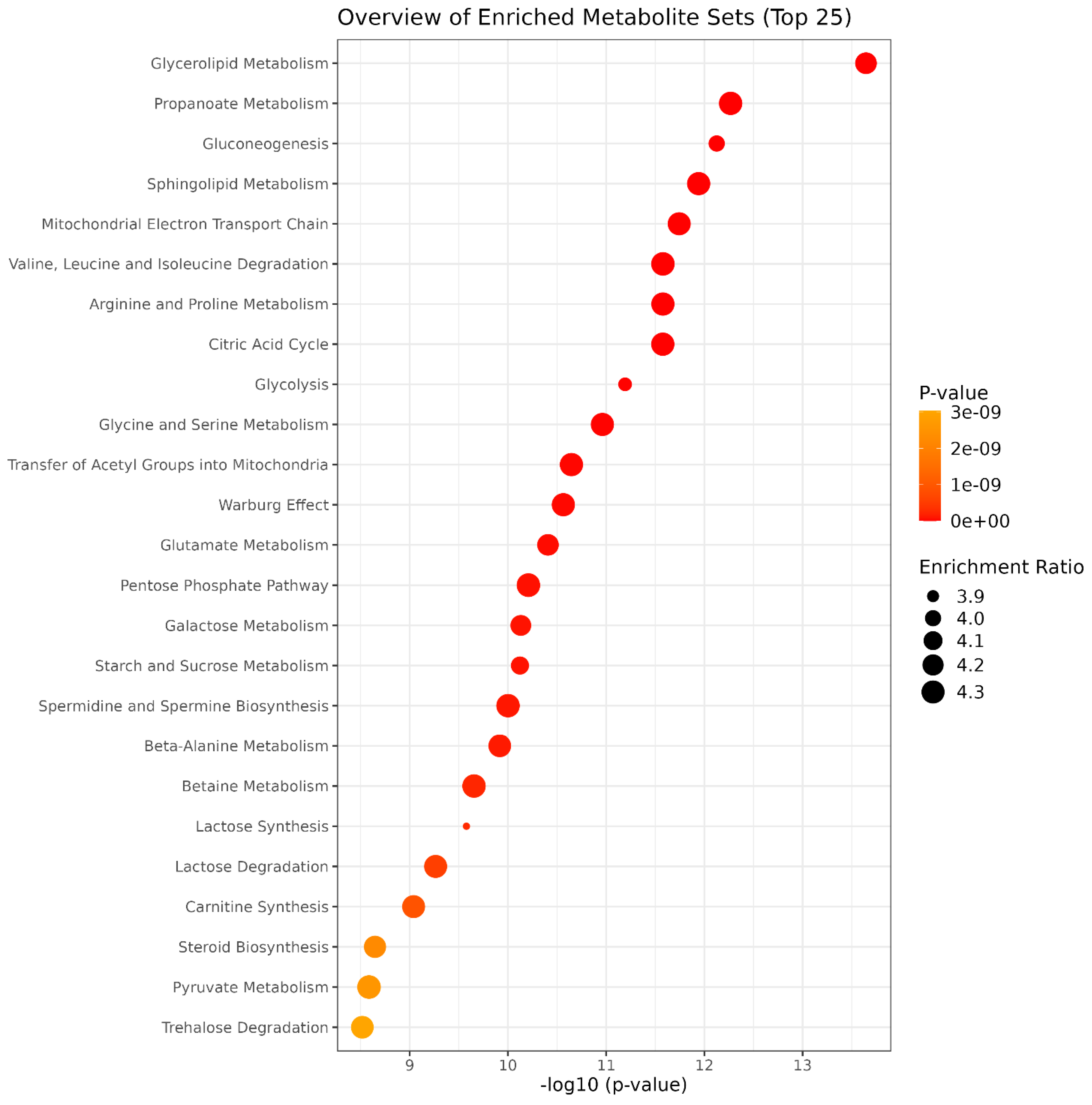
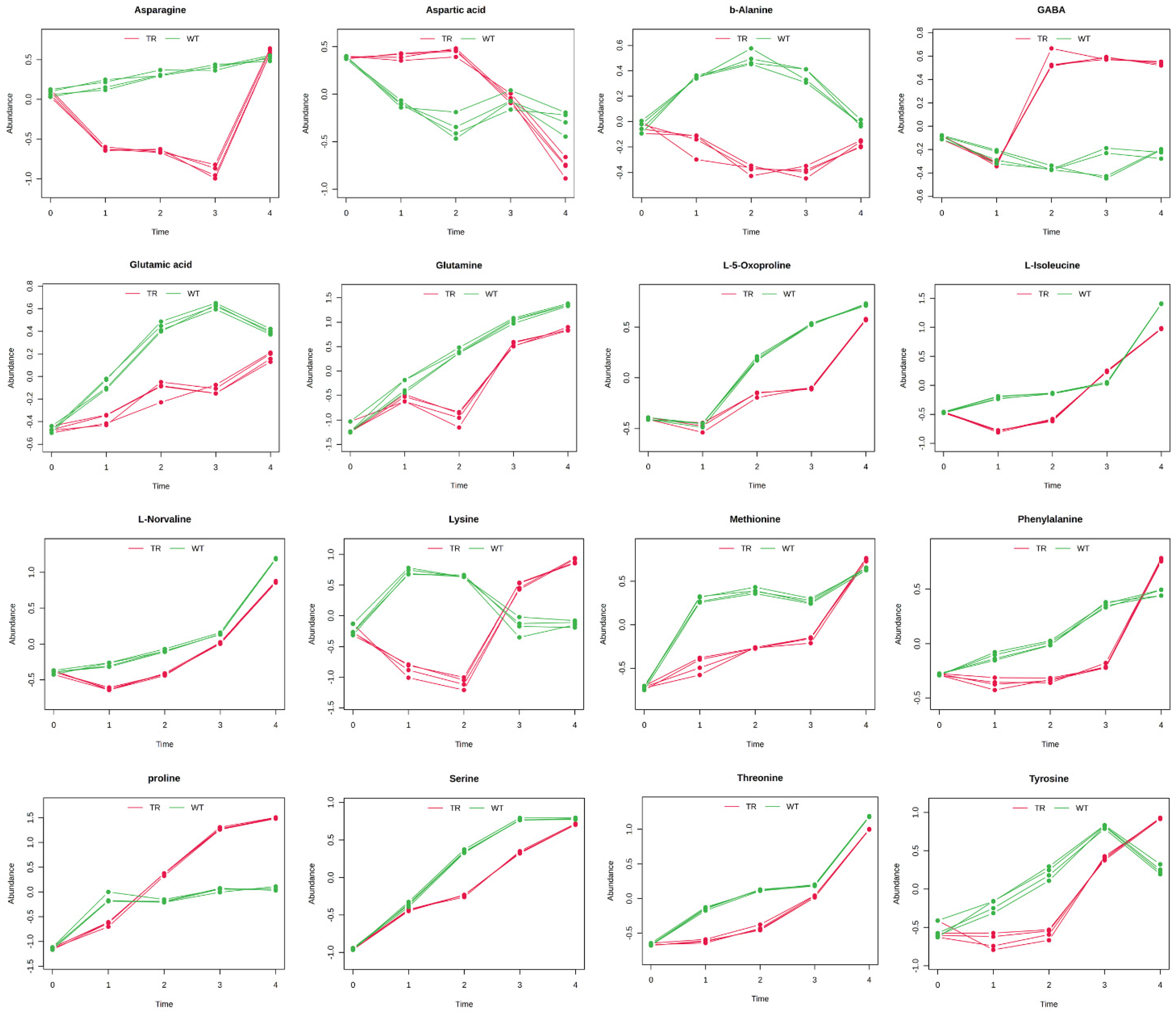
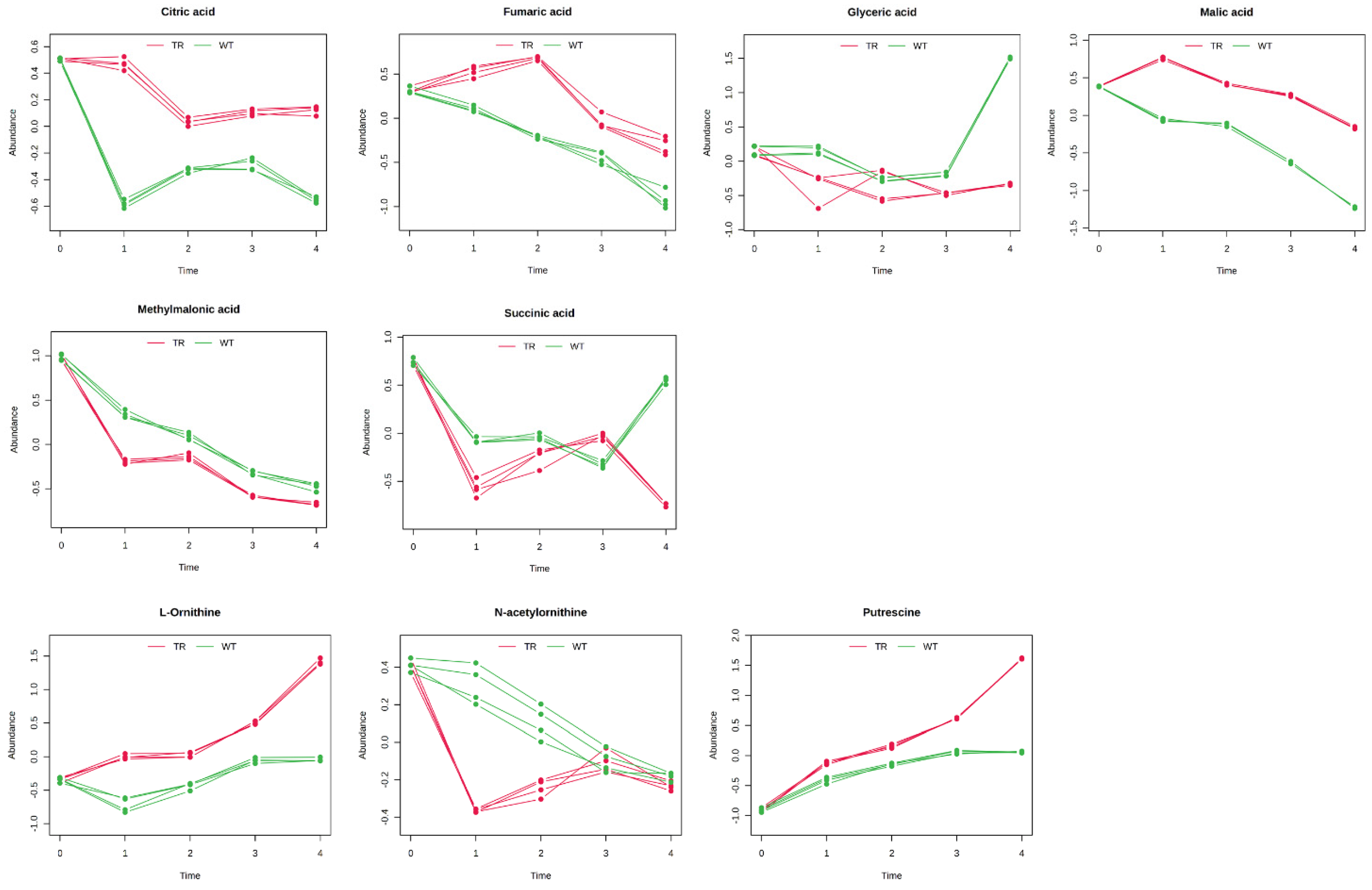
3.3. GC–MS Untargeted Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ueda, H.; Kikuta, Y.; Matsuda, K. Plant communication: Mediated by individual or blended VOCs? Plant Signal. Behav. 2012, 7, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2010, 15, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tian, L.; Han, X.; Yang, Y. Research advances in allelopathy of volatile organic compounds (VOCs) of plants. Horticulturae 2021, 7, 278. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sunseri, F.; Abenavoli, M.R. Allelopathic potential of Dittrichia viscosa (L.) W. Greuter mediated by VOCs: A physiological and metabolomic approach. PLoS ONE 2017, 12, e0170161. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.H. The role of chemical inhibition (allelopathy) in vegetational composition. Bull. Torrey Bot. Club 1966, 93, 332–351. [Google Scholar] [CrossRef]
- Arminante, F.; De Falco, E.; De Feo, V.; De Martino, L.; Mancini, E.; Quaranta, E. Allelopathic activity of essential oils from Mediterranean Labiatae. In Proceedings of the I International Symposium on the Labiatae: Advances in Production, Biotechnology and Utilisation, Sanremo, Italy, 22–25 February 2006; ISHS: Leuven, Belgium, 2006; Volume 723, pp. 347–356. [Google Scholar] [CrossRef]
- Singh, H.; Batish, D.; Setia, N.; Kohli, R. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 2005, 146, 89–94. [Google Scholar] [CrossRef]
- Kang, G.; Mishyna, M.; Appiah, K.S.; Yamada, M.; Takano, A.; Prokhorov, V.; Fujii, Y. Screening for plant volatile emissions with allelopathic activity and the identification of L-Fenchone and 1,8-Cineole from star anise (Illicium verum) leaves. Plants 2019, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Maninang, J.S.; Fujii, Y. Identification of octanal as a plant growth inhibitory volatile compound released from Heracleum sosnowskyi fruit. Nat. Prod. Commun. 2015, 10, 1934578X1501000518. [Google Scholar] [CrossRef]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function, and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef]
