Genetic Characterization, Transmission Pattern and Health Risk Analysis of Intestinal Colonization ESBL-Producing Escherichia coli in Vegetable Farming Population
Abstract
1. Introduction
2. Materials and Methods
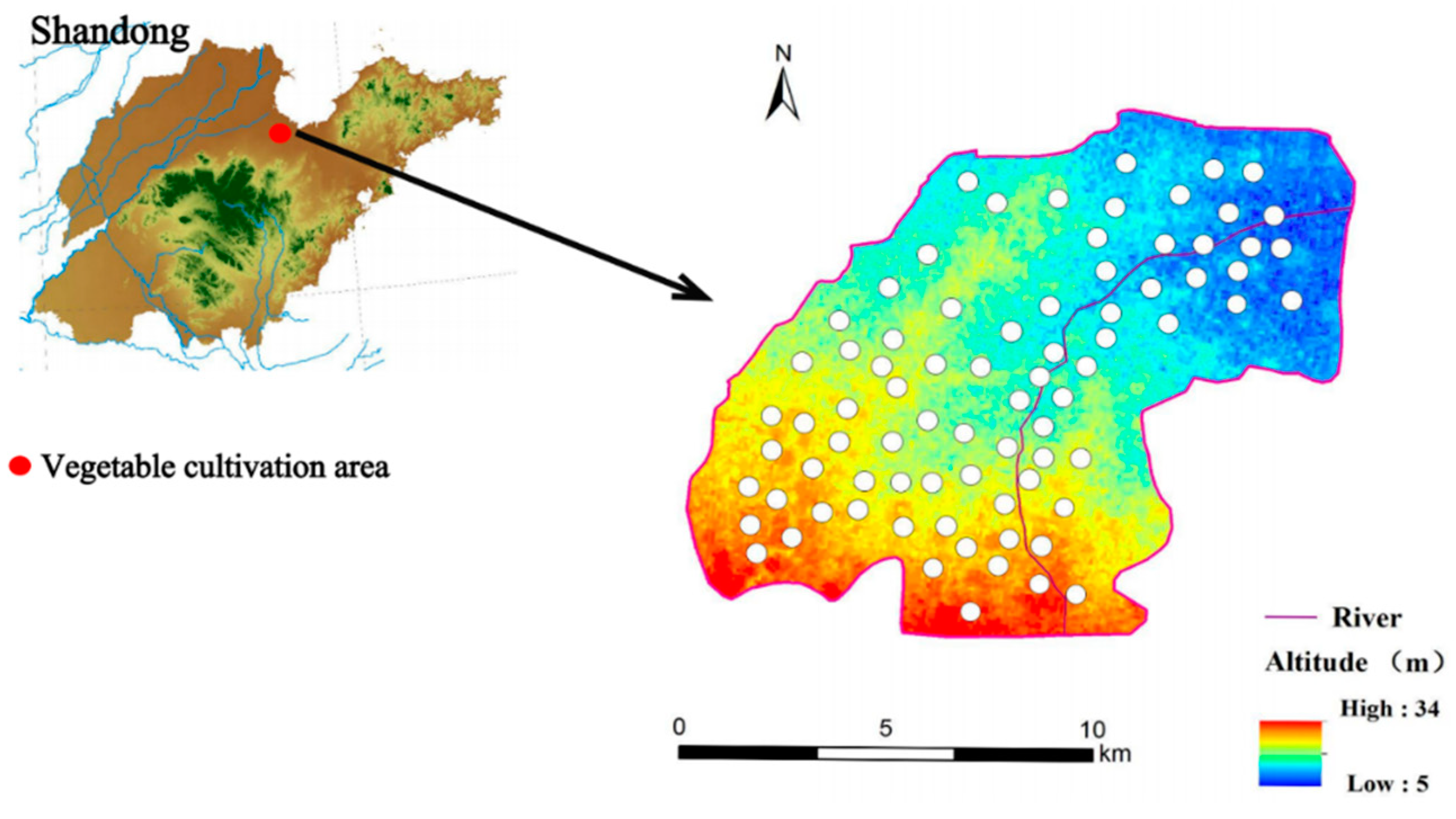
2.1. Study Site and Samples Collection
2.2. Screening of ESBL-Producing Escherichia coli
2.3. Antimicrobial Susceptibility Testing
2.4. Plasmid Transferability-Related Series of Experiments
2.5. Pulsed Field Gel Electrophoresis
2.6. Whole Genome Sequencing and Biosignature Analysis
2.7. Phylogenetic Analysis of Intestinal Colonization of ESBL-Ec
3. Results
3.1. Detection of ESBL-Ec
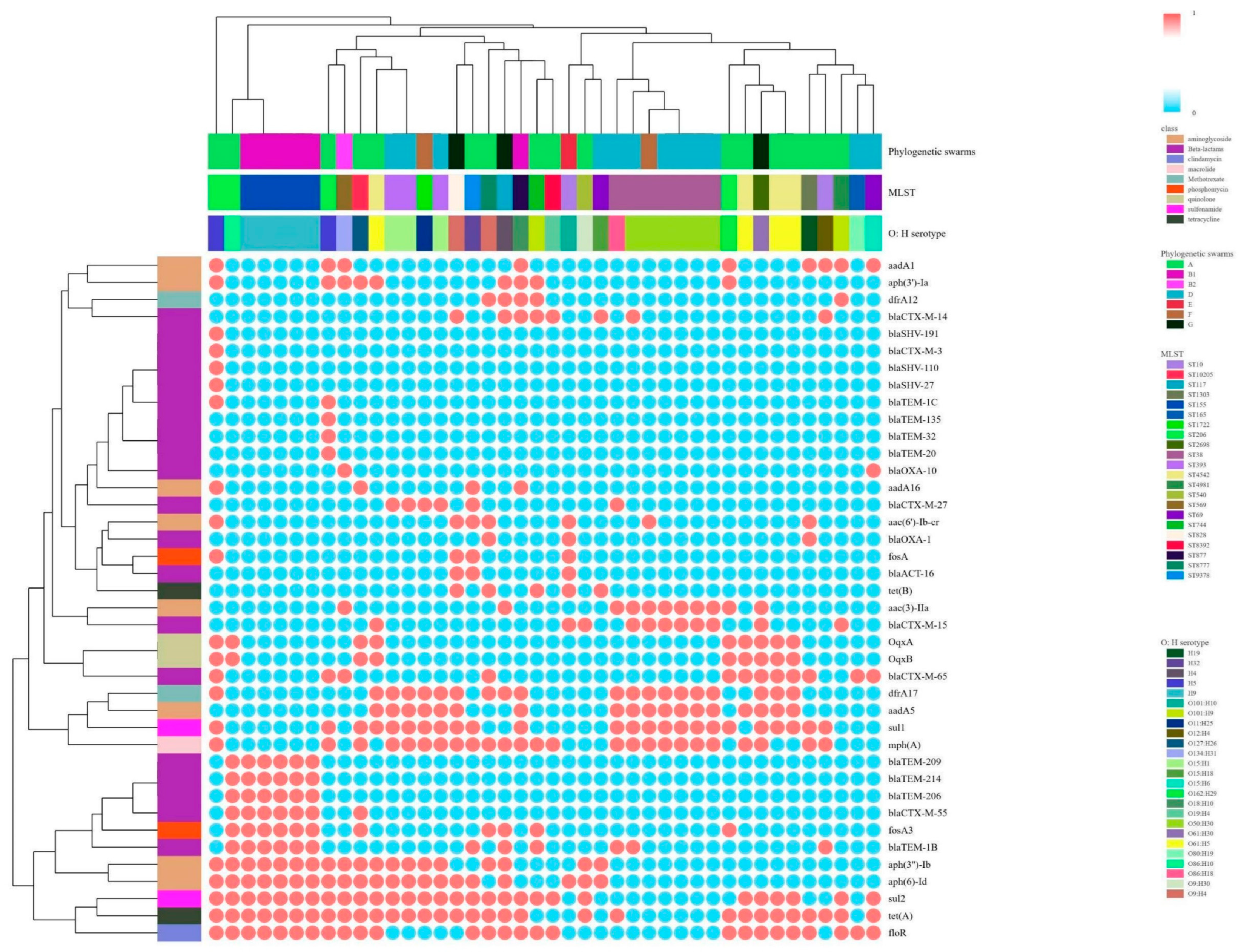
3.2. Characterization of ESBL-Ec Resistance in Intestinal Colonization of Vegetable Farmers
3.2.1. Resistance Phenotypes and Resistance Genes
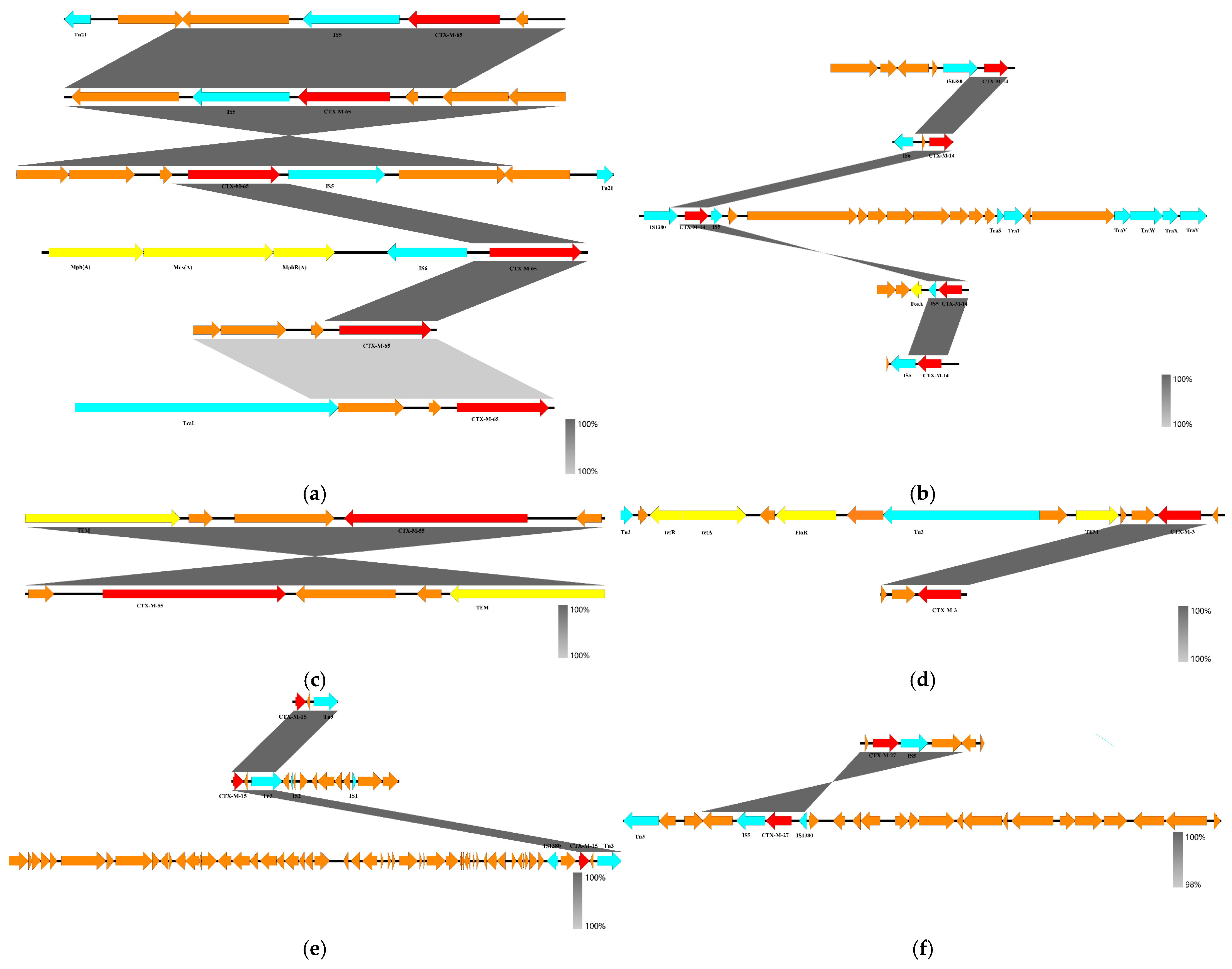
3.2.2. blaCTX-M Gene Skeleton and Plasmid Characterization
3.3. Correlations Among MLST Types, Phylogenetic Groups and Serotypes
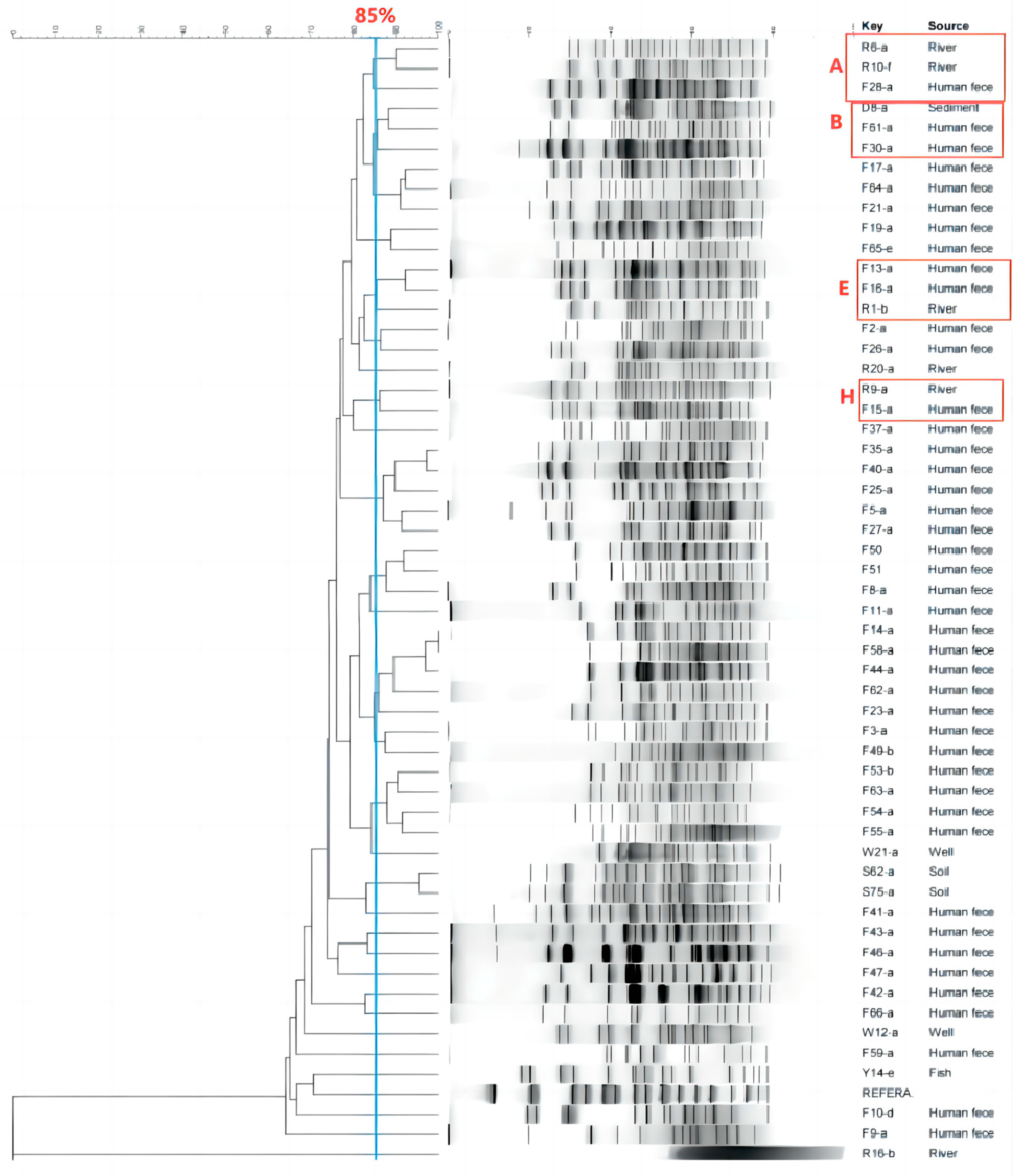
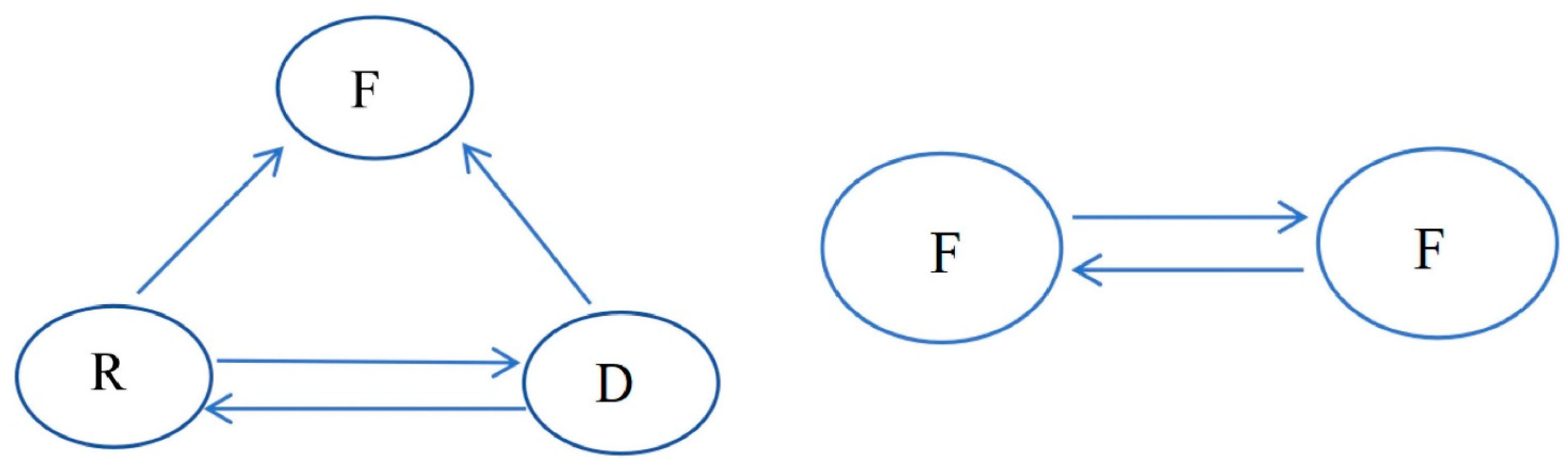
3.4. Analysis of the Transmission of ESBL-Ec for Intestinal Colonization by Vegetable Farmers
3.4.1. Transmission Inside the Vegetable Area
3.4.2. Transmission Outside the Vegetable Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gay, N.; Rabenandrasana, M.A.N.; Panandiniaina, H.P.; Rakotoninidrina, M.F.; Ramahatafandry, I.T.; Enouf, V.; Roger, F.; Collard, J.-M.; Cardinale, E.; Rieux, A.; et al. One Health Compartment Analysis of ESBL-Producing Escherichia coli Reveals Multiple Transmission Events in a Rural Area of Madagascar. J. Antimicrob. Chemother. 2023, 78, 1848–1858. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef]