- McCalla, T.; Norstard, F.A. Toxicity problems in mulch tillage. Agric. Environ. 1974, 1, 153–174. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy—An overview. In Chemically Mediated Interactions Between Plants and Other Organisms; Thompson, L., Ed.; Springer: Boston, MA, USA, 1985; pp. 81–105. [Google Scholar] [CrossRef]
- Soler, J.; Izquierdo, J. The invasive Ailanthus altissima: A biology, ecology, and control review. Plants 2024, 13, 931. [Google Scholar] [CrossRef]
- Naveed, S.; Ullah, I.; Akhtar, B.; Azam, B.; Noreen, A.; Khalid, N.; Momin, A.; Parveen, A. Allelopathic effect of Ailanthus altissima on germination and growth parameters of Pennesitum glaucum and Brassica nigra seeds. Pure Appl. Biol. 2024, 13, 407–421. [Google Scholar] [CrossRef]
- Bostan, C.; Borlea, F.; Mihoc, C.; Selesan, M. Ailanthus altissima species invasion on biodiversity caused by potential allelopathy. Res. J. Agric. Sci. 2014, 46, 95–103. [Google Scholar]
- Rojas-Sandoval, J. Bidens pilosa (blackjack); CABI Compendium: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Al-Qthanin, R.; Radwan, A.M.; Donia, A.M.; Balah, M.A. Potentials of invasive Bidens pilosa, Conyza bonariensis and Parthenium hysterophorus species based on germination patterns and growth traits. PLoS ONE 2024, 19, e0309568. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Araniti, F.; Prinsi, B.; Espen, L. The delay of Raphanus raphanistrum subsp. sativus (L.) domin seed germination induced by coumarin is mediated by a lower ability to sustain the energetic metabolism. Plants 2022, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Das, V.; Landi, M.; Abenavoli, M.; Araniti, F. Short-term effects of the allelochemical umbelliferone on Triticum durum L. metabolism through GC–MS-based untargeted metabolomics. Plant Sci. 2020, 298, 110548. [Google Scholar] [CrossRef] [PubMed]
- Misra, B. Steps for building an open-source EI-MS mass spectral library for GC-MS-based metabolomics. Protocols.io 2019. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Viant, M.R. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis, and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Sladonja, B.; Poljuha, D.; Sušek, M.; Dudaš, S. Herbicidal effect of Ailanthus altissima leaves water extracts on Medicago sativa seeds germination. In Proceedings of the Conference VIVUS: Transmission of Innovations, Knowledge and Practical Experience into Everyday Practice, Naklo, Slovenia, 14–15 November 2014; pp. 14–15. [Google Scholar]
- Camacho-Coronel, X.; Molina-Torres, J.; Heil, M. Sequestration of exogenous volatiles by plant cuticular waxes as a mechanism of passive associational resistance: A proof of concept. Front. Plant Sci. 2020, 11, 121. [Google Scholar] [CrossRef]
- Bradow, J.M.; Connick, W.J. Volatile seed germination inhibitors from plant residues. J. Chem. Ecol. 1990, 16, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Tan, H.; Jian, G.; Zhou, X.; Huo, L.; Jia, Y.; Zeng, L.; Yang, Z. Herbivore-induced (Z)-3-hexen-1-ol is an airborne signal that promotes direct and indirect defenses in tea (Camellia sinensis) under light. J. Agric. Food Chem. 2021, 69, 12608–12620. [Google Scholar] [CrossRef]
- Mao, K.; Li, C.; Zhai, H.; Wang, Y.; Lou, Y.; Xue, W.; Zhou, G. OsRCI-1-Mediated GLVs enhance rice resistance to brown planthoppers. Plants 2024, 13, 1494. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, R.H.; Willems, L.A.; Joosen, R.V.; Khan, N.; Ligterink, W.; Hilhorst, H.W. Metabolomic analysis of tomato seed germination. Metabolomics 2017, 13, 145. [Google Scholar] [CrossRef]
- Qu, C.; Zuo, Z.; Cao, L.; Huang, J.; Sun, X.; Zhang, P.; Yang, C.; Li, L.; Xu, Z.; Liu, G. Comprehensive dissection of transcript and metabolite shifts during seed germination and post-germination stages in poplar. BMC Plant Biol. 2019, 19, 279. [Google Scholar] [CrossRef]