- Flokas, M.E.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Prevalence of ESBL-Producing Enterobacteriaceae in Paediatric Urinary Tract Infections: A Systematic Review and Meta-Analysis. J. Infect. 2016, 73, 547–557. [Google Scholar] [CrossRef]
- Stewart, A.G.; Harris, P.N.A.; Chatfield, M.D.; Littleford, R.; Paterson, D.L. Ceftolozane-Tazobactam versus Meropenem for Definitive Treatment of Bloodstream Infection Due to Extended-Spectrum Beta-Lactamase (ESBL) and AmpC-Producing Enterobacterales (“MERINO-3”): Study Protocol for a Multicentre, Open-Label Randomised Non-Inferiority Trial. Trials 2021, 22, 301. [Google Scholar] [CrossRef]
- Gaviria, L.P.; Montsant, L.; Azuaje, C.; González-Díaz, A.; Horcajada, J.P.; Limón, E.; Viñas, M.; Espinal, P.; Fusté, E. A Descriptive Analysis of Urinary ESBL-Producing-Escherichia Coli in Cerdanya Hospital. Microorganisms 2022, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zou, H.; Wang, D.; Zhao, L.; Meng, M.; Wang, Z.; Wu, T.; Wang, S.; Li, X. Tracking Spatio-Temporal Distribution and Transmission of Antibiotic Resistance in Aquatic Environments by Using ESBL-Producing Escherichia Coli as an Indicator. J. Environ. Manag. 2023, 344, 118534. [Google Scholar] [CrossRef]
- Sudatip, D.; Mostacci, N.; Tiengrim, S.; Thamlikitkul, V.; Chasiri, K.; Kritiyakan, A.; Phanprasit, W.; Thinphovong, C.; Abdallah, R.; Baron, S.A.; et al. The Risk of Pig and Chicken Farming for Carriage and Transmission of Escherichia coli Containing Extended-Spectrum Beta-Lactamase (ESBL) and Mobile Colistin Resistance (Mcr) Genes in Thailand. Microb. Genom. 2023, 9, 000951. [Google Scholar] [CrossRef]
- Rodríguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.-F.; Kemper, M.; Marutescu, L.; Gradisteanu, G.P.; Popa, M.; Spießberger, B.; Weinmann, T.; et al. Carriage of ESBL-Producing Enterobacterales in Wastewater Treatment Plant Workers and Surrounding Residents—The AWARE Study. Eur. J. Clin. Microbiol. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Zhao, Q. Dissemination of blaNDM-5 via IncX3 Plasmids in Carbapenem-Resistant Enterobacteriaceae among Humans and in the Environment in an Intensive Vegetable Cultivation Area in Eastern China. Environ. Pollut. 2021, 273, 116370. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Oh, S.-S.; Kim, J.; Shin, J. Extended-Spectrum β-Lactamase-Producing Escherichia Coli Isolated from Raw Vegetables in South Korea. Sci. Rep. 2020, 10, 19721. [Google Scholar] [CrossRef] [PubMed]
- Kon, H.; Lurie-Weinberger, M.; Cohen, A.; Metsamber, L.; Keren-Paz, A.; Schwartz, D.; Carmeli, Y.; Schechner, V. Occurrence, Typing, and Resistance Genes of ESBL/AmpC-Producing Enterobacterales in Fresh Vegetables Purchased in Central Israel. Antibiotics 2023, 12, 1528. [Google Scholar] [CrossRef] [PubMed]
- Colosi, I.A.; Baciu, A.M.; Opriș, R.V.; Peca, L.; Gudat, T.; Simon, L.M.; Colosi, H.A.; Costache, C. Prevalence of ESBL, AmpC and Carbapenemase-Producing Enterobacterales Isolated from Raw Vegetables Retailed in Romania. Foods 2020, 9, 1726. [Google Scholar] [CrossRef]
- Baquero, F.; Negri, M.; Morosini, M.; Blázquez, J. Antibiotic-selective Environments. Clin. Infect. Dis. 1998, 27 (Suppl. S1), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Swarthout, J.M.; Chan, E.M.G.; Garcia, D.; Nadimpalli, M.L.; Pickering, A.J. Human Colonization with Antibiotic-Resistant Bacteria from Nonoccupational Exposure to Domesticated Animals in Low- and Middle-Income Countries: A Critical Review. Environ. Sci. Technol. 2022, 56, 14875–14890. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rojas, V.; Villanueva-García, D.; Miranda-Vega, A.L.; Aldana-Vergara, R.; Aguilar-Rodea, P.; López-Marceliano, B.; Reyes-López, A.; Alcántar-Curiel, M.D. Gut Colonization and Subsequent Infection of Neonates Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli and Klebsiella Pneumoniae. Front. Cell. Infect. Microbiol. 2024, 13, 1322874. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zheng, B.; Sun, M.; Ottoson, J.; Li, Y.; Berglund, B.; Chi, X.; Ji, X.; Li, X.; Stålsby Lundborg, C.; et al. Evaluating Dissemination Mechanisms of Antibiotic-Resistant Bacteria in Rural Environments in China by Using CTX-M-Producing Escherichia coli as an Indicator. Microb. Drug Resist. 2019, 25, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; León, S.L.; Blanco, M.; Blanco, J.E.; López, C.; Dahbi, G.; Echeita, A.; González, E.A.; Blanco, J. Phage Types, Virulence Genes and PFGE Profiles of Shiga Toxin-Producing Escherichia Coli O157:H7 Isolated from Raw Beef, Soft Cheese and Vegetables in Lima (Peru). Int. J. Food Microbiol. 2007, 114, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Verschuuren, T.D.; Van Hout, D.; Arredondo-Alonso, S.; Fluit, A.C.; Reuland, E.A.; Top, J.; Schürch, A.C.; Bosch, T.; Bonten, M.J.M.; Kluytmans, J.A.J.W.; et al. Comparative Genomics of ESBL-Producing Escherichia coli (ESBL-Ec) Reveals a Similar Distribution of the 10 Most Prevalent ESBL-Ec Clones and ESBL Genes among Human Community Faecal and Extra-Intestinal Infection Isolates in The Netherlands (2014–17). J. Antimicrob. Chemother. 2021, 76, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S.N.; Hilty, M.; Kronenberg, A.; Droz, S.; Perreten, V.; Endimiani, A. Extended-Spectrum Cephalosporin-Resistant Escherichia Coli in Community, Specialized Outpatient Clinic and Hospital Settings in Switzerland. J. Antimicrob. Chemother. 2013, 68, 2249–2254. [Google Scholar] [CrossRef]