- Han, C.; Yang, P.; Sakata, K.; Komatsu, S. Quantitative proteomics reveals the role of protein phosphorylation in rice embryos during early stages of germination. J. Proteome Res. 2014, 13, 1766–1782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Sun, M.; He, C.; Yu, K.; Zhao, B.; Li, R.; Li, J.; Yang, Z.; Wang, X.; et al. Multi-Omics analyses reveal systemic insights into maize vivipary. Plants 2021, 10, 2437. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Nie, Y.; Cui, K.; Sun, J. Integrative transcriptome and metabolome profiles reveal common and unique pathways involved in seed initial imbibition under artificial and natural salt stresses during germination of halophyte quinoa. Front. Plant Sci. 2022, 13, 853326. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ong, W.D.; Wang, J.; Huang, C.; Hong, J.; Xi, J.; Lv, S.; Li, X.; Huang, J. Comparative Metabolome Analyses of Chinese Hickory Nut Kernels Highlight the Importance of Allocation Shift and Accumulation on Primary and Secondary Metabolites During In-Situ Germination. Correspondence Affiliation, City, State, Country. 2020; preprint. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- Guo, S.; Klinkesorn, U.; Lorjaroenphon, Y.; Ge, Y.; Na Jom, K. Effects of germinating temperature and time on metabolite profiles of sunflower (Helianthus annuus L.) seed. Food Sci. Nutr. 2021, 9, 2810–2822. [Google Scholar] [CrossRef]
- Rosental, L.; Perelman, A.; Nevo, N.; Toubiana, D.; Samani, T.; Batushansky, A.; Sikron, N.; Saranga, Y.; Fait, A. Environmental and genetic effects on tomato seed metabolic balance and its association with germination vigor. BMC Genom. 2016, 17, 1047. [Google Scholar] [CrossRef] [PubMed]
- Gangl, R.; Tenhaken, R. Raffinose family oligosaccharides act as galactose stores in seeds and are required for rapid germination of Arabidopsis in the dark. Front. Plant Sci. 2016, 7, 1115. [Google Scholar] [CrossRef]
- Höftberger, M.; Althammer, M.; Foissner, I.; Tenhaken, R. Galactose induces formation of cell wall stubs and cell death in Arabidopsis roots. Planta 2022, 256, 26. [Google Scholar] [CrossRef] [PubMed]
- Althammer, M.; Blöchl, C.; Reischl, R.; Huber, C.G.; Tenhaken, R. Phosphoglucomutase is not the target for galactose toxicity in plants. Front. Plant Sci. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Hacham, Y.; Shitrit, O.; Nisimi, O.; Friebach, M.; Amir, R. Elucidating the importance of the catabolic enzyme, methionine-gamma-lyase, in stresses during Arabidopsis seed development and germination. Front. Plant Sci. 2023, 14, 1143021. [Google Scholar] [CrossRef]
- Silva, A.T.; Ligterink, W.; Hilhorst, H.W. Metabolite profiling and associated gene expression reveal two metabolic shifts during the seed-to-seedling transition in Arabidopsis thaliana. Plant Mol. Biol. 2017, 95, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraan, N.; Samarah, N.; Rasheed, E. The role of chitosan priming in induction of GABA shunt pathway during wheat seed germination under salt stress. Biol. Plant. 2023, 67, 234–248. [Google Scholar] [CrossRef]
- Toyoizumi, T.; Kosugi, T.; Toyama, Y.; Nakajima, T. Effects of high-temperature cooking on the gamma-aminobutyric acid content and antioxidant capacity of germinated brown rice (Oryza sativa L.). CyTA-J. Food 2021, 19, 360–369. [Google Scholar] [CrossRef]
- Peng, L.; Sun, S.; Yang, B.; Zhao, J.; Li, W.; Huang, Z.; Li, Z.; He, Y.; Wang, Z. Genome-wide association study reveals that the cupin domain protein OsCDP3.10 regulates seed vigour in rice. Plant Biotechnol. J. 2022, 20, 485–498. [Google Scholar] [CrossRef] [PubMed]


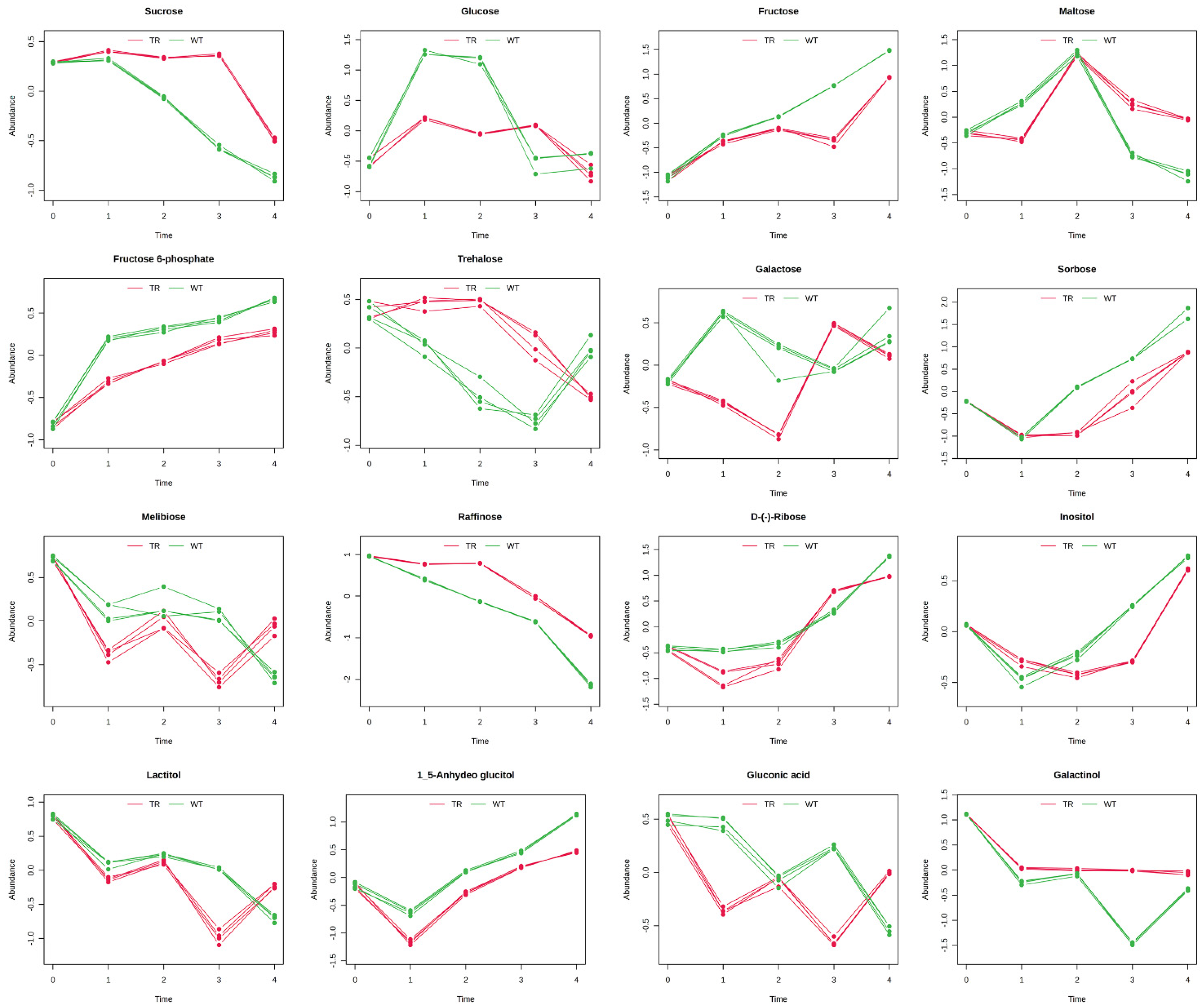
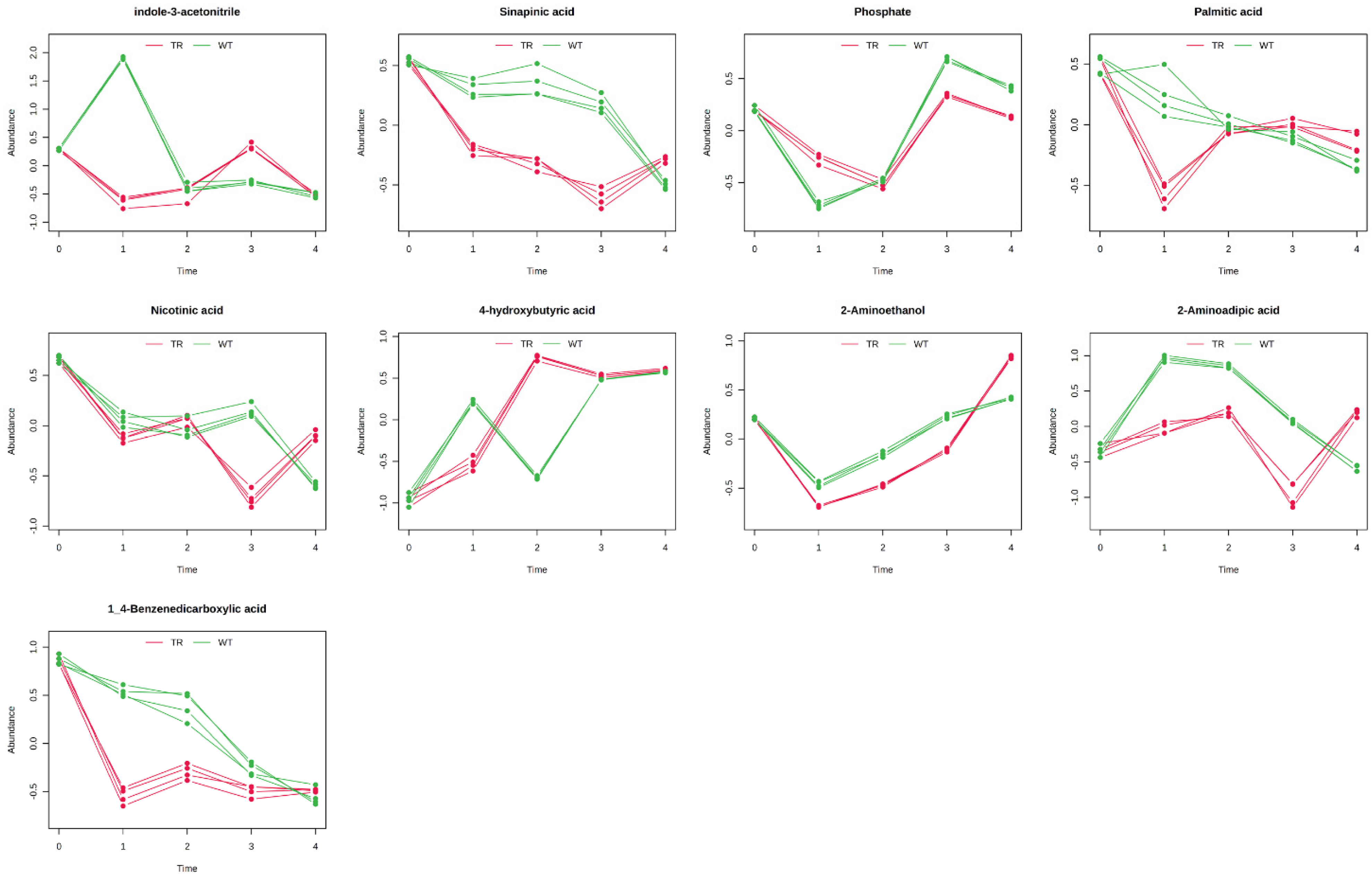
| RT | RI | Metabolite Name | SubClass | RAP% |
|---|---|---|---|---|
| 2.517 | 681.83 | 2-ethyl-furan | Furanoid | 0.45 (±0.08) |
| 2.867 | 706.23 | 2-Methyl-1-butanol | Alcohols and polyols | 0.57 (±0.17) |