- Song, W.; Lee, H.; Lee, K.; Jeong, S.H.; Bae, I.K.; Kim, J.-S.; Kwak, H.-S. CTX-M-14 and CTX-M-15 Enzymes Are the Dominant Type of Extended-Spectrum β-Lactamase in Clinical Isolates of Escherichia Coli from Korea. J. Med. Microbiol. 2009, 58, 261–266. [Google Scholar] [CrossRef]
- Pietsch, M.; Simon, S.; Meinen, A.; Trost, E.; Banerji, S.; Pfeifer, Y.; Flieger, A. Third Generation Cephalosporin Resistance in Clinical Non-Typhoidal Salmonella Enterica in Germany and Emergence of Bla CTX-M-Harbouring pESI Plasmids. Microb. Genom. 2021, 7, 000698. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, M.; Liu, J.; Zhou, Y.; Miao, Z. Prevalence and Antibiotic Resistance Profiles of Extended-Spectrum β-Lactamase–Producing Escherichia Coli Isolated from Healthy Broilers in Shandong Province, China. J. Food Prot. 2016, 79, 1169–1173. [Google Scholar] [CrossRef]
- Vinueza-Burgos, C.; Ortega-Paredes, D.; Narváez, C.; De Zutter, L.; Zurita, J. Characterization of Cefotaxime Resistant Escherichia Coli Isolated from Broiler Farms in Ecuador. PLoS ONE 2019, 14, e0207567. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Dorado-García, A.; Van Duijkeren, E.; Van Den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable Sources of Community-Acquired Carriage of Escherichia Coli Containing β-Lactam Antibiotic Resistance Genes: A Population-Based Modelling Study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Guo, S.; Chang, W. Prevalence and Characteristics of Extended-Spectrum Beta-Lactamase (ESBL)-Producing Enterobacteriaceae Isolated from Rural Well Water in Taian, China, 2014. Environ. Sci. Pollut. Res. 2015, 22, 11488–11492. [Google Scholar] [CrossRef]
- Sun, J.; Dai, J.; Chen, J.; He, Y.; Su, L.; Gong, M.; Cao, M.; Wei, K.; You, Y.; Liu, L.; et al. Antibiotic Susceptibility and Genomic Analysis of Ciprofloxacin-Resistant and ESBLs-Producing Escherichia Coli in Vegetables and Their Irrigation Water and Growing Soil. Int. J. Food Microbiol. 2024, 414, 110629. [Google Scholar] [CrossRef] [PubMed]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic Resistance Genes Identified in Wastewater Treatment Plant Systems—A Review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- Woerther, P.-L.; Andremont, A.; Kantele, A. Travel-Acquired ESBL-Producing Enterobacteriaceae: Impact of Colonization at Individual and Community Level. J. Travel. Med. 2017, 24 (Suppl. S1), S29–S34. [Google Scholar] [CrossRef]
- Johnson, J.R.; Delavari, P.; Kuskowski, M.; Stell, A.L. Phylogenetic Distribution of Extraintestinal Virulence-Associated Traits in Escherichia coli. J. Infect. Dis. 2001, 183, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, E.O.; Beshiru, A.; Igbinosa, I.H.; Cho, G.S.; Franz, C.M. Multidrug-Resistant Extended Spectrum β-Lactamase (ESBL)-Producing Escherichia Coli from Farm Produce and Agricultural Environments in Edo State, Nigeria. PLoS ONE 2023, 18, e0282835. [Google Scholar] [CrossRef] [PubMed]
- Paris, T.; Kiss, A.; Signor, L.; Lutfalla, G.; Blaise, M.; Boeri Erba, E.; Chaloin, L.; Yatime, L. The IbeA Protein from Adherent Invasive Escherichia coli Is a Flavoprotein Sharing Structural Homology with FAD-Dependent Oxidoreductases. FEBS J. 2024, 291, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Carattoli, A.; Karisik, E.; Underwood, A.; Ellington, M.J.; Livermore, D.M. Complete Nucleotide Sequences of Plasmids pEK204, pEK499, and pEK516, Encoding CTX-M Enzymes in Three Major Escherichia coli Lineages from the United Kingdom, All Belonging to the International O25:H4-ST131 Clone. Antimicrob. Agents Chemother. 2009, 53, 4472–4482. [Google Scholar] [CrossRef]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L.; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective Depletion of Uropathogenic E. coli from the Gut by a FimH Antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef] [PubMed]
- dos Anjos Adur, M.; Châtre, P.; Métayer, V.; Drapeau, A.; Pillonetto, M.; Penkal, M.L.; Lopes, J.K.; Beirão, B.C.B.; Madec, J.-Y.; de Macedo, R.E.F.; et al. Escherichia coli ST224 and IncF/blaCTX-M-55 Plasmids Drive Resistance to Extended-Spectrum Cephalosporins in Poultry Flocks in Parana, Brazil. Int. J. Food Microbiol. 2022, 380, 109885. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids Carrying Antimicrobial Resistance Genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Spychala, C.N.; Hu, F.; Sheng, J.-F.; Doi, Y. Complete Nucleotide Sequences of blaCTX-M-Harboring IncF Plasmids from Community-Associated Escherichia coli Strains in the United States. Antimicrob. Agents Chemother. 2015, 59, 3002–3007. [Google Scholar] [CrossRef]


| Primer Genotype | Primer Sequence | Clip Size | Annealing Temperature |
|---|---|---|---|
| CTX-M-F | TTTGCGATGTGCAGTACCAGTAA | 544 bp | 56 °C |
| CTX-M-R | CGATATCGTTGGTGGTGCCATA | ||
| TEM-F | TTGATCGTTGGGAACCGGAG | 861 bp | 55 °C |
| TEM-R | TCCGCCTCCATCCAGTCTAT | ||
| SHV-F | TGCTCATCATGGGAAAGCGT | 861 bp | 55 °C |