| 2.99 | 714.66 | (E)-2-methyl-2-Butenal | Carbonyl compounds | 0.19 (±0.10) |
| 3.446 | 746.61 | 1-Pentanol | Alcohols and polyols | 0.12 (±0.04) |
| 3.594 | 756.92 | 1-Penten-3-ol, 2-methyl- | Alcohols and polyols | 0.41 (±0.06) |
| 3.684 | 763.25 | Methyl α-methylbutanoate | Fatty acid esters | 0.11 (±0.04) |
| 4.192 | 798.74 | 3-Hexenal | Carbonyl compounds | 1.11 (±1.15) |
| 4.23 | 800.16 | Heptane, 2,4-dimethyl- | Alkanes | 0.77 (±0.04) |
| 4.244 | 800.81 | Hexanal | Carbonyl compounds | 0.57 (±0.28) |
| 4.435 | 805.44 | 3-Octene, (Z)- | Unsaturaed aliphatic hydrocarbons | 0.28 (±0.27) |
| 5.332 | 826.58 | 3-Hexene, 1-methoxy-, (Z)- | Ethers | 0.24 (±0.05) |
| 6.18 | 846.69 | Butanoic acid, 2-methyl-, ethyl ester | Fatty acid esters | 0.30 (±0.23) |
| 6.224 | 847.76 | 3-Hexen-1-ol, (E)- | Fatty alcohols | 0.60 (±0.22) |
| 6.395 | 851.78 | 3-Hexen-1-ol, (Z)- | Fatty alcohols | 47.52 (±12.25) |
| 6.573 | 856.22 | 4-Hexen-1-ol, (Z)- | Fatty alcohols | 10.73 (±1.72) |
| 6.976 | 865.51 | 2-Hexen-1-ol, (Z)- | Fatty alcohols | 1.52 (±1.07) |
| 7.63 | 881.05 | 1-Butanol, 2-methyl-, acetate | Carboxylic acids derivatives | 0.38 (±0.17) |
| 8.423 | 899.9 | 4,4-Dimethyl octane | Alkanes | 0.12 (±0.05) |
| 9.052 | 921 | Amyl acetate | Carboxylic acids derivatives | 0.11 (±0.04) |
| 9.349 | 931.05 | Dimethylallyl acetate | Carboxylic acids derivatives | 0.32 (±0.26) |
| 9.541 | 937.55 | alpha-Pinene | Monoterpenoids | 1.49 (±0.22) |
| 10.74 | 978.03 | β-Thujene | Monoterpenoids | 0.13 (±0.01) |
| 11.205 | 993.75 | p-Mentha-1(7),8-diene | Monoterpenoids | 1.66 (±1.26) |
| 11.54 | 1009.79 | 3-Methyl-4-penten-1-ol acetate | Carboxylic acids derivatives | 58.58 (±47.89) |
| 11.659 | 1017.68 | Hexyl acetate | Carboxylic acids derivatives | 4.33 (±1.23) |
| 11.711 | 1020.99 | 2-Hexen-1-yl-acetate | Carboxylic acids derivatives | 3.50 (±2.65) |
| 11.872 | 1032.82 | 3-Carene | Monoterpenoids | 0.31 (±0.20) |
| 11.892 | 1032.82 | L-Limonene | Monoterpenoids | 0.76 (±0.31) |
| 12.064 | 1044.02 | 1,4-p-Menthadien-7-ol | Alcohols and polyols | 0.28 (±0.03) |
| 12.083 | 1045.27 | o-Cresol | Cresols | 0.18 (±0.16) |
| 12.23 | 1054.92 | cis-β-Ocimene | Monoterpenoids | 1.03 (±0.58) |
| 12.345 | 1062.39 | Artemisia alcohol | Alcohols and polyols | 0.19 (±0.20) |
| 12.445 | 1068.61 | Artemesia ketone | Carbonyl compounds | 0.55 (±0.12) |
| 12.792 | 1091.65 | Isoterpinolene | Monoterpenoids | 0.49 (±0.20) |
| 12.935 | 1101.41 | Linalyl formate | Monoterpenoids | 0.39 (±0.26) |
| 12.987 | 1106.3 | Nonanal | Carbonyl compounds | 0.21 (±0.09) |
| 13.134 | 1119.96 | Perillene | Monoterpenoids | 8.25 (±5.77) |
| 13.43 | 1147.7 | (Z)-3-Hexenyl butanoate | Fatty acid esters | 0.24 (±0.10) |
| 13.678 | 1170.83 | α-Acetoxytoluene | Benzyloxycarbonyls | 0.20 (±0.23) |
| 13.768 | 1179.3 | Ethyl benzoate | Benzoic acids and derivatives | 0.15 (±0.03) |
| 13.864 | 1188.19 | (E)-2,6-Dimethyl-3,7-octadien-2,6-diol | Alcohols and polyols | 0.15 (±0.13) |
| 14.022 | 1203.86 | Methyl salicylate | Benzoic acids and derivatives | 7.92 (±8.17) |
| 14.284 | 1235.04 | cis-3-Hexenyl valerate | Fatty acid esters | 2.84 (±1.02) |
| 14.319 | 1239.2 | Hexyl 2-methylbutyrate | Fatty acid esters | 0.19 (±0.02) |
| 14.797 | 1296.07 | 2-Undecanone | Carbonyl compounds | 0.04 (±0.05) |
| 14.84 | 1301.33 | Dihydroedulan II | Sesquiterpenoids | 0.05 (±0.01) |
| 15.192 | 1348.96 | α-Longipinene | Sesquiterpenoids | 0.67 (±0.60) |
| 15.297 | 1363.12 | Copaene | Sesquiterpenoids | 11.52 (±9.81) |
| 15.353 | 1370.63 | Longicyclene | Sesquiterpenoids | 0.48 (±0.35) |
| Pathway | Total Cmpd | Hits | Raw p | FDR | Impact |
|---|---|---|---|---|---|
| Alanine aspartate and glutamate metabolism | 22 | 7 | 3.37 × 10−11 | 9.26 × 10−10 | 0.77698 |
| Galactose metabolism | 27 | 9 | 2.02 × 10−10 | 2.77 × 10−9 | 0.64052 |
| Starch and sucrose metabolism | 22 | 7 | 3.83 × 10−12 | 2.11 × 10−10 | 0.60856 |
| Isoquinoline alkaloid biosynthesis | 6 | 1 | 4.33 × 10−6 | 4.98 × 10−6 | 0.5 |