| SHV-R | ATCTCCCTGTTAGCCACCCT | ||
| OXA-F | TCCTGTAAGTGCGGACACAA | 762 bp | 53 °C |
| OXA-R | TGGGAAAACTGGTGCAGGAT |
| Source | Sample Type | Number of Samples | ESBL-Ec | Detection Rate (%) |
|---|---|---|---|---|
| Human | Human feces (F) | 59 | 45 | 76.27 |
| Environment | River (R) | 20 | 6 | 30.00 |
| Well (W) | 24 | 2 | 8.33 | |
| River sediments (D) | 20 | 1 | 5.00 | |
| Soil (S) | 87 | 2 | 2.30 | |
| Fish intestines (Y) | 9 | 1 | 11.11 | |
| Vegetable swab (V) | 106 | 0 | 0.00 | |
| total | 325 | 57 | 17.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, F.; Zhao, Q.; Wang, D.; Li, X. Genetic Characterization, Transmission Pattern and Health Risk Analysis of Intestinal Colonization ESBL-Producing Escherichia coli in Vegetable Farming Population. Microorganisms 2024, 12, 2646. https://doi.org/10.3390/microorganisms12122646
Yao F, Zhao Q, Wang D, Li X. Genetic Characterization, Transmission Pattern and Health Risk Analysis of Intestinal Colonization ESBL-Producing Escherichia coli in Vegetable Farming Population. Microorganisms. 2024; 12(12):2646. https://doi.org/10.3390/microorganisms12122646
Chicago/Turabian StyleYao, Fanghui, Qian Zhao, Di Wang, and Xuewen Li. 2024. "Genetic Characterization, Transmission Pattern and Health Risk Analysis of Intestinal Colonization ESBL-Producing Escherichia coli in Vegetable Farming Population" Microorganisms 12, no. 12: 2646. https://doi.org/10.3390/microorganisms12122646
APA StyleYao, F., Zhao, Q., Wang, D., & Li, X. (2024). Genetic Characterization, Transmission Pattern and Health Risk Analysis of Intestinal Colonization ESBL-Producing Escherichia coli in Vegetable Farming Population. Microorganisms, 12(12), 2646. https://doi.org/10.3390/microorganisms12122646