| Phenylalanine metabolism | 12 | 1 | 2.16 × 10−6 | 3.49 × 10−6 | 0.42308 |
| Arginine biosynthesis | 18 | 6 | 8.27 × 10−9 | 4.13 × 10−8 | 0.36117 |
| Glycine serine and threonine metabolism | 33 | 4 | 4.92 × 10−9 | 3.01 × 10−8 | 0.32415 |
| Fructose and mannose metabolism | 18 | 2 | 1.12 × 10−6 | 2.00 × 10−6 | 0.29099 |
| Arginine and proline metabolism | 32 | 5 | 7.14 × 10−9 | 3.93 × 10−8 | 0.2634 |
| Beta-alanine metabolism | 18 | 2 | 1.04 × 10−8 | 4.42 × 10−8 | 0.25397 |
| Citrate cycle (TCA cycle) | 20 | 4 | 1.94 × 10−10 | 2.77 × 10−9 | 0.21839 |
| Amino sugar and nucleotide sugar metabolism | 52 | 3 | 2.36 × 10−7 | 6.50 × 10−7 | 0.2096 |
| Tryptophan metabolism | 29 | 1 | 2.54 × 10−7 | 6.64 × 10−7 | 0.20611 |
| Tyrosine metabolism | 17 | 2 | 8.72 × 10−7 | 1.81 × 10−6 | 0.20112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, L.; Mircea, D.M.; Araniti, F. Metabolomic Insights into the Allelopathic Effects of Ailanthus altissima (Mill.) Swingle Volatile Organic Compounds on the Germination Process of Bidens pilosa (L.). Metabolites 2025, 15, 12. https://doi.org/10.3390/metabo15010012
Bruno L, Mircea DM, Araniti F. Metabolomic Insights into the Allelopathic Effects of Ailanthus altissima (Mill.) Swingle Volatile Organic Compounds on the Germination Process of Bidens pilosa (L.). Metabolites. 2025; 15(1):12. https://doi.org/10.3390/metabo15010012
Chicago/Turabian StyleBruno, Leonardo, Diana M. Mircea, and Fabrizio Araniti. 2025. "Metabolomic Insights into the Allelopathic Effects of Ailanthus altissima (Mill.) Swingle Volatile Organic Compounds on the Germination Process of Bidens pilosa (L.)" Metabolites 15, no. 1: 12. https://doi.org/10.3390/metabo15010012
APA StyleBruno, L., Mircea, D. M., & Araniti, F. (2025). Metabolomic Insights into the Allelopathic Effects of Ailanthus altissima (Mill.) Swingle Volatile Organic Compounds on the Germination Process of Bidens pilosa (L.). Metabolites, 15(1), 12. https://doi.org/10.3390/metabo15010012









